Abstract
Among the F2-isoprostanes, the 15- and the 5-series are currently used as markers of lipid peroxidation in vascular diseases. 15-F2t-IsoP (also named iPF2α-III) exerts a vasoconstriction in most vessels, whereas no data is available concerning 5-F2t-IsoP (also named iPF2α-VI), which is more abundant in plasma.
The aim of this study was to determine whether 5-F2t-IsoP possess any vascular effects on various vessels including the isolated rat thoracic aorta, the human internal mammary artery and the saphenous vein.
In organ baths, 5-F2t-IsoP and its 5-epimer did not affect the basal tone of any vessel, unlike 15-F2t-IsoP. These compounds possessed no antagonist effects on 15-F2t-IsoP-induced contractions, No dilator effect was observed in comparison with sodium nitroprusside and acetylcholine on the rat aorta.
In conclusion, we show that unlike 15-F2t-IsoP, 5-F2t-IsoP and its 5-epimer possess no vasomotor effects and as such are unlikely to be involved in the pathogenesis of vascular diseases. Further studies are required to test whether these mediators may have effects on systems not being measured in the current study.
Keywords: Isoprostane, 5-F2t-IsoP, lipid peroxidation, vascular reactivity, internal mammary artery, saphenous vein
Introduction
Isoprostanes are a family of compounds produced in vivo by non-enzymatic free radical-induced peroxidation of arachidonic acid (Morrow et al., 1990). Depending on which of the labile hydrogen atoms is first abstracted by free radicals, three initial arachidonoyl radicals can be formed, which then form four prostaglandin H2 isomers. Fully reduced, they can form four F2-isoprostane regioisomers (Figure 1), each of which is comprised of eight diastereoisomers. F2-isoprostanes are initially formed esterified on phospholipids and then released in free form by phospholipases (Morrow et al., 1992). Among these F2-isoprostanes, 15-F2t-IsoP (Taber et al., 1997) also named isoprostaglandin F2α type III (Rokach et al., 1997) or 8-iso PGF2α, and 5-F2t-IsoP (isoprostaglandin F2α type VI), respectively from the 15-series and the 5-series, are currently quantifiable in plasma and urine, and are extensively used as clinical markers of lipid peroxidation in vascular diseases (Patrono & Fitzgerald, 1997, Cracowski et al., 2001). Elevated levels of 5-F2t-IsoP have been described in atherosclerosis (Pratico et al., 1997), myocardial reperfusion following thrombolysis (Reilly et al., 1997) and percutaneous transluminal coronary angioplasty (Iuliano et al., 2001).
Figure 1.
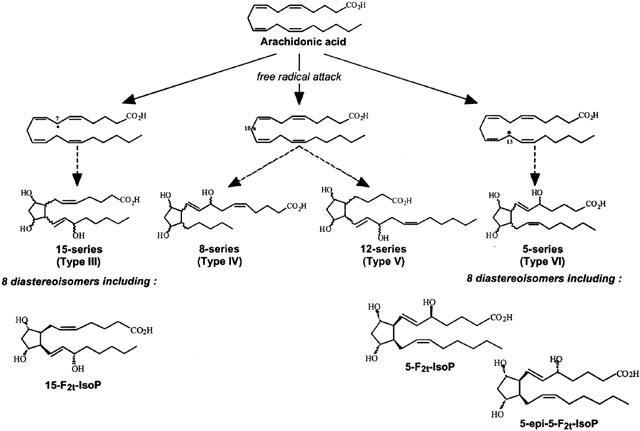
F2-Isoprostanes formation from arachidonic acid, leading to four F2-Isoprostane regioisomers. For simplicity, the intermediate compounds are not shown.
15-F2t-IsoP induces vasoconstriction in numerous animals and human vessels, via. TP-receptor activation (Kromer & Tippins, 1996; 1998; Oliveira et al., 2000; Cracowski et al., 2001). In contrast, no data is available concerning the vascular properties of 5-F2t-IsoP. Such observations are of the utmost interest since 5-F2t-IsoP and its 5-epimer have been shown to be generated in greater concentrations than 15-F2t-IsoP in humans (Li et al., 1999).
Therefore, the aim of this study was to determine the vascular effects of 5-F2t-IsoP and its 5-epimer on the isolated rat thoracic aorta, the human internal mammary artery and the human saphenous vein.
Methods
Isolated preparations
In accordance with French law and the local ethical committee guidelines for animal research, male Wistar rats (370 – 430 g IFFA CREDO, Lyon, France) were housed in climate controlled conditions and provided with standard rat chow. Animals were anaesthetized with an intraperitoneal injection of 60 mg kg−1 sodium pentobarbital (Sanofi, Libourne, France). Heparin (150 IU, Sanofi Winthrop, Gentilly, France) was injected intravenously. Then, the thoracic aorta was quickly excised, cleaned of connective tissue and cut into 4-mm lengths. Six rings were taken from each thoracic aorta. The endothelium was removed from some aortic rings by gently rolling the tip of a plastic forceps inside the vessel.
Human internal mammary arteries and saphenous veins were obtained from patients undergoing coronary bypass surgery. The discarded distal ends of the arterial and venous grafts were immediately placed in oxygenated HEPES-buffered Krebs solution maintained at 4°C and transferred to the laboratory within 2 h. The HEPES-buffered Krebs solution had following composition (mM): NaCl (130), KCl (3.8), CaCl2 (2.1), MgSO4 (1.2), KH2PO4 (1.2), NaHCO3 (14.8), glucose (10.4) and HEPES (10). Blood vessels were dissected free from connective tissue and cut into 4-mm lengths.
Experimental design
The methods used for the measurement of isometric tension were as previously reported (Cracowski et al., 2000; Stanke-Labesque et al., 2001). Briefly, rings were suspended in organ chambers filled with 6 ml of Krebs solution maintained at 37°C and gassed with a mixture of 95% oxygen and 5% carbon dioxide. Segments were mounted between two stainless steel wires. The upper wire was fixed to a force transducer through which changes in isometric forces were continuously displayed on a recorder. The rings were initially stretched and were allowed to equilibrate for 60 min. The rings were then challenged twice with KCl (90 mM) at a 10-min interval. The endothelial function was assessed by testing the relaxant effect of acetycholine (1 μM) on aortic rings precontracted with methoxamine (3 μM). Following a further 60-min period, concentration – contraction curves were made. Only one cumulative concentration – contraction curve was established in each ring. Four rings were run in parallel.
The vasomotor effects of 5-F2t-IsoP and 5-epi-5-F2t-IsoP were tested on rat thoracic aortic rings: (1) The contractile responses were compared to 15-F2t-IsoP. The role of the endothelium was assessed by comparing the response to 5-F2t-IsoP and 5-epi-5-F2t-IsoP in rings with an intact or denuded endothelium. (2) In order to test an antagonist activity of 5-F2t-IsoP or 5-epi-5-F2t-IsoP on the contractile response to 15-F2t-IsoP, concentration – responses curves to 15-F2t-IsoP were obtained 30 min after pretreatment with 5-F2t-IsoP and 5-epi-5-F2t-IsoP (10−5 M). (3) To determine the potential dilator effects, the rings were contracted by phenylephrine (10−7 M). When a stable plateau was reached, 5-F2t-IsoP and 5-epi-5-F2t-IsoP were added in a cumulative fashion (10−10 – 10−5 M), and compared to sodium nitroprusside and acetylcholine-induced relaxation (10−9 – 10−4 M).
Contractile experiments were performed on rings of internal mammary arteries and saphenous veins in order to study 5-F2t-IsoP and 5-epi-5-F2t-IsoP effects on both arterial and venous human vessels in comparison with 15-F2t-IsoP.
Drugs
15-F2t-IsoP (8-iso-prostaglandin F2α) was purchased from Cayman (Ann Arbor, U.S.A.), sodium nitroprusside (SNP), acetylcholine (Ach) and phenylephrine from Sigma (Saint Quentin Fallavier, France). 5-F2t-IsoP and 5-epi-5-F2t-IsoP were synthesized according to our procedure (Durand et al., 2001). All isoprostanes were dissolved in methanol at 10−2 M. Stocks solutions were then diluted in distilled water before being added to the organ baths. The highest concentration of methanol was 0.1%, which had no direct effect on the vascular tone in preliminary experiments.
The 5-series of isoprostanes (5-F2t-isoP) is the only group of isoprostanes that have an OH function on the C-5 relative to the COOH and are expected to form a six-membered-ring lactone 2 (Figure 2). The lactone 2 being much less polar than the unlactonized hydroxy acid 1b, it was easily to check the stability of such isoprostanes by thin layer chromatography. We have also, checked the stability of the methyl ester of 5-F2t-isoP 1a and analogues by 1H and 13C NMR spectroscopy. Using these two different techniques, we are able to conclude that the 5-F2t-isoP 1b and all epimers are stable compounds, when they are kept at −20°C in a solution of methyl alcohol under nitrogen, up to 1 year.
Figure 2.
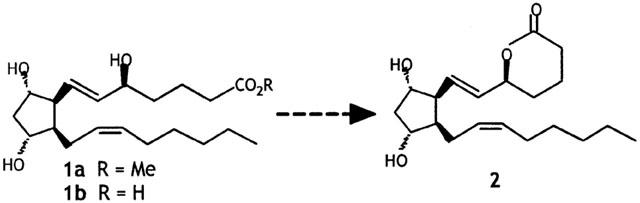
Potential lactonization of 5-series F2-isoprostanes.
The stability of 5-F2t-IsoP in Krebs' solution was checked by gas chromatography-electronic impact mode mass spectrometry, using 15 F2t-IsoP-d4 as the internal standard with a methodology derived from 15-F2t-IsoP quantification (Bessard et al., 2001). We added 100 μl of 5-F2t-IsoP (10−3 M) in 900 μl Krebs' solution, and maintained at 37°C during 0, 30 and 60 min. The respective concentrations were 10−4 M, 9.2 10−5 M and 9.4 10−5 M (ANOVA: NS).
Data analysis
Concentration – contraction curves were expressed as a percentage of KCl 90 mM-induced contraction. Relaxation curves were expressed as a percentage of the initial phenylephrine (10−7 M) contraction. Maximum contraction (Emax) and potency (pEC50) were calculated to determine the arterial segment reactivity. Emax was expressed as a percentage of KCl 90 mM-induced maximal contraction. The effective concentration of agent that caused 50% of maximum contraction (EC50) was calculated from each curve by a logistic, curve-fitting equation. EC50 values were expressed as pEC50 (−log EC50). Data were expressed as mean±s.e.m. Unpaired t-tests were used to test the statistical significance between two means. More than two means were compared with the use of analysis of variance. Values of P<0.05 were considered significant.
Results
Vasomotor effects on rat aorta
5-F2t-IsoP and 5-epi-5-F2-IsoP induced no variation of the baseline on the rat aorta (Figure 3A), unlike 15-F2t-IsoP (pEC50=5.98±0.17; Emax=106±15%). Similarly no contraction was observed in endothelium denuded rings. Pretreatment with 5-F2t-IsoP (10−5 M) and 5-epi-5-F2t-IsoP (10−5 M) had no effect on 15-F2t-IsoP concentration – response curves (pEC50=5.98±0.17; 5.91±0.26 and 5.72±0.34; Emax=106± 15%; 94±22% and 105±7% in the presence of vehicle, 5-F2t-IsoP (10−5 M) and 5-epi-5-F2t-IsoP (10−5 M) respectively, NS). SNP and Ach (10−9 – 10−4 M) induced a significant aortic relaxation (SNP: pEC50 8.14±0.26, Emax 106±5% and Ach: pEC50 6.7±0.87, Emax 90±3%), whereas both 5-F2t-IsoP and 5-epi-5-F2t-IsoP, had no effect on rat aortic rings precontracted with phenylephrine (10−7 M) (Figure 4).
Figure 3.
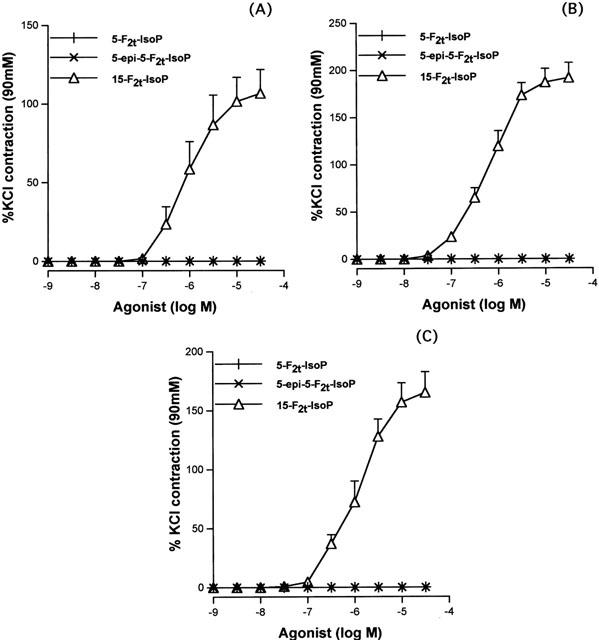
Concentration – contraction curves to 5-F2t-IsoP and its epimer (5-epi-5-F2t-IsoP) in comparison with 15-F2t-IsoP in the rat aorta (A), the human internal mammary artery (B) and the saphenous vein (C). (n=6 in all groups).
Figure 4.
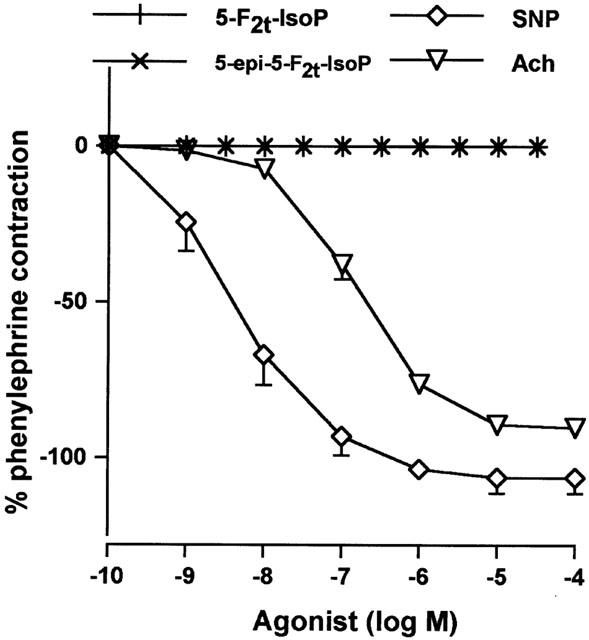
Concentrations – relaxation curves to 5-F2t-IsoP and its epimer (5-epi-5-F2t-IsoP) in comparison to sodium nitroprusside (SNP) and acetylcholine (Ach) in rat aortic rings precontracted with phenylephrine (10−7 M) (n=6 in all groups).
Contractile responses on human internal mammary arteries and saphenous veins
5-F2t-IsoP and 5-epi-5-F2t-IsoP had no vasoconstrictor effect on human internal mammary arteries and saphenous veins (Figure 3B,C), unlike 15-F2t-IsoP that induced a concentration – dependent vasoconstriction (pEC50=6.21±0.1 and 5.85±0.1; Emax=191±16% and 165±18% in internal mammary arteries and saphenous veins, respectively).
Discussion
5-F2t-IsoP and its 5-epimer did not affect the basal tone of the rat thoracic aorta as well as the human internal mammary artery and the saphenous vein. In addition, these compounds had neither antagonist effects on 15-F2t-IsoP-induced contractions, nor dilator effects on the rat thoracic aorta. Therefore, 5-F2t-IsoP, unlike 15-F2t-IsoP, had no vasomotor effect on arterial and venous blood vessels, which remained consistent between species.
15-F2t-IsoP induces a vasoconstriction, mediated by TP-receptor stimulation, which may be modulated by the endothelium (Cracowski et al., 2001). Although the 5- and the 15-series F2-Isoprostanes are both produced through free radical peroxidation of arachidonic acid, they differ from their initial arachidonoyl radical that leads to major differences in the lateral chain structure (see Figure 1). The 15-F2t-IsoP shares similar side chains with the classical prostanoids thromboxane A2 and prostaglandin F2α in contrast to the lack of 15S-hydroxyl in the 5-series isoprostanes, which is essential to high agonist potency on prostanoid receptors. In line with this hypothesis, the 5-series F2-Isoprostanes had no vasomotor effect in the vessels examined.
Substantial evidence has been accumulated to support the use of urinary isoprostanes analysis as a non invasive index of lipid peroxidation in vivo (Roberts & Morrow, 2000). Attention was initially focused on 15-F2t-IsoP quantification. Recently, other isomers, 5-F2t-IsoP, 5-F2c-IsoP and 5-epi- 5-F2t-IsoP, were detected and quantified in plasma, coronary sinus or urine by mass spectrometry (Pratico et al., 1998; Li et al., 1999; Iuliano et al., 2001). To date, the major differences between the 5- and the 15-series F2-Isoprostanes were their respective concentrations: 5-F2t-IsoP levels were found to be approximately 3 – 4 times higher than 15-F2t-IsoP (Li et al., 1999; Iuliano et al., 2001), enabling an easier quantification. The second difference is that their formation in human diseases may be dissociated: a proportional increase was found in cigarette smoking (Pratico et al., 1998), hypercholesterolemia (Reilly et al., 1998; Li et al., 1999) and percutaneous coronary angioplasty (Iuliano et al., 2001), whereas the urinary levels of the 15-series F2-Isoprostanes, but not of the 5-series were elevated in cardiac failure (Li et al., 1999). The present study shows a third difference between 15-F2t-IsoP and 5-F2t-IsoP that is of the utmost importance: 15-F2t-IsoP is a vasoconstrictor, which has been hypothesized to be involved in the pathogenesis of coronary vasospasm (Iuliano et al., 2001), whereas 5-F2t-IsoP has no vasomotor effects, and as such is not likely to be involved in the pathogenesis of vascular diseases. Our study does not rule out the possibility that the 5-series may share other biological activity of 15-F2t-IsoP such as platelet aggregation inhibition (Cranshaw et al., 2001), neutrophil adhesion (Fontana et al., 2001), cardiomyocyte hypertrophy (Kunapuli et al., 1998). Further studies are required to test whether these mediators may have effects on systems not being measured in the current study.
In conclusion, most attention on isoeicosanoid analysis has focused recently on 5-F2t-IsoP, which is more abundant in biologicals fluids than 15-F2t-IsoP. We show that unlike 15-F2t-IsoP, 5-F2t-IsoP and its epimer possess no vasomotor effects and as such are unlikely to contribute to the pathogenesis of vascular diseases.
Acknowledgments
The editorial assistance of Mark Hunt and the technical assistance of Françoise Caron and Jocelyne Truchet are gratefully acknowledged.
Abbreviations
- 5-epi-5-F2t-IsoP
5-epi-5-F2t-Isoprostane
- 5-F2t-IsoP
5-F2t-Isoprostane
- 15-F2t-IsoP
15-F2t-Isoprostane
- Ach
acetylcholine
- SNP
sodium nitroprusside
- TP-receptor
thromboxane/PGH2-receptor
References
- BESSARD J., CRACOWSKI J.L., STANKE-LABESQUE F., BESSARD G. Determination of isoprostaglandin F2α type III in human urine by gas chromatography-electronic impact mass spectrometry. Comparison with enzyme immunoassay. J. Chromatogr. B. 2001;754:333–343. doi: 10.1016/s0378-4347(00)00621-6. [DOI] [PubMed] [Google Scholar]
- CRACOWSKI J.L., DEVILLIER P., DURAND T., STANKE-LABESQUE F., BESSARD G. Vascular biology of the isoprostanes. J. Vasc. Res. 2001;38:93–103. doi: 10.1159/000051036. [DOI] [PubMed] [Google Scholar]
- CRACOWSKI J.L., STANKE-LABESQUE F., DEVILLIER P., CHAVANON O., HUNT M., SOUVIGNET C., BESSARD G. Human internal mammary artery contraction by isoprostaglandin F2α type-III (8-iso-prostaglandin F2α) Eur. J. Pharmacol. 2000;397:161–168. doi: 10.1016/s0014-2999(00)00217-x. [DOI] [PubMed] [Google Scholar]
- CRANSHAW J.H., EVANS T.W., MITCHELL J.A. Characterization of the effects of isoprostanes on platelet aggregation in human whole blood. Br. J. Pharmacol. 2001;132:1699–1706. doi: 10.1038/sj.bjp.0704019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURAND T., CRACOWSKI J.L., GUY A., ROSSI J.C. Syntheses and preliminary pharmacological evaluation of the two epimers of the 5-F2t-isoprostane. Bioorg. Med. Chem. Lett. 2001;11:2495–2498. doi: 10.1016/s0960-894x(01)00473-5. [DOI] [PubMed] [Google Scholar]
- FONTANA L., GIAGULLI C., MINUZ P., LECHI A., LAUDANNA C. 8-Iso-PGF2α induces beta 2-integrin-mediated rapid adhesion of human polymorphonuclear neutrophils: a link between oxidative stress and ischemia/reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2001;21:55–60. doi: 10.1161/01.atv.21.1.55. [DOI] [PubMed] [Google Scholar]
- IULIANO L., PRATICO D., GRECO C., MANGIERI E., SCIBILIA G., FITZGERALD G.A., VIOLI F. Angioplasty increases coronary sinus F2 isoprostane formation: evidence for in vivo oxydative stress during PTCA. J. Am. Coll. Cardiol. 2001;37:76–80. doi: 10.1016/s0735-1097(00)01040-8. [DOI] [PubMed] [Google Scholar]
- KROMER B., TIPPINS J.R. Coronary artery constriction by the isoprostane 8-epi-prostaglandin F2α. Br. J. Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROMER B., TIPPINS J.R. Actions of 8-epi-prostaglandin F2α. on isolated rat aorta. J. Cardiovasc. Pharmacol. 1998;32:471–478. doi: 10.1097/00005344-199809000-00019. [DOI] [PubMed] [Google Scholar]
- KUNAPULI P., LAWSON J.A., ROKACH J.A., MEINKOTH J.L., FITZGERALD G.A. Prostaglandin F2α (PGF2α) and the isoprostane, 8, 12-iso-isoprostane F2α-III, induce cardiomyocyte hypertrophy. Differential activation of downstream signaling pathways. J. Biol. Chem. 1998;273:22442–22452. doi: 10.1074/jbc.273.35.22442. [DOI] [PubMed] [Google Scholar]
- LI H., LAWSON J.A., REILLY M., ADIYAMAN M., HWANG S.W., ROKACH J., FITZGERALD G.A. Quantitative high performance liquid chromatography / tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urine. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J.D., AWAD J., BOSS H., BLAIR I., ROBERTS L., II Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J.D., HILL K.E., BURK R.F., NAMMOUR T.M., BADR K.F., ROBERTS L.J. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVEIRA L., STALLWOOD N.A., CRANKSHAW D.J. Effects of some isoprostanes on the human umbilical artery in vitro. Br. J. Pharmacol. 2000;129:509–514. doi: 10.1038/sj.bjp.0703083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRONO C., FITZGERALD G.A. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler. Thromb. Vasc. Biol. 1997;17:2309–2315. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- PRATRICO D., BARRY O.P., LAWSON J.A., ADIYAMAN M., HWANG S.W., KHANAPURE S.P., IULIANO L., ROKACH J., FITZGERALD G.A. IPF2α-I: an index of lipid peroxidation in humans. Proc. Natl. Acad. Sci, USA. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRATICO D., IULIANO J., MAURIELLO A., SPAGNOLI S., LAWSON J., MACLOUF J., VIOLI F., FITZGERALD G.A. Localisation of distinct F2 isoprostanes in human atherosclerotic lesions. J. Clin. Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REILLY N., DELANTY N., ROY L., ROKACH J., CALLAGHAN P.O., CREAN P., LAWSON J.A., FITZGERALD G.A. Increased formation of the Isoprostanes IPF2α-I and 8-epi PGF2α in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–3320. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- REILLY N., PRATICO D., DELANTY N., DIMINNO G., TREMOLI E., RADER D.J., KAPOOR S., ROKACH J., LAWSON J.A., FITZGERALD G.A. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–2828. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- ROBERTS L.J., MORROW J.D. Measurement of F2 isoprostanes as an index of oxydative stress in vivo. Free Radic. Biol. Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- ROKACH J., KHANAPURE S.P., HWANG S.W., ADIYAMAN M., LAWSON J.A., FITZGERALD G.A. Nomenclature of the isoprostanes: a proposal. Prostaglandins. 1997;54:853–873. doi: 10.1016/s0090-6980(97)00184-6. [DOI] [PubMed] [Google Scholar]
- STANKE-LABESQUE F., DEVILLIER P., VEITL S., CARON F., CRACOWSKI J.L., BESSARD G. Cysteinyl leukotrienes are involved in angiotensin II-induced contraction of aorta from spontaneously hypertensive rats. Cardiovasc Res. 2001;49:152–160. doi: 10.1016/s0008-6363(00)00238-8. [DOI] [PubMed] [Google Scholar]
- TABER D.F., MORROW J.D., ROBERTS L.J., II A nomenclature system for the isoprostanes. Prostaglandins. 1997;53:63–67. doi: 10.1016/s0090-6980(97)00005-1. [DOI] [PubMed] [Google Scholar]


