Abstract
The flavonol quercetin has been shown to activate a Cl− secretion in rat colon. Unlike the secretory activity of the related isoflavone genistein, quercetin's secretory activity does not depend on cyclic AMP; instead, it depends on Ca2+. We investigated the possible involvement of Ca2+ dependent basolateral K+ channels using apically permeabilized rat distal colon epithelium mounted in Ussing chambers.
In intact epithelium, quercetin induced an increase in short-circuit current (Isc), which was diminished by the Cl− channel blockers NPPB and DPC, but not by glibenclamide, DIDS or anthracene-9-carboxylic acid. The effect of the flavonol was also inhibited by several serosally applied K+ channel blockers (Ba2+, quinine, clotrimazole, tetrapentylammonium, 293B), whereas other K+ channel blockers failed to influence the quercetin-induced increase in Isc (tetraethylammonium, charybdotoxin).
The apical membrane was permeabilized by mucosal addition of nystatin and a serosally directed K+ gradient was applied. The successful permeabilization was confirmed by experiments demonstrating the failure of bumetanide to inhibit the carbachol-induced current.
In apically permeabilized epithelium, quercetin induced a K+ current (IK), which was neither influenced by ouabain nor by bumetanide. Whereas DPC, NPPB, charybdotoxin and 293B failed to inhibit this IK, quinine, Ba2+, clotrimazole and tetrapentylammonium were effective blockers of this current.
We conclude from these results that at least part of the quercetin-induced Cl− secretion can be explained by an activation of basolateral K+ channels.
Keywords: Cl− secretion, colon, quercetin, flavonoids, K+ channels
Introduction
The flavonol quercetin is widely distributed in higher plants and, therefore, the most abundant flavonoid in the diet of humans and herbivorous animals (Herrmann, 1988; Hertog et al., 1993). We have recently demonstrated that this flavonol induces a Cl− and HCO3− secretion in rat colon epithelium (Cermak et al., 1998). Intestinal Cl− secretion depends on the combined activity of several transport mechanisms. The Na+/K+ ATPase generates the Na+ gradient, which is responsible for the combined influx of Na+, K+, and Cl− via basolateral Na+/K+/2 Cl− cotransport. The Cl− ions are secreted via the cystic fibrosis transmembrane conductance regulator (CFTR), which represents the Cl− channel in the apical membrane of the colon mucosa. The K+ ions can recycle via basolateral K+ channels (Greger et al., 1997). Changes in any of these mechanisms will alter epithelial Cl− secretion.
Secretagogues principally activate two different second messenger systems. Cyclic adenosine monophosphate (cyclic AMP) activates Cl− secretion via protein kinase A dependent phosphorylation of CFTR (Greger, 2000). Furthermore, cyclic AMP activates a certain class of basolateral K+ channels (Warth et al., 1996). Basolateral K+ channels are mandatory for Cl− secretion. Activation of these channels hyperpolarizes the membrane potential, thereby providing the electrochemical driving force for the apical exit of Cl− ions (Greger et al., 1997). Another second messenger is used by a variety of secretagogues such as muscarinic agonists. They increase intracellular Ca2+ via Ca2+ release from intracellular stores and via Ca2+ influx through cation selective channels (Frings et al., 1999; Greger et al., 1997). The rise in cytosolic Ca2+ leads to a hyperpolarization of the membrane potential due to the opening of basolateral and apical K+ channels, which are distinct from the ones activated by cyclic AMP. Hence, Cl− secretion increases without direct involvement of CFTR (Mall et al., 1998; Schultheiss & Diener, 1997; Strabel & Diener, 1995).
In an attempt to identify the underlying mechanism of the secretory activity of quercetin, we observed in previous studies that quercetin's effect is independent of cyclic AMP. Instead, it depends on Ca2+/calmodulin. Quercetin diminishes the secretion evoked by the muscarinic agonist carbachol. However, we demonstrated that the flavonol does not act via muscarinic receptors (Cermak et al., 1998; 2000). Since quercetin's activity is dependent on Ca2+, the flavonol should activate basolateral K+ channels in a similar manner to carbachol.
The objective of the present study was to test this hypothesis. For this purpose, we used intact rat distal colon mucosa as well as apically permeabilized tissue sheets mounted in Ussing chambers.
Methods
Solutions
The standard buffer solution contained (in mM) NaCl, 107; KCl, 4.5; NaHCO3, 25; Na2HPO4, 1.8; NaH2PO4, 0.2; CaCl2, 1.25; MgCl2, 1; and glucose 12. The solution was gassed with 5% CO2 in 95% O2 and kept at 37°C; pH was adjusted to 7.4. When Ba2+ was used, bicarbonate was replaced by Cl− and 10 mM HEPES (N-[2-Hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]) was added to the solution which was gassed with 100% O2. In the experiments with apically permeabilized epithelia, a serosally directed K+ gradient was applied by changing the standard solution on both sides of the epithelium. The mucosal buffer contained (in mM): KCl, 114.5; NaHCO3, 25; Na2HPO4, 1.8; NaH2PO4, 0.2; MgCl2, 1 and glucose 12; the serosal buffer contained (in mM) KCl, 4.5; N-methyl-D-glucamine (NMDG+) chloride, 110; NaHCO3, 25; Na2HPO4, 1.8; NaH2PO4, 0.2; CaCl2, 1.25; MgCl2, 1 and glucose, 12. In the experiments with Ba2+ and Gd3+, bicarbonate was replaced by Cl−; 10 mM HEPES and 10 mM Tris (2-amino-2-hydroxymethyl-propan-1,3-diol) were added to the mucosal and serosal solution, respectively, which were gassed with 100% O2.
Tissue preparation
Male Sprague Dawley rats (180 – 250 g) (RCC Ltd., Füllinsdorf, Switzerland) were used. The animals had free access to water and food (Eberle Nafag AG, Gossau, Switzerland) until the day of the experiment. Animals were stunned by a blow on the head and killed by exsanguination. The distal colon was taken out immediately and the serosa and muscularis were dissected free.
Determination of electrophysiological parameters
Sheets of tissue were mounted in a modified Ussing chamber, bathed with a volume of 4 ml buffer solution on each side of the epithelium and continuously short-circuited by an automatic voltage clamp device (Aachen Microclamp, AC Copy Datentechnik, Aachen, Germany) with correction for solution resistance. Tissue conductance (Gt) was measured by recording the voltage resulting from bipolar current pulses (±100 μA) applied across the tissue at one minute intervals. The continuously applied short-circuit current (Isc) and Gt were printed out every minute. Before the addition of any drugs, there was an equilibration time of at least 60 min to stabilize basal values. When effects of blockers were tested, control experiments without the blocker were performed with tissue from the same animal. The baseline of the electrical parameters was determined as the mean over a 5 min period (five values) immediately prior to administration of a drug. The maximal changes in Isc and Gt induced by a drug were expressed as the difference from the baseline (ΔIsc and ΔGt).
Drugs and statistics
Anthracene-9-carboxylic acid (9-AC), bumetanide, carbachol, clotrimazole, diphenylamine-2-carboxylate (DPC), glibenclamide, ouabain, quercetin, quinine, tetraethylammonium (TEA), and tetrapentylammonium (TPeA) were obtained from Sigma or Fluka (Buchs, Switzerland); 4,4′-diisothiocyano-stilbene-2,2′-disulphonic acid (DIDS) and 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) were from Calbiochem (Bad Soden, Germany). Charybdotoxin was purchased from Sigma and from Latoxan (Valence, France). Trans-6-Cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane (293B) was a generous gift from Professor R Greger (University of Freiburg, Germany). Charybdotoxin was dissolved in a solution containing (mM): NaCl, 100; Tris, 10; ethylenediaminetetraacetic acid (EDTA) 1, and 0.1% (w/w) bovine albumin and stored in glass containers. TEA and TPeA were dissolved in aqueous stock solutions, quinine was dissolved in ethanol, and all other drugs were dissolved in dimethyl sulphoxide (DMSO).
Data are presented as mean values±s.e.mean. Comparisons between the experimental group and the respective control were done with the unpaired t-test. P values <0.05 were considered to be statistically significant.
Results
Blockers of CFTR inhibit quercetin's effect in intact colon mucosa
In all experiments with quercetin, the flavonol was applied at a concentration of 100 μM to the serosal side only.
Quercetin increased Isc in intact distal colon tissue sheets bathed in buffer solution as previously demonstrated (Cermak et al., 1998; 2000). A first set of experiments was carried out in order to identify the apical Cl− channels involved. Since it had been demonstrated that NPPB, a compound which is able to block CFTR (Schultz et al., 1999), abolishes this flavonol-induced Isc in proximal colon (Cermak et al., 1998), NPPB and other Cl− channel blockers were applied to the mucosal side in order to investigate their influence on the quercetin-induced secretion in the distal segment. Among the inhibitors used in this study, NPPB, glibenclamide, and DPC have been shown to block CFTR. DIDS and 9-AC, blockers of Ca2+-activated or volume-sensitive Cl− channels, respectively, are ineffective in this respect (Begenisich & Melvin, 1998; Clarke et al., 1994; Diener et al., 1992; Schultz et al., 1999).
DPC (1 mM) significantly decreased Isc by 1.0±0.3 μEq h−1 cm−2 (P<0.05; n=5), whereas 9-AC (1 mM), DIDS (100 μM), glibenclamide (100 μM) and NPPB (100 μM) had no effect on basal Isc. Gt rose significantly after the administration of NPPB (ΔGt=8.9±1.8 mS cm−2, P<0.001, n=6). All other compounds did not influence this parameter. Among the Cl− channel blockers used, only NPPB and DPC were able to inhibit the quercetin-induced rise in Isc (Figure 1a). The rise in Gt induced by the flavonol was not altered by any of the blockers used. These results indicate that Cl− channels other than CFTR are not involved in the secretion induced by quercetin.
Figure 1.
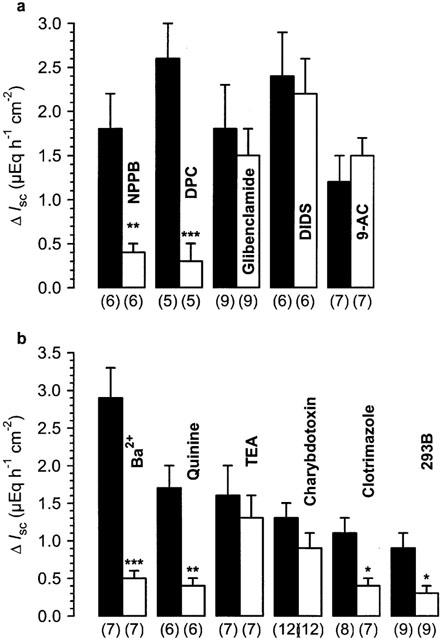
Effects of Cl− (a) and K+ (b) channel blockers on quercetin-induced Isc in rat distal colon. The Cl− channel blockers DPC (1 mM), glibenclamide (100 μM), DIDS (100 μM), and 9-AC (1 mM) were added 20 min prior to quercetin (100 μM serosal) to the mucosal side of the tissue; NPPB (100 μM) was added 40 min before the flavonol. The K+ channel blockers Ba2+ (10 mM) and quinine (1 mM) were added 30 min before quercetin (100 μM), TEA (5 mM), charybdotoxin (200 nM), clotrimazole (30 μM) and 293B (30 μM) were added 20 min prior to the flavonol to the serosal side. The experiments with Ba2+ and the respective controls were performed in HEPES-buffer. Black bars represent the control experiments without the blocker. Numbers in parentheses indicate the number of experiments. *P<0.05, **P<0.01, ***P<0.001 versus respective controls.
Blockers of K+ channels also inhibit quercetin's effect in intact colon mucosa
The basolateral K+ channels involved in colonic Cl− secretion belong to either one of two classes. The channels which are activated by intracellular Ca2+ were recently identified in rat colonic crypts and named rSK4 due to their homology to the human small-conductance Ca2+ activated K+ channels hSK4 (Warth et al., 1999). They are sensitive to Ba2+, quinine, and charybdotoxin, but insensitive to the K+ channel blocker TEA (Bleich et al., 1996; Strabel & Diener, 1995). The antifungal antibiotic, clotrimazole, is able to block the analogous human SK4 channel expressed in Xenopus oocytes, but is rather ineffective in preventing carbachol-induced secretion in rat colon mucosa (Warth et al., 1999). The other relevant class of K+ channels is activated by cyclic AMP and was also recently identified. These channels consist of two subunits in colon epithelium: KCNQ1 (or KvLQT1) and KCNE3 (Schroeder et al., 2000). This conductance is specifically blocked by the chromanol 293B as well as by clotrimazole, Ba2+ and quinine, but not by TEA or by charybdotoxin (Diener et al., 1996; Schroeder et al., 2000; Warth et al., 1996).
In order to differentiate between the K+ channels possibly involved in the secretory activity of quercetin, we added the above-mentioned blockers to the serosal side. Ba2+ (10 mM) induced a transient increase in Isc by 3.4±0.3 μEq h−1 cm−2 and in Gt by 6.9±1.0 mS cm−2 (P<0.001, n=7). Thereafter, Isc decreased towards basal values. The serosal addition of quinine (1 mM) induced a similar transient Isc increase by 1.5±0.4 μEq h−1 cm−2 (P<0.01, n=6) with a subsequent fall in the short-circuit current below basal values (ΔIsc=−0.6±0.3 μEq h−1 cm−2, P>0.05); Gt was not significantly altered by quinine.
The effect of quercetin on Isc was blocked in the presence of Ba2+ and quinine (Figure 1b). Tetraethylammonium (TEA, 5 mM) and charybdotoxin (200 nM) failed to influence the effect of the flavonol (Figure 1b). Due to our original hypothesis that quercetin activates the same Ca2+ dependent K+ channels as carbachol, this last result came quite unexpectedly. In order to prove the effectiveness of charybdotoxin, we checked the interaction of this compound with the effect of carbachol. Charybdotoxin itself increased Isc by 0.4±0.1 μEq h−1 cm−2 (n=14, P<0.001). The muscarinic agonist carbachol (50 μM serosal) induced a transient increase in Isc by 9.3±0.7 μEq h−1 cm−2 in control tissues (n=14) which was partially inhibited by charybdotoxin (ΔIsc=6.6±1.0 μEq h−1 cm−2, n=14, P<0.05). However, in two further experimental series charybdotoxin failed to inhibit the effect of carbachol (data not shown). Thus, the effectiveness of charybdotoxin is questionable, at least under our experimental conditions. TEA increased Isc by 0.3±0.1 μEq h−1 cm−2 (P<0.01, n=7). Isc did not return towards basal values over 20 min. Clotrimazole (30 μM) and 293B (30 μM) had the opposite effect on basal Isc, decreasing Isc by 0.7±0.2 μEq h−1 cm−2 (P<0.05, n=7) and by 0.6±0.1 μEq h−1 cm−2 (P<0.05, n=9), respectively. Both compounds inhibited the effect of quercetin effectively (Figure 1b). Especially the result obtained with 293B may suggest an involvement of the cyclic AMP-induced conductance in the effect of the flavonol.
Tetrapentylammonium (TPeA), which is a very effective blocker of Ca2+ activated K+ channels in human distal colon (Mcnamara et al., 1999), had a strong inhibitory effect on basal Isc at a concentration of 100 μM (−1.6±0.3 μEq h−1 cm−2, P<0.01, n=7). Furthermore, it abolished the effect of quercetin (Figure 2).
Figure 2.
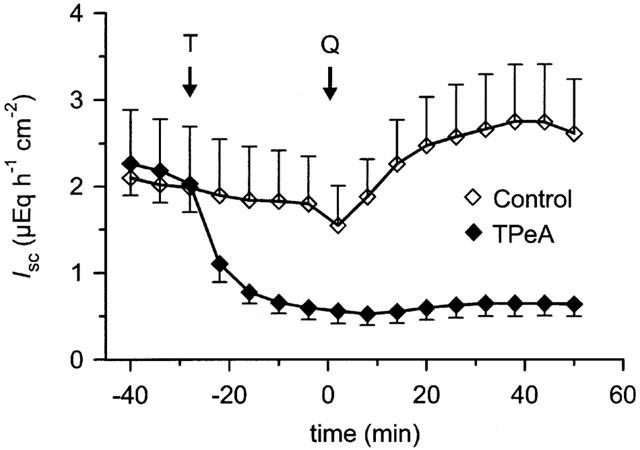
Effect of tetrapentylammonium (TPeA) on quercetin-induced Isc in rat distal colon. TPeA (T, 100 μM) or vehicle were applied at the time indicated to the serosal side. Quercetin (Q, 100 μM serosal) was added at the time indicated to both the control group (n=7) and to the TPeA-pretreated group (n=7).
Apical permeabilization of rat distal colon epithelium
Next, we tried to bypass the apical membrane of the epithelium in order to selectively investigate electrogenic transport via the basolateral membrane. For this purpose, we permeabilized the apical membrane by using the polyene antibiotic nystatin. Nystatin forms channels in lipid membranes, which are permeable for monovalent cations and Cl− (selectivity for cations over Cl− is ∼9 : 1), but impermeable for divalent cations or larger molecules like ATP (Rae & Fernandez, 1991).
In preliminary experiments, we observed that the effect of nystatin was maximal at 200 mg l−1. Thus, we used this concentration in all of the following experiments. The standard buffer on the mucosal surface was replaced by the high K+ solution while the serosal surface was perfused with the low K+ solution. Since the concentration of all other permeable ions was the same on both sides of the tissue, this procedure yielded a K+ gradient across the basolateral membrane, thereby mimicking the physiological K+ gradient. Afterwards, the polyene antibiotic was added to the mucosal solution and remained throughout the rest of the experiment. Nystatin induced very large currents which led to saturation of the voltage-clamp device. Ten to 30 min after the addition of nystatin, these currents decreased and returned back to the measurable range. Similarly, Gt increased rapidly and returned afterwards slowly towards basal values. After 2 h, Gt had decreased to values that were not much higher than the pre-nystatin values. Substances were added during the phase of a constant Isc decrease, usually around 80 min after the addition of nystatin.
Ouabain (1 mM), a Na+/K+ ATPase inhibitor, caused a decrease in Isc by 2.8±0.5 μEq h−1 cm−2 (n=10, P<0.001), demonstrating the existence of an electrogenic pump current in the nystatin-treated epithelia. The remaining current must have been carried mainly by K+ ions due to the applied gradient. For this reason, it was named IK.
In order to prove the successful permeabilization of the apical membrane, we investigated the effect of bumetanide, a blocker of Na+/K+/2 Cl− cotransport, on the IK induced by carbachol. In intact tissue, carbachol induces a Cl− secretion due to the activation of K+ channels. Since this Cl− secretion depends on the basolateral influx of Cl− via the Na+/K+/2 Cl− cotransporter; bumetanide is able to block this effect (Strabel & Diener, 1995). First, we performed control experiments with intact distal colon mucosa. In these tissues, carbachol (50 μM serosal) induced a transient increase in Isc by 11.1±0.6 μEq h−1 cm−2 and in Gt by 8.4±0.5 mS cm−2 (n=6). Preincubation with serosal bumetanide (100 μM) nearly abolished this Isc transient (ΔIsc=1.9±0.3 μEq h−1 cm−2, n=6; P<0.001), whereas the carbachol-induced increase in Gt was not significantly smaller in the presence of bumetanide (ΔGt=6.3±0.8 mS cm−2, P>0.05).
In nystatin treated tissues, bumetanide (100 μM serosal) failed to alter IK, something which is in accordance with the electroneutral mechanism of Na+/K+/2 Cl− cotransport. In contrast to intact epithelia, bumetanide failed to influence the current induced by 50 μM carbachol (controls: IK=0.7±0.4 μEq h−1 cm−2, n=5; bumetanide-group: IK=0.5±0.1 μEq h−1 cm−2, n=5; P>0.05). Thus, the carbachol-induced current was solely due to a K+ current via the basolateral membrane and did not depend on transepithelial Cl− transport as in intact tissue. This demonstrated that the apical membrane was successfully permeabilized by nystatin.
Basolateral Na+/K+/2 Cl− cotransport and Na+/K+ ATPase are not directly involved in the activity of the flavonol
As mentioned in the introduction, transepithelial Cl− secretion depends on the cofunction of Na+/K+/2 Cl− cotransport and Na+/K+ ATPase. We investigated the influence of both transport mechanisms on the effect of the flavonol.
Block of the Na+/K+ ATPase by ouabain (1 mM) in intact distal colon mucosa induced a transient increase in Isc (Figure 3) and in Gt (ΔGt=1.8±0.4 mS cm−2, n=7; controls: ΔGt=−0.4±0.2 mS cm−2, n=6, P<0.001), something which was similar to the effect of Ba2+ and quinine. The effect of quercetin on Isc (Figure 3) and Gt was nearly abolished in the presence of ouabain (ouabain-group: ΔGt=0.3±0.1 mS cm−2, controls: ΔGt=1.5±0.4 mS cm−2, P<0.01).
Figure 3.
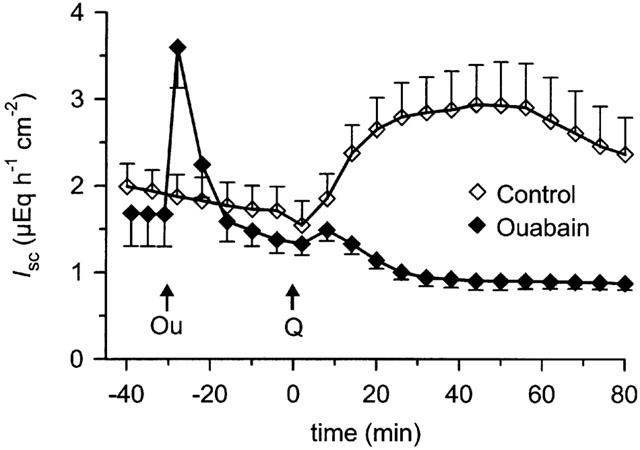
Effect of ouabain on quercetin-induced Isc in rat distal colon. Ouabain (Ou, 1 mM) or vehicle were applied at the time indicated to the serosal side; quercetin (Q, 100 μM serosal) was added at the time indicated to both the control group (n=6) and to the ouabain-pretreated group (n=7).
In nystatin-permeabilized mucosa, ouabain induced a marked decrease in Isc by 6.6±0.3 μEq h−1 cm−2 (n=7) which was clearly different from the slight Isc decrease in control tissues (ΔIsc=−1.4±0.5 μEq h−1 cm−2, n=7, P<0.001) (Figure 4a). In control tissues, quercetin induced an increase in Isc (ΔIsc=1.7±0.4 μEq h−1 cm−2), which was similar to the one in intact epithelium. This increase was not altered by ouabain in permeabilized tissues (ΔIsc=2.0±0.3 μEq h−1 cm−2). This demonstrates that the Na+/K+ ATPase does not directly affect quercetin's activity.
Figure 4.
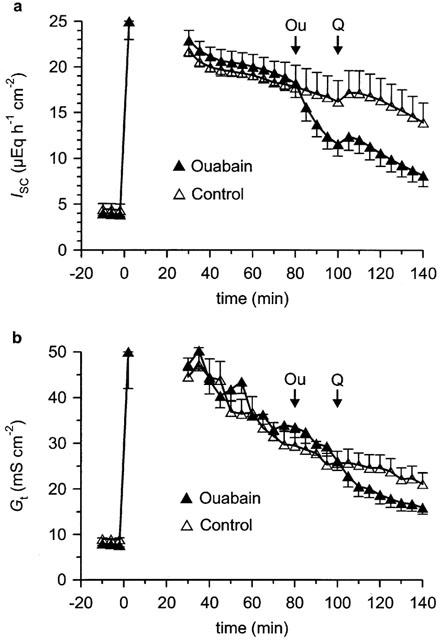
Effect of ouabain on quercetin-induced Ik (a) and Gt (b) in rat distal colon mucosa after apical permeabilization with nystatin. Nystatin (200 mg l−1) was added to the mucosal side at time 0 in the presence of a serosally directed K+ gradient. Since the voltage clamp was saturated directly after this manoeuvre, data up to 30 min after the addition of nystatin are not indicated. Either ouabain (Ou, 1 mM serosal) or vehicle was added at the time indicated. Both groups received quercetin (Q, 100 μM serosal) at the time indicated. n=7 for ouabain group and for control group.
In agreement with the model of Cl− secretion, inhibition of Na+/K+/2 Cl− cotransport diminishes quercetin-induced secretion in rat colon (Cermak et al., 1998). In nystatin-treated tissues, basal IK decreased by 2.2±0.8 μEq h−1 cm−2 (n=7) in the presence of bumetanide (100 μM serosal). This decrease in basal IK was not different from the commonly observed decrease in control tissues (ΔIK=−3.7±0.9 μEq h−1 cm−2, n=7; P>0.05). Due to the electroneutral nature of the cotransporter, bumetanide's lack of effect on the basal current was expected. Quercetin induced an increase in IK which was not different between controls (ΔIK=1.1±0.4 μEq h−1 cm−2) and the bumetanide group (ΔIK=0.9±0.4 μEq h−1 cm−2, n=7). Thus, the cotransporter is also not directly involved in the effect of the flavonol.
Quercetin activates basolateral K+ channels
After excluding a direct effect of quercetin on Na+/K+/2 Cl− cotransport and Na+/K+ ATPase in the previous experiments, we investigated a possible participation of basolateral K+ channels in the effect of the flavonol. In nystatin-permeabilized mucosal sheets, the K+ channel blockers Ba2+ (5 mM serosal) and quinine (1 mM serosal) induced a marked decrease in basal IK which was clearly different from the commonly observed continuous current decrease in control tissues (Figure 5a). On the other hand, TEA (5 mM serosal), charybdotoxin (200 nM serosal) and clotrimazole (30 μM serosal) had no significant effect on basal IK. Ba2+, quinine and clotrimazole inhibited the current induced by quercetin, whereas TEA and charybdotoxin were without effect (Figure 5b). Similar to its effect in intact colon mucosa, TPeA (100 μM serosal) drastically decreased basal IK by 10.8±1.3 μEq h−1 cm−2; n=10) in comparison with controls (ΔIsc=−1.1±0.3 μEq h−1 cm−2; n=10; P<0.001). The quercetin-induced current that was visible in control tissues (ΔIsc=1.8±0.6 μEq h−1 cm−2) was nearly abolished by TPeA (ΔIsc=0.2±0.1 μEq h−1 cm−2; P<0.05) (Figure 6). In contrast to the experiments with intact epithelium, the chromanol 293B did not affect basal IK or the current induced by the flavonol (Figure 7a,b).
Figure 5.
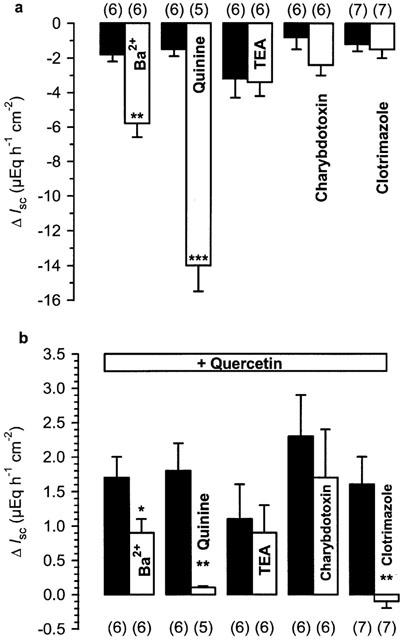
(a) Effect of different blockers in nystatin-permeabilized rat distal colon (200 mg nystatin l−1). Ba2+ (5 mM), quinine (1 mM), TEA (5 mM), charybdotoxin (200 nM) and clotrimazole (30 μM) were applied to the serosal side of the tissue. (b) Effect of quercetin (100 μM), added to the serosal bath 20 min after the blockers in the same experiments. The experiments with Ba2+ and the respective controls were performed in HEPES/Tris-buffer. Black bars represent the control experiments without the blocker. Numbers in parentheses indicate the number of experiments. *P<0.05, **P<0.01, ***P<0.001 versus respective controls.
Figure 6.
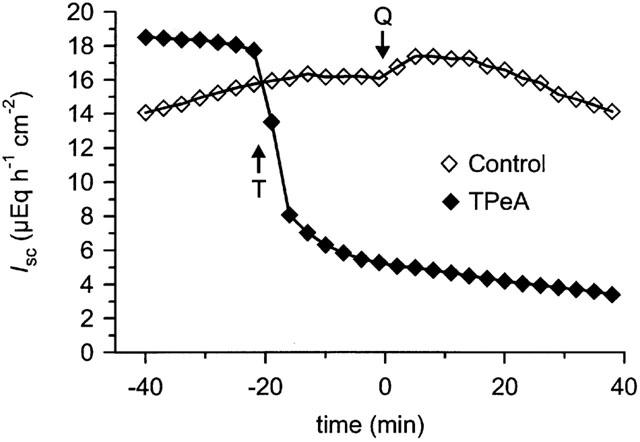
Effect of tetrapentylammonium (TPeA) on quercetin-induced Isc in a mucosal sheet of rat distal colon after apical permeabilization with nystatin. TPeA (T, 100 μM) was applied at the time indicated to the serosal side; the control tissue received only vehicle. Quercetin (Q, 100 μM serosal) was added at the time indicated to both the control tissue and the TPeA-pretreated tissue.
Figure 7.
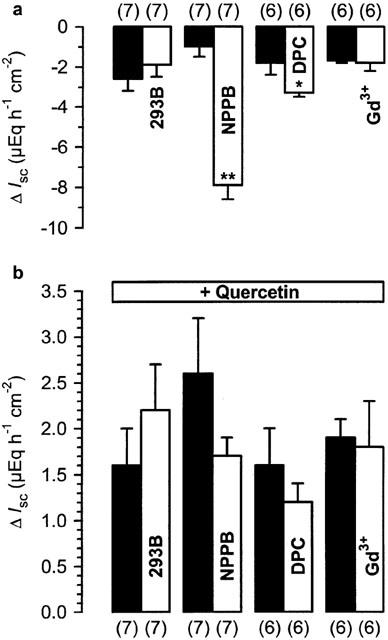
(a) Effect of different blockers in nystatin-permeabilized rat distal colon (200 mg nystatin l−1). 293B (30 μM) and Gadoliniumchloride (100 μM) were applied to the serosal side, NPPB (100 μM) and DPC (1 mM) to the mucosal side of the tissue. (b) Effect of quercetin (100 μM), added to the serosal bath 20 min after the blockers in the same experiments. The experiments with Gd3+ and the respective controls were performed in HEPES/Tris-buffer. Black bars represent the control experiments without the blocker. Numbers in parentheses indicate the number of experiments. *P<0.05, **P<0.001 versus respective controls.
Interestingly, the mucosally applied Cl− channel blockers DPC (1 mM) and NPPB (100 μM) diminished basal IK (Figure 7a). In contrast to intact colon mucosa, both blockers failed to significantly alter the quercetin-induced current (Figure 7b). In a previous study, we showed that Gd3+, a lanthanide which is able to inhibit the carbachol-induced Ca2+ influx into colon mucosa (Frings et al., 1999), had no effect on the short-circuit current induced by quercetin (Cermak et al., 2000). In distal colon epithelium permeabilized with nystatin, the serosal addition of Gd3+ (100 μM) affected neither basal IK nor the current induced by the flavonol (Figure 7a,b). The latter experiments were conducted in HEPES/Tris buffer due to the fact that Gd3+ ions can be effectively bound by CO32− and PO43− anions (Caldwell et al., 1998).
Discussion
Intestinal chloride secretion depends on the coordinated activity of apical Cl− channels as well as on basolateral Na+/K+ ATPases, Na+/K+/2 Cl− cotransporters and K+ channels. Inhibition of one of these transport mechanisms leads to the cessation of secretion. Accordingly, the short-circuit current induced by quercetin, which is indicative of the Cl− secretion induced by this flavonol, is inhibited by several manoeuvres: block of the Na+/K+ pump with ouabain, inhibition of Na+/K+/2 Cl− cotransport (Cermak et al., 1998), block of basolateral K+ channels, and inhibition of CFTR. In intestinal mucosa, additional Ca2+ activated Cl− channels do not play a role in Cl− secretion, something which is different from other secretory epithelia (Clarke et al., 1994; Greger, 2000; Strabel & Diener, 1995). Accordingly, a blocker of Ca2+ activated Cl− channels, DIDS (Begenisich & Melvin, 1998; Clarke et al., 1994; Schultz et al., 1999), as well as a blocker of volume-sensitive Cl− channels, 9-AC (Begenisich & Melvin, 1998; Diener et al., 1992), failed to inhibit the quercetin-induced current. On the other hand, NPPB and DPC, which are able to block CFTR at the concentrations used in this study (Schultz et al., 1999), diminished quercetin's effect. However, another blocker of CFTR, namely glibenclamide (Schultz et al., 1999), failed to inhibit the flavonol-induced secretion in our study. We do not have an explanation for the latter observation at present.
Several K+ channel blockers were also able to block the secretion induced by quercetin in intact mucosa. At present, two classes of K+ channels have been identified which take part in chloride secretion in the rat colon: Ca2+ dependent rSK4 channels and cyclic AMP-activated KCNQ1/KCNE3 channels (Schroeder et al., 2000; Warth et al., 1999). All of the inhibitors which block the cyclic AMP-activated K+ conductance, especially the chromanol 293B, were also effective in diminishing the quercetin-induced Isc in intact mucosa. On the other hand, charybdotoxin, which is specific for the Ca2+ dependent K+ conductance, failed to alter the effect of the flavonol.
At first sight, these results seem to be contrary to our previous studies in which we could clearly demonstrate that the secretion evoked by quercetin is independent of cyclic AMP, but dependent on Ca2+ (Cermak et al., 1998; 2000). One possible explanation is that quercetin acts on KCNQ1, which might also be activated by Ca2+ other than cyclic AMP (Warth et al., 1999).
However, the failure of charybdotoxin to block the effect of quercetin could have been due to the tissue preparation we used. In contrast to its marked effect on Ca2+ dependent K+ channels in isolated epithelial cells, charybdotoxin seems to be rather ineffective in epithelial preparations with several submucosal cell layers present. This is indicated by its weak effect on the carbachol-induced Isc in our experiments. Another blocker of Ca2+ activated K+ channels, TPeA (Mcnamara et al., 1999), nearly abolished the effect of the flavonol. In addition, 293B is also known to block CFTR at the concentration that we used in our experiments (Bachmann et al., 2001). It is possible that the chromanol could have blocked the quercetin-induced current independently of cyclic AMP-activated K+ channels.
Therefore, we went on to examine the ion transport mechanisms involved in the effect of the flavonol more selectively. By using nystatin-permeabilized epithelium, we bypassed the apical Cl− conductance in order to specifically investigate the possible involvement of the basolateral Na+/K+ pump, Na+/K+/2 Cl− cotransport and K+ channels. In agreement with a recent study that used the same approach (Schultheiss & Diener, 1997), we also found that a major part of the observed current under this condition was due to the electrogenic Na+/K+ pump. The remaining current was carried by K+ ions due to the applied transepithelial gradient.
In apically permeabilized colon mucosa, quercetin induced a current which was independent of the Na+/K+ pump and Na+/K+/2 Cl− cotransport. This observation indicated that the current induced by the flavonol must have been solely due to an activation of basolateral K+ channels (IK). This assumption was further supported by the observation that the Cl− channel blockers NPPB and DPC, which nearly abolished the quercetin-induced Isc in intact epithelium, failed to alter this current in permeabilized mucosa. Thus, apical Cl− channels were bypassed.
The results obtained with several inhibitors of K+ channels in nystatin-treated tissue were similar to our previous experiments in intact epithelium. Interestingly, the only K+ channel blocker which acted differently in intact and permeabilized mucosa was 293B. The chromanol affected neither basal IK nor the current increase induced by quercetin in nystatin-treated epithelium. This observation very likely rules out a participation of cyclic AMP-activated KCNQ1/KCNE3 channels in the effect of the flavonol. Thus, the effect of 293B observed in intact epithelium can be explained by an inhibition of CFTR, which was absent in the nystatin experiments (Bachmann et al., 2001).
Basal IK in nystatin-treated colon mucosa was sensitive to Ba2+ and quinine. IK was insensitive to TEA which is in accordance with another study (Schultheiss & Diener, 1997). Surprisingly, the Cl− channel blockers NPPB and DPC inhibited a part of the basal IK as well. Since this cannot be explained by block of a residual Cl− current due to incomplete permeabilization (otherwise the quercetin-induced current in the same experiments would have been affected as well, which was not the case), we suggest that this was an unspecific effect of NPPB and DPC on K+ channels (Chang & Dawson, 1988; Illek et al., 1992).
Because carbachol induces a Ca2+ influx through Gd3+-sensitive cation channels in rat colon crypts (Frings et al., 1999), we additionally investigated if quercetin might activate a similar mechanism. Gd3+ neither affected basal IK nor did it influence the current induced by the flavonol, observations which are consistent with a previous study performed with intact epithelium (Cermak et al., 2000).
Illek & Fischer (1998) suggested that quercetin directly interacts with CFTR in airway epithelium in a similar manner to the related isoflavone genistein. The recent demonstration of a direct interaction of genistein and quercetin with the second nucleotide-binding fold of CFTR strongly supports this assumption (Randak et al., 1999). In our experiments, we could not determine if quercetin acts additionally on the apical Cl− conductance. In contrast to monolayers of cultured cell lines, permeabilization of the basolateral membrane by nystatin was not feasible in our preparation. This was probably due to the presence of subepithelial tissue. Based on the published data, a direct interaction of quercetin with CFTR might be an additional means of inducing Cl− secretion in colon mucosa. This has to be investigated in future studies.
In summary, we have demonstrated that the flavonol quercetin activates basolateral K+ channels in rat distal colon mucosa. Cyclic AMP-activated K+ channels of the KCNQ1/KCNE3 type do not seem to be involved in the effect of the flavonol. The K+ conductance altered by quercetin is likely activated by Ca2+. Activation of such K+ channels is probably the major mechanism by which quercetin induces chloride secretion in rat colon.
Acknowledgments
We want to thank Professor Erwin Scharrer for his continuous support of this study and Zoran Vujicic for his expert technical assistance. 293B was a generous gift from Professor Rainer Greger, Universität Freiburg, Germany.
Abbreviations
- 293B
trans-6-cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane
- 9-AC
anthracene-9-carboxylic acid
- cyclic AMP
cyclic adenosine monophosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- DIDS
4,4′-diisothiocyanostilbene-2,2′-disulphonic acid
- DPC
diphenylamine-2-carboxylate
- Gt
tissue conductance
- IK
K+ current
- Isc
short-circuit current
- NPPB
5-nitro-2-(3-phenylpropylamino)-benzoic acid
- rSK4
rat small-conductance Ca2+-activated K+ channel
- TEA
tetraethylammonium
- TPeA
tetrapentylammonium
References
- BACHMANN A., QUAST U., RUSS U. Chromanol 293B, a blocker of the slow delayed rectifier K+ current (IKs), inhibits the CFTR Cl− current. Naunyn Schmied. Arch. Pharmacol. 2001;363:590–596. doi: 10.1007/s002100100410. [DOI] [PubMed] [Google Scholar]
- BEGENISICH T., MELVIN J.E. Regulation of chloride channels in secretory epithelia. J. Membrane Biol. 1998;163:77–85. doi: 10.1007/s002329900372. [DOI] [PubMed] [Google Scholar]
- BLEICH M., RIEDEMANN N., WARTH R., KERSTAN D., LEIPZIGER J., HOR M., VAN DRIESSCHE W., GREGER R. Ca2+ regulated K+ and nonselective cation channels in the basolateral membrane of rat colonic crypt base cells. Pflügers Arch. 1996;432:1011–1022. doi: 10.1007/s004240050229. [DOI] [PubMed] [Google Scholar]
- CALDWELL R.A., CLEMO H.F., BAUMGARTEN C.M. Using gadolinium to identify stretch-activated channels: technical considerations. Am. J. Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- CERMAK R., FÖLLMER U., WOLFFRAM S. Dietary flavonol quercetin induces chloride secretion in rat colon. Am. J. Physiol. 1998;275:G1166–G1172. doi: 10.1152/ajpgi.1998.275.5.G1166. [DOI] [PubMed] [Google Scholar]
- CERMAK R., VUJICIC Z., KUHN G., WOLFFRAM S. The secretory response of the rat colon to the flavonol quercetin is dependent on Ca2+-calmodulin. Exp. Physiol. 2000;85:255–261. [PubMed] [Google Scholar]
- CHANG D., DAWSON D.C. Digitonin-permeabilized colonic cell layers. Demonstration of calcium-activated basolateral K+ and Cl− conductances. J. Gen. Physiol. 1988;92:281–306. doi: 10.1085/jgp.92.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE L.L., GRUBB B.R., YANKASKAS J.R., COTTON C.U., MCKENZIE A., BOUCHER R.C. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(−/−) mice. Proc. Natl. Acad. Sci. USA. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENER M., HUG F., STRABEL D., SCHARRER E. Cyclic AMP-dependent regulation of K+ transport in the rat distal colon. Br. J. Pharmacol. 1996;118:1477–1487. doi: 10.1111/j.1476-5381.1996.tb15563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENER M., NOBLES M., RUMMEL W. Activation of basolateral Cl− channels in the rat colonic epithelium during regulatory volume decrease. Pflügers Arch. 1992;421:530–538. doi: 10.1007/BF00375048. [DOI] [PubMed] [Google Scholar]
- FRINGS M., SCHULTHEISS G., DIENER M. Electrogenic Ca2+ entry in the rat colonic epithelium. Pflügers Arch. 1999;439:39–48. doi: 10.1007/s004249900159. [DOI] [PubMed] [Google Scholar]
- GREGER R. Role of CFTR in the colon. Annu. Rev. Physiol. 2000;62:467–491. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- GREGER R., BLEICH M., LEIPZIGER J., ECKE D., MALL M., KUNZELMANN K. Regulation of ion transport in colonic crypts. News Physiol. Sci. 1997;12:62–66. [Google Scholar]
- HERRMANN K. On the occurence of flavonol and flavone glycosides in vegetables. Z. Lebensm. Unters. Forsch. 1988;186:1–5. [Google Scholar]
- HERTOG M.G.L., HOLLMAN P.C.H., VAN DE PUTTE B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agr. Food Chem. 1993;41:1242–1246. [Google Scholar]
- ILLEK B., FISCHER H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am. J. Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- ILLEK B., FISCHER H., KREUSEL K.M., HEGEL U., CLAUSS W. Volume-sensitive basolateral K+ channels in HT-29/B6 cells: block by lidocaine, quinidine, NPPB, and Ba2+ Am. J. Physiol. 1992;263:C674–C683. doi: 10.1152/ajpcell.1992.263.3.C674. [DOI] [PubMed] [Google Scholar]
- MALL M., BLEICH M., SCHÜRLEIN M., KUEHR J., SEYDEWITZ H.H., BRANDIS M., GREGER R., KUNZELMANN K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am. J. Physiol. 1998;275:G1274–G1281. doi: 10.1152/ajpgi.1998.275.6.G1274. [DOI] [PubMed] [Google Scholar]
- MCNAMARA B., WINTER D.C., CUFFE J.E., O'SULLIVAN G.C., HARVEY B.J. Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J. Physiol. 1999;519:251–260. doi: 10.1111/j.1469-7793.1999.0251o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAE J.L., FERNANDEZ J. Perforated patch recordings in physiology. News Physiol. Sci. 1991;6:273–277. [Google Scholar]
- RANDAK C., AUERSWALD E.A., ASSFALG-MACHLEIDT I., REENSTRA W.W., MACHLEIDT W. Inhibition of ATPase, GTPase and adenylate kinase activities of the second nucleotide-binding fold of the cystic fibrosis transmembrane conductance regulator by genistein. Biochem. J. 1999;340:227–235. [PMC free article] [PubMed] [Google Scholar]
- SCHROEDER B.C., WALDEGGER S., FEHR S., BLEICH M., WARTH R., GREGER R., JENTSCH T.J. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- SCHULTHEISS G., DIENER M. Regulation of apical and basolateral K+ conductances in the rat colon. Br. J. Pharmacol. 1997;122:87–94. doi: 10.1038/sj.bjp.0701353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ B.D., SINGH A.K., DEVOR D.C., BRIDGES R.J. Pharmacology of CFTR chloride channel activity. Physiol. Rev. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- STRABEL D., DIENER M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur. J. Pharmacol. 1995;274:181–191. doi: 10.1016/0014-2999(94)00728-p. [DOI] [PubMed] [Google Scholar]
- WARTH R., HAMM K., BLEICH M., KUNZELMANN K., VON HAHN T., SCHREIBER R., ULLRICH E., MENGEL M., TRAUTMANN N., KINDLE P., SCHWAB A., GREGER R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Arch. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- WARTH R., RIEDEMANN N., BLEICH M., VAN DRIESSCHE W., BUSCH A.E., GREGER R. The cAMP regulated and 293B-inhibited K+ conductance of rat colonic crypt base cells. Pflügers Arch. 1996;432:81–88. doi: 10.1007/s004240050108. [DOI] [PubMed] [Google Scholar]


