Abstract
E3040 (6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole), is a novel dual inhibitor of 5-lipoxygenase (5-LOX) and thromboxane synthase (Tx synthase). Here, we examined the effects of E3040 sulphate, a sulphate conjugate of E3040, on these enzyme activities in cell-free systems and on the thromboxane A2 (TxA2)-mediated Cl− secretion induced by platelet-activating factor (PAF) in isolated rat colons.
E3040 sulphate inhibited Tx synthase activity in a concentration-dependent manner (IC50=0.013 μM), whereas it induced little effects on 5-LOX and cyclo-oxygenase activities (IC50>100 μM) with the cell-free enzyme assay.
With isolated rat colonic mucosa, E3040 sulphate in a concentration-dependent manner (IC50=1.8 μM) inhibited the Cl− secretion induced by 10 μM PAF. On the other hand, E3040 sulphate (30 μM) induced no effect on the prostaglandin E2 (0.5 μM)- and leukotriene D4 (1 μM)-induced Cl− secretion in the colon.
PAF (10 μM) increased a release of TxB2, a stable metabolite of TxA2, from the colonic mucosa. This increase was significantly inhibited by subsequent addition of E3040 sulphate (30 μM).
Probenecid (100 μM), an inhibitor of organic anion transporter, abolished the inhibitory effect of E3040 sulphate on the PAF-induced Cl− secretion. Another inhibitor, sulphobromophthalein (30 μM) partially but significantly attenuated the effect of E3040 sulphate. p-Aminohippuric acid (1 mM) had no effect.
These findings suggest that E3040 sulphate is a novel Tx synthase inhibitor, and that E3040 sulphate taken up into the colonic cells by organic anion transporters inhibits the PAF-induced Cl− secretion by blocking a release of TxA2.
Keywords: Thromboxane A2, thromboxane synthase, E3040, E3040 sulphate, platelet-activating factor, colon, chloride secretion, inflammatory bowel disease, organic anion transporter
Introduction
E3040 (6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole), a new class of anti-inflammatory drug, is a dual inhibitor of 5-lipoxygenase (5-LOX) and thromboxane synthase (Tx synthase) (Hibi et al., 1994). E3040 potently inhibits production of leukotriene B4 (LTB4) and thromboxane B2 (TxB2), a stable metabolite of TxA2, in isolated rat peritoneal leukocytes and human blood cells (Oketani et al., 2001b). In lipopolysaccharide-immunized rats, E3040 prevents the lipopolysaccharide-induced bowel damage by inhibiting the production of both LTB4 and TxB2, suggesting that the drug may be clinically useful for management of endotoxin-related response in inflammatory bowel disease (Oketani et al., 2001a).
E3040 rapidly disappears from the plasma after intravenous injection of the drug to rats, and the major metabolite of E3040 is a sulphate conjugate (E3040 sulphate) (Takenaka et al., 1995, 1997). The transporting mechanism of E3040 sulphate across liver and kidney cell membranes was recently studied (Hirohashi et al., 1999; Kouzuki et al., 1999; Takenaka et al., 1995, 1997; Yamada et al., 2000). Here, we found for the first time that E3040 sulphate acts as a specific Tx synthase inhibitor but not as a 5-LOX inhibitor in a cell-free system.
We found that a stable analog of TxA2 (9,11-epithio-11,12-methano-TxA2) stimulates Cl− secretion in isolated rat colonic mucosa (Sakai et al., 1997). TxA2 has been suggested to play a major pathogenic role in inflammatory bowel disease in human and experimental animal models (Rampton & Collins, 1993).
Recently, we have reported that the platelet-activating factor (PAF)-induced Cl− secretion in the rat colon is mainly mediated by a release of TxA2 (Suzuki et al., 2000b). Similar to the function of TxA2 in the colon, PAF has been suggested as a mediator of pathogenesis of inflammatory bowel disease such as ulcerative colitis (Eliakim et al., 1988; Wardle et al., 1996) and Crohn's disease (Kald et al., 1990; Denizot et al., 1992; Sobhani et al., 1992) in human. PAF was also suggested as an inflammatory mediator in a rat model of chronic colitis (Wallace, 1988; Wallace et al., 1989). Stimulation of Cl− secretion by PAF may contribute to the production of diarrhea in inflammatory bowel disease (Wardle et al., 1996; Borman et al., 1998).
In the present study, we tested effects of E3040 sulphate on the TxA2-mediated Cl− secretion induced by PAF in isolated rat colon, and found that the PAF response is potently inhibited by E3040 sulphate.
Methods
Chemicals
E3040 sulphate (6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole O-sulphate, sodium salt; Figure 1) and OKY-1581 (sodium (E)-3-[4-(3-pyridylmethyl)phenyl]-2-methylacrylate) were synthesized at Eisai Co. (Tsukuba, Japan). Arachidonic acid, prostaglandin H2 (PGH2), (±)5-hydroxy-(6E,8Z,11Z,14Z)-eicosatetraenoic acid (racemic 5-HETE), (±)13-hydroxy-(9Z,11E)-octadecadienoic acid (racemic 13-HODE) were obtained from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). Prostaglandin E2 (PGE2) was a generous gift from Toray Industries, (Tokyo, Japan). PAF (1-alkyl-2-acetoyl-sn-glycero-3-phosphocholine) was obtained from Avanti Polar Lipids (Alabaster, AL, U.S.A.). LTD4 and p-aminohippuric acid (PAH) were obtained from Wako Pure Chemical Industries (Osaka, Japan). Sulphobromophthalein (BSP) was from Sigma (St. Louis, MO, U.S.A.).
Figure 1.
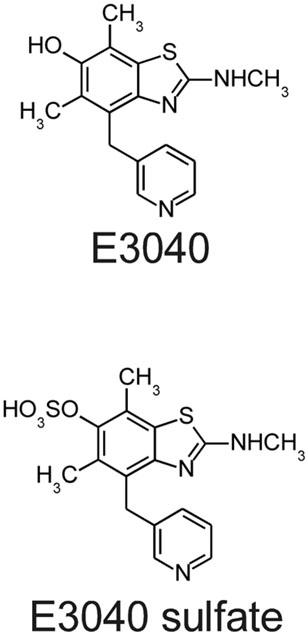
Chemical structures of E3040 and E3040 sulphate.
Measurement of enzyme activities of Tx synthase, 5-LOX and cyclo-oxygenase (COX)
For a measurement of Tx synthase activity, human platelets were isolated from blood of healthy volunteers. The platelets were homogenized and centrifuged at 105,000×g for 60 min at 4°C, and then the pellets were suspended in 50 mM Tris-HCl, pH 8.0 (approx. 1.2 mg of protein ml−1). The suspension supplemented with indomethacin (10 μM) was incubated with E3040 sulphate or vehicle for 5 min at 25°C. Then, the enzyme reaction was initiated by the addition of PGH2 (10 μg ml−1). One minute later, the reaction was terminated by the addition of acidic ice-cold citric acid/ethanol solution (5.5 mM/10%). After centrifugation at 1000×g for 10 min at 4°C, the amount of TxB2 in the supernatant was measured by using an enzyme immunoassay kit (Cayman Chemical Co.).
For a measurement of 5-LOX activity, adherent rat basophilic leukemia (RBL-1) cells were homogenized and centrifuged at 105,000×g for 60 min at 4°C. The supernatant supplemented with 2 mM glutathione was incubated with E3040 sulphate or vehicle for 5 min at 37°C. Then, the enzyme reaction was initiated by the addition of arachidonic acid (200 μM) and CaCl2 (2 mM). Five minutes later, the reaction was terminated by acidification with ice-cold formic acid (0.2 N). 13-HODE (0.6 μg) was added to each sample as an internal standard for high-performance liquid chromatography (HPLC) analysis of 5-HETE. Extraction was carried out by the addition of ethyl acetate. The organic phase was evaporated to dryness under a nitrogen stream and dissolved in 200 μl of methanol. The samples were injected onto a Hypersil BDS C18 column (Shandon Scientific Co., Cheshire, U.K.). The quantification of 5-HETE was performed by measuring UV absorbance at 235 nm.
For a measurement of COX activity, sheep seminal vesicle gland microsomes were dissolved in 100 mM Tris-HCl, pH 7.4 containing 5 mM tryptophan and 5 mM glutathione (approx. 2.3 mg of protein ml−1). The solution was incubated with E3040 sulphate or vehicle for 10 min at 25°C. Then, the enzyme reaction was initiated by the addition of arachidonic acid (5 μg ml−1). Twenty minutes later, the reaction was terminated by the addition of indomethacin (100 μM). After centrifugation at 1000×g for 10 min at 4°C, the amount of PGE2 in the supernatant was measured by using an enzyme immunoassay kit (Cayman Chemical Co.).
Isolation of rat colonic mucosa
The following procedures were performed in accordance with the guidelines presented by the Animal Care and Use Committee of Toyama Medical and Pharmaceutical University. The mucosa-submucosa preparation (hereafter, simply described as the mucosa) was obtained from female Wistar rats (Japan SLC, Shizuoka, Japan) with a weight of 140 – 200 g. The animals had free access to water and food until the day of the experiment. Animals were killed rapidly by stunning and cervical dislocation. The serosa and muscularis propria were stripped away by hand to obtain the mucosa-submucosa preparation of distal part of the colon descendens. The Parsons solution for tissue preparation and Ussing chamber experiments consisted of (in mM): NaCl 107, KCl 4.5, NaHCO3 25, Na2HPO4 1.8, NaH2PO4 0.2, CaCl2 1.25, MgSO4 1 and glucose 12. The solution was gassed with carbogen (5% CO2 – 95% O2) at a pH of 7.4.
Ussing chamber experiments
The tissue was set between modified Ussing chambers and bathed with 4 ml of the Parsons solution on each side of the mucosa at 37°C. The exposed surface of the tissue was 0.3 cm2. Short-circuit current (Isc) was continuously measured at zero voltage difference with an amplifier (CEZ-9100, Nihon Kohden Co., Tokyo, Japan). The fluid resistance was compensated. The direction of Isc from the mucosal-to-serosal side was expressed as positive: that is, an increase in Cl− movement from the serosal-to-mucosal side (Cl- secretion) corresponded to an increase in Isc. The transepithelial potential difference (Pd) under open-circuit conditions was measured in a current-clamp mode of the amplifier, and the reference was taken on the serosal side. Tissue conductance (Gt) was determined from the deviation of Isc in response to the command voltage pulse of 0.5 mV (its duration was 100 ms).
To assess effects of inhibitor on the PAF-induced increase in Isc, the inhibitor was added when the plateau phase of Isc was observed after the addition of PAF, and the value of Isc was read when the effect of the inhibitor had become steady. Data were then expressed as net increases from the baseline Isc (just before addition of PAF) (ΔIsc). Therefore, it should be noted that smaller value of ΔIsc means larger extent of inhibition. To determine concentration-dependent effects of E3040 sulphate on the PAF-induced increase in Isc, the drug was added cumulatively and data were expressed as ΔIsc.
Assay of TxB2
The mucosa was set between the Ussing chambers. TxA2 quickly transforms into TxB2 in aqueous solutions. We measured the concentration of TxB2 in the serosal solution using an enzyme immunoassay kit (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). The mucosa was incubated in the absence of PAF, then in the presence of PAF, and in the presence of PAF and E3040 sulphate. Three sample solutions from the serosal side were collected from each mucosa: that is, the first solution after the 30 min-incubation period in the absence of PAF (control), the second solution after the 30 min-incubation period in the presence PAF (during the PAF-elicited plateau phase of Isc), and the third solution after the 30 min-incubation period in the presence of PAF and E3040 sulphate (during the E3040 sulphate-elicited plateau phase of Isc). These sample solutions (1 ml, each) were immediately frozen, freeze-dried and dissolved with 0.25 ml of the assay kit buffer to determine the concentration of TxB2.
Statistical analysis
Results are presented as the means±s.e.m. Differences between groups were analysed by one-way analysis of variance (ANOVA), and correction for multiple comparisons was made by using Dunnett's multiple comparison test. If necessary, Tukey's multiple comparison test was used. Comparison between the two groups was made by using Student's t-test. Statistically significant differences were assumed at P<0.05. The IC50 values of data were calculated by using the KaleidaGraph program, version 3.08 (Synergy Software, Reading, PA, U.S.A.).
Results
Effects of E3040 sulphate on activities of Tx synthase, 5-LOX and COX
First, we tested effects of E3040 sulphate on enzyme activities in the cell-free assay system. E3040 sulphate inhibited Tx synthase in a concentration-dependent manner with an IC50 of 0.013 μM (Figure 2). In contrast, E3040 sulphate (10 – 100 μM) had little effects on activities of 5-LOX and COX (Figure 2). These results suggest that E3040 sulphate is a specific inhibitor of Tx synthase.
Figure 2.
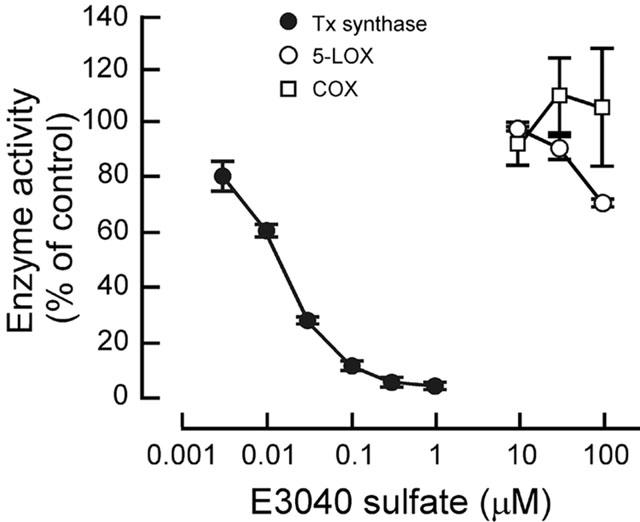
Effects of E3040 sulphate on enzyme activities of Tx synthase, 5-LOX and COX in the cell-free system. Data are means±s.e.m. of three experiments, each performed in duplicate.
The TxA2-mediated Cl− secretion induced by PAF in isolated rat colon
In the present study, we used 10 μM PAF because this concentration of PAF was known to give a maximal response in the rat colon (Wardle et al., 1996). PAF (10 μM at the serosal side) significantly increased Isc from 27.4±1.1 to 70.0±3.2 μA cm−2 at a sustained plateau phase (P<0.01, n=36). The plateau phase started at 35±3 min (n=36) after the addition of PAF, and the phase sustained at least for 60 min (Figure 3A,B). We found recently that the Cl− secretion induced by PAF in isolated rat colon is mediated by a release of TxA2 (Suzuki et al., 2000b). In fact, the PAF-elicited Cl− secretion was inhibited by a selective TxA2 receptor antagonist (KW-3635) and a selective thromboxane synthase inhibitor (Y-20811) (Suzuki et al., 2000b).
Figure 3.
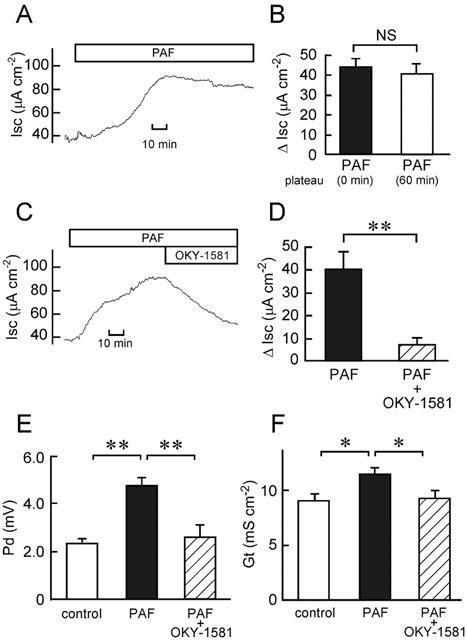
Increase in Cl− current by PAF (A,B) and inhibitory effects of OKY-1581 on the PAF-induced increases in Cl− current (C,D), Pd (E) and Gt (F) in isolated rat colon. Ten μM PAF was added at the serosal side. (A) A typical trace of the PAF-induced current is shown. (B) The Isc values just after (0 min; left column) and 60 min after (right column) the beginning of PAF-elicited plateau phase were read, and expressed as net increases from the Isc just before addition of PAF (ΔIsc). Data are means±s.e.m. from five experiments. NS, not significantly different (P>0.05). (C) One μM OKY-1581 was added at both the serosal and mucosal sides after the PAF-elicited plateau phase was observed. A typical trace of Isc is shown. (D) The values of Isc just before addition of OKY-1581 (1 μM) were read, and expressed as net increases from the Isc just before addition of PAF (ΔIsc) (left column). When the effect of OKY-1581 had become steady, the Isc values were read, and expressed as ΔIsc (right column). Data are means±s.e.m. from five experiments. **, significantly different (P<0.01). (E,F) The Pd (E) and Gt (F) values were measured just before addition of PAF (open column), when values of Isc had established their steady states after addition of PAF (filled column), and after the additional application of 1 μM OKY-1581 (hatched column). Data are means±s.e.m. from five experiments. * and **, significantly different (P<0.05 and 0.01).
To confirm again the involvement of TxA2 production in the PAF response, we used OKY-1581 (Feuerstein & Ramwell, 1981) as another specific Tx synthase inhibitor. OKY-1581 (1 μM) inhibited the PAF-increased Cl− secretion by 81% (Figure 3C,D), while it did not affect the baseline Isc (in the absence of PAF) in the rat colon: the values before and after the addition of OKY-1581 were 31.6±1.4 and 31.2±2.1 μA cm−2, respectively (n=5, P>0.05). Increases in Pd and Gt elicited by PAF were significantly inhibited by 1 μM OKY-1581 (Figure 3E,F).
Inhibition of the PAF-induced Cl− secretion by E3040 sulphate
The PAF (10 μM)-induced Cl− secretion was inhibited in a concentration-dependent manner by E3040 sulphate added at the serosal side (Figure 4A,B). Its IC50 value was 1.8 μM (Figure 4B). Increases in Pd and Gt elicited by PAF were also significantly inhibited by 30 μM E3040 sulphate (Figure 4C,D). E3040 sulphate (30 μM), as found with OKY-1581, did not affect the baseline Isc (in the absence of PAF): the values before and after the addition of E3040 sulphate were 32.3±0.8 and 32.7±1.1 μA cm−2, respectively (n=5, P>0.05). When PAF (10 μM) was added in the presence of E3040 sulphate (30 μM), the effect of PAF on Isc was significantly inhibited: the values of ΔIsc (at 60 min after the addition of PAF) in the absence and presence of E3040 sulphate were 41.7±2.0 and 7.9±4.0 μA cm−2, respectively (n=4, P<0.01).
Figure 4.

Inhibitory effects of E3040 sulphate on the PAF-induced increases in Cl− current (A,B,E), Pd (C) and Gt (D) in isolated rat colon. Ten μM PAF was added at the serosal side. (A) Thirty μM E3040 sulphate was added at the serosal side after the PAF-elicited plateau phase was observed. A typical trace of Isc is shown. (B) Concentration-dependent effects of E3040 sulphate. The values of Isc are expressed as net increases from the Isc just before addition of PAF (ΔIsc). Smaller value of ΔIsc means larger extent of inhibition. Data are means±s.e.m. from five experiments. * and **, significantly different from the value in the absence of E3040 sulphate (P<0.05 and 0.01). (C,D) The Pd (C) and Gt (D) values were measured just before addition of PAF (open column), when values of Isc had established their steady states after addition of PAF (filled column), and after the additional application of 30 μM E3040 sulphate (hatched column). Data are means±s.e.m. from five experiments. * and **, significantly different (P<0.05 and 0.01). (E) Effect of E3040 sulphate on the Cl− current in the presence of PAF and OKY-1581. The experimental protocol was the same as that in the legend of Figure 3C and D, except E3040 sulphate (30 μM) was subsequently added when the effect of OKY-1581 (1 μM) had become steady. The values of Isc are expressed as ΔIsc. Data are means±s.e.m. from seven experiments. NS, not significantly different (P>0.05).
After the inhibition of PAF-induced increase in Isc by OKY-1581 (1 μM), E3040 sulphate (30 μM) did not further decrease the Isc (Figure 4E).
Effects of E3040 sulphate on PGE2- and LTD4-induced increases in Isc
PGE2 (Diener et al., 1988) and LTD4 (Hyun & Binder, 1993) have been reported to stimulate Cl− secretion in isolated rat colonic mucosa. E3040 sulphate (30 μM) did not significantly affect the increases in Isc evoked by PGE2 (0.5 μM; Figure 5) and LTD4 (1 μM; Figure 6). E3040 sulphate also had no effect on the LTD4-elicited downward deflection of Isc observed just after the transient increase (Figure 6D). These results suggest that E3040 sulphate does not antagonize prostaglandin EP receptors and LTD4 receptor present in the colonic mucosa, and that it does not block the PGE2- and LTD4-elicited signalling pathways in the colonic cells.
Figure 5.
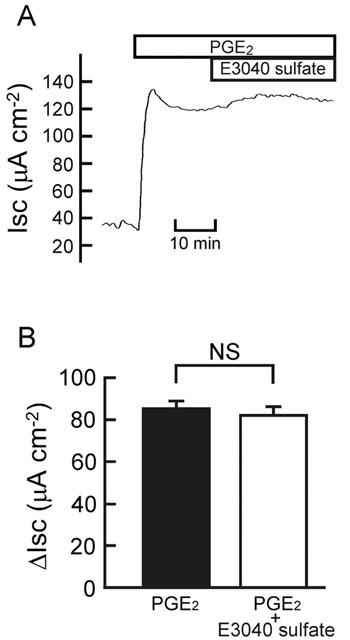
Effect of E3040 sulphate on the PGE2-elicited current. (A) At the plateau phase observed after addition of PGE2 (0.5 μM at the serosal side), E3040 sulphate (30 μM) was added at the serosal side. A typical trace is shown. (B) The values of Isc were read just before addition of E3040 sulphate, and data are expressed as net increases from the Isc just before addition of PGE2 (ΔIsc) (left column). At 30 min after addition of E3040 sulphate, the Isc values were read, and data are expressed as ΔIsc (right column). Data are means±s.e.m. from five experiments. NS, not significantly different (P>0.05).
Figure 6.
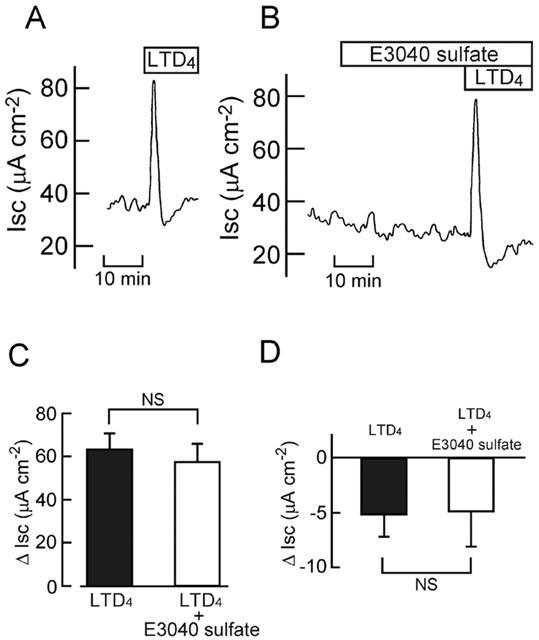
Effect of E3040 sulphate on the LTD4-elicited current. LTD4 (1 μM) was added at the serosal side in the absence (A) and presence (B) of E3040 sulphate (30 μM at the serosal side). Typical traces are shown. (C,D) The LTD4-induced transient peak (C) and subsequent downward deflection (D) of Isc were measured in the absence (left column) and presence (right column) of E3040 sulphate, and the values are expressed as net increases (C) and decreases (D) from the Isc just before addition of LTD4 (ΔIsc). Data are means±s.e.m. from five experiments. NS, not significantly different (P>0.05).
Inhibition of the PAF-stimulated release of TxB2 by E3040 sulphate from the colonic mucosa
In concert with above results, PAF (10 μM at the serosal side) stimulated a release of TxB2, a stable analogue of TxA2, from the colonic mucosa into the serosal solution (Figure 7). The PAF-increased release of TxB2 was significantly inhibited by E3040 sulphate (30 μM at the serosal side) (Figure 7).
Figure 7.
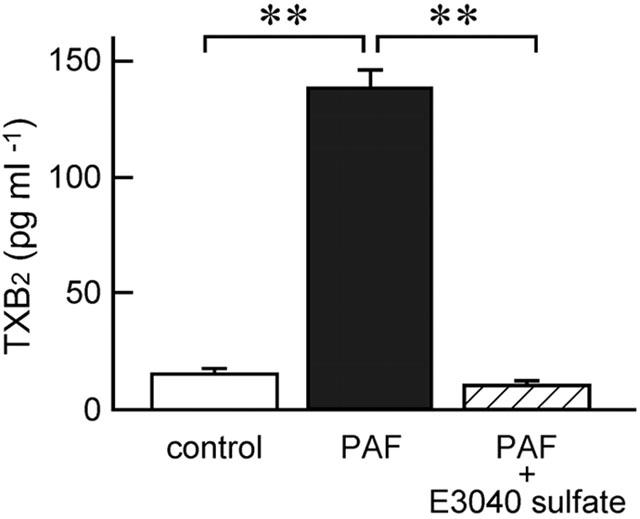
PAF-induced release of TxB2 into the serosal solution from the mucosa set between Ussing chambers, and inhibitory effect of E3040 sulphate. TxB2 concentrations in serosal solutions incubated without PAF and E3040 sulphate (control), with 10 μM PAF (PAF), and with 10 μM PAF plus 30 μM E3040 sulphate (PAF+E3040 sulphate) were determined in each mucosa as described in Methods. The values are expressed as the amount of TxB2 (pg) in 1 ml of serosal solution. Data are means±s.e.m. from five experiments. **, significantly different (P<0.01).
Effects of inhibitors of organic anion transporters on the E3040 sulphate-induced inhibition of the PAF response
It has been suggested that the hepatic uptake of E3040 sulphate in the rat may be mediated by the Na+-independent multispecific organic anion transporter, because E3040 sulphate is efficiently taken up into the liver in spite of its hydrophilic nature (Takenaka et al., 1997). We, therefore, tested whether the E3040 sulphate (30 μM)-induced inhibition of the PAF response (Figure 8A) was attenuated by organic anion transport inhibitors such as probenecid, p-aminohippuric acid (PAH) and sulphobromophthalein (BSP).
Figure 8.
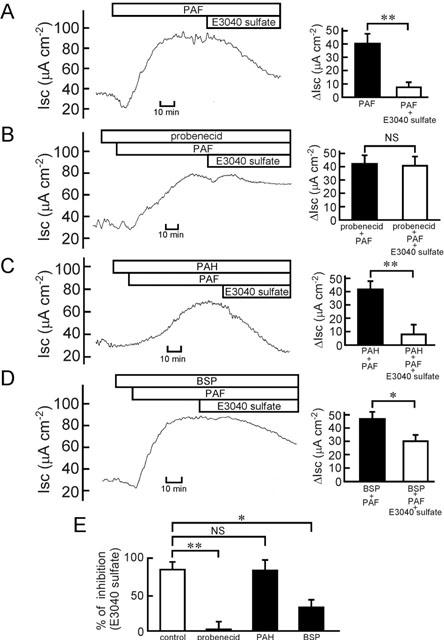
Effects of organic anion transport inhibitors on the E3040 sulphate-induced inhibition of the PAF-induced Cl− current. (A – D, left) Before addition of PAF (10 μM at the serosal side), the mucosa was pre-treated without (A) and with probenecid (100 μM at the serosal side) (B), PAH (1 mM at the serosal side) (C) or BSP (30 μM at the serosal side) (D). At the plateau phases observed after addition of PAF, E3040 sulphate (30 μM) was added at the serosal side. Typical traces are shown. (A – D, right) The values of Isc just before addition of E3040 sulphate were read, and data are expressed as net increases from the Isc just before addition of PAF (ΔIsc) (filled column). At 60 min after addition of E3040 sulphate in the absence (A) and presence of probenecid (B), PAH (C) or BSP (D), the Isc values were read, and data are expressed as ΔIsc (open column). Data are means±s.e.m. from five experiments. * and **, significantly different (P<0.05 and 0.01). NS, not significantly different (P>0.05). (E) The inhibitory effects of E3040 sulphate on the PAF response in the absence (control) and presence of the drug (probenecid, PAH or BSP) are expressed as per cent of inhibition. The values were calculated using the data of Figure 8A – D. One hundred per cent means complete inhibition of the PAF response by E3040 sulphate. * and **, significantly different (P<0.05 and 0.01). NS, not significantly different (P>0.05).
Probenecid (100 μM) abolished the inhibitory effect of E3040 sulphate (Figure 8B,E). It did not affect the baseline Isc (in the absence of PAF and E3040 sulphate): the values before and after the addition of 100 μM probenecid were 31.2±1.8 and 29.2±2.8 μA cm−2, respectively (n=5, P>0.05). PAH (1 mM) did not attenuate the effect of E3040 sulphate (Figure 8C,E). BSP (30 μM) partially but significantly attenuated the inhibitory effect of E3040 sulphate (Figure 8D,E). It did not affect the baseline Isc: the values before and after the addition of 30 μM BSP were 30.3±1.6 and 29.3±2.3 μA cm−2, respectively (n=5, P>0.05). These results suggest that E3040 sulphate is taken up into the colon by an organic anion transporter, which is sensitive to probenecid and BSP but not to PAH.
Discussion
Conjugative metabolic reactions, such as sulphation and glucuronidation, are important pathways for inactivation or detoxification of drugs. We found in the present study that a metabolite of E3040, E3040 sulphate, has potent activity for inhibition of Tx synthase (IC50=0.013 μM; Figure 2), as does its parent compound, E3040 (IC50=0.01 μM; Oketani et al., 2001b), in the cell-free system. Hibi et al. (1994) indicated that the 3-pyridylmethyl moiety of E3040 plays an essential role for inhibition of Tx synthase. In fact, this moiety is conserved between E3040 sulphate and E3040 (Figure 1). In contrast, E3040 sulphate did not show any significant activity for inhibition of 5-LOX (IC50>100 μM; Figure 2), whereas E3040 has potent activity against the enzyme (IC50=0.23 μM; Oketani et al., 2001b). The difference of chemical structure between E3040 and E3040 sulphate is only one moiety (-OH in E3040 and −OSO3H in E3040 sulphate; Figure 1), suggesting that the 6-hydroxy moiety played a key role for inhibition of 5-LOX. Both E3040 sulphate (Figure 2) and E3040 (Oketani et al., 2001b) had no activity against COX in the cell-free system. Taken together, E3040 sulphate is suggested to be a specific Tx synthase inhibitor, whereas E3040 is a dual inhibitor of 5-LOX and Tx synthase (Hibi et al., 1994).
E3040 sulphate was also effective in isolated rat colonic mucosa (Figure 4). We found recently that TxA2 is a novel secretagogue in the colonic mucosa (Sakai et al., 1995, 1997; Suzuki et al., 2000a), and that it acts on TxA2 receptor in the epithelial crypt cells (Ikari et al., 1999; Suzuki et al., 2000a). We also found that PAF-induced Cl− secretion in the rat colon is mainly mediated by TxA2 production (Suzuki et al., 2000b). In fact, PAF stimulated a release of TxB2 from the colonic mucosa (Figure 7), and OKY-1581, a specific Tx synthase inhibitor, blocked the PAF response (Figure 3). Leukotrienes are not involved in the PAF response in the rat colon (Suzuki et al., 2000b). In the present study, E3040 sulphate inhibited the PAF-induced Cl− secretion in a concentration-dependent manner with an IC50 of 1.8 μM (Figure 4). E3040 sulphate did not significantly affect the Cl− secretion induced by PGE2 (Figure 5) or LTD4 (Figure 6). Furthermore, E3040 sulphate significantly inhibited the PAF-stimulated release of TxB2 from the colon (Figure 7). These results suggest that E3040 sulphate inhibits the PAF-induced Cl− secretion by preventing TxA2 production in the rat colon.
Although the IC50 value for E3040 sulphate in isolated rat colon (1.8 μM) is about 100 times higher than that in the cell-free system (0.013 μM; Figure 2), the effect of E3040 sulphate observed in the isolated colon may be physiologically relevant because of following studies. Oral administration of E3040 at 10 mg kg−1 to lipopolysaccharide-immunized rats inhibited the production of TxB2 in the colon (Oketani et al., 2001a). When [14C]-E3040 (10 mg kg−1) was orally administered to rats, the maximal radioactivity in the plasma was observed 15 min after the administration (4.94 μg eq. ml−1). At the time, 97.4% of total radioactivity was derived from [14C]-E3040 sulphate (O. Takenaka, Eisai Co.; personal communication). Based on these facts, the maximum plasma concentration of E3040 sulphate is calculated to be 13 μM after an oral administration of E3040 at a pharmacologically effective dose.
After intravenous injection of [14C]-E3040 to the rat, it rapidly disappeared from plasma with a half life of 2.5 – 4 min (Takenaka et al., 1995). On the other hand, the disappearance of E3040 sulphate from plasma was markedly slow compared with E3040 and E3040 glucuronide, and the area under the plasma concentration-time profiles from zero to infinity (AUC∞) of E3040 sulphate was approximately seven times greater than those of E3040 and E3040 glucuronide (Takenaka et al., 1995). These results and our present data generate a new idea that the inhibitory effect of E3040 on Tx synthase activity in the rat in vivo (Oketani et al., 2001a) is not mainly elicited by E3040 itself, but by E3040 sulphate.
The membrane permeability of E3040 sulphate is markedly reduced compared with E3040 (Takenaka et al., 1997), suggesting that E3040 sulphate is taken up into the rat colon by a specific uptake mechanism as reported in the liver (Takenaka et al., 1997). The hepatic uptake of E3040 sulphate has been suggested to be mediated by Na+-independent multispecific organic anion transporters such as Oatp1. E3040 sulphate was taken up into COS-7 cells transiently expressing Oatp1 protein (Kouzuki et al., 1999). In the present study, we found that the E3040 sulphate-induced effect in the rat colonic mucosa was attenuated by inhibitors for Oatp1 such as probenecid and BSP (Jacquemin et al., 1994; Kullak-Ublick et al., 1994; Sugiyama et al., 2001) (Figure 8). Our results suggest that Oatp1 and/or Oatp1-related homologue(s) that can transport E3040 sulphate may be expressed in the rat colon.
What kind of the cells are targets for PAF and E3040 sulphate in the rat colonic mucosa? At present, we do not have a definitive answer to this question. But PAF-targeting cells may be present in the submucosal layer, because 99% of total eicosanoids such as PGE2, PGF2α, 6-keto-PGF1α and TxB2 are populated in this layer (Craven & DeRubertis, 1983). Eosinophils may be one of possible candidates for the PAF-targeting cells that selectively release TxA2 in the colon, because Giembycz et al. (1990) found that PAF stimulated the release of TxB2 and PGE1/PGE2 from purified guinea-pig eosinophils at a relative molar ratio of 30 : 1 (TxB2 : PGE1/PGE2). Apparently, further studies are necessary to clarify these points.
Interestingly, both PAF (Nassif et al., 1996) and TxA2 (Rampton & Collins, 1993) are suggested as pathophysiological mediators of inflammatory bowel disease. In fact, PAF antagonists (Longo et al., 1995; Meenan et al., 1996), Tx synthase inhibitors (Vilaseca et al., 1990; Tozaki et al., 1999), and a TxA2 receptor antagonist (Taniguchi et al., 1997) significantly ameliorated mucosal inflammation in animal models of colitis. Oral administration of a Tx synthase inhibitor (ridogrel) for patients with active ulcerative colitis showed clinical and colonoscopic improvement, and the inhibitor significantly reduced the release of TxB2, while the release of PGE2 and LTD4 was not affected (Casellas et al., 1995).
In conclusion, we found that E3040 sulphate is a specific Tx synthase inhibitor, and that the drug taken up into subepithelial cells in the colon by multispecific organic anion transporters inhibits the PAF-induced colonic Cl− secretion by blocking a release of TxA2.
Acknowledgments
This work was supported in part by Grants-in-Aid from Japan Society for the Promotion of Science (to H. Sakai and N. Takeguchi) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H. Sakai and N. Takeguchi), and by the grants from Uehara Memorial Foundation, Takeda Science Foundation, Suzuken Memorial Foundation, The Research Foundation for Pharmaceutical Sciences and Tamura Foundation for Promotion of Science and Technology (to H. Sakai.). T. Suzuki is a research fellow of Japan Society for the Promotion of Science.
Abbreviations
- BSP
sulphobromophthalein
- COX
cyclo-oxygenase
- E3040
6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl) benzothiazole
- E3040 sulphate
6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl) benzothiazole O-sulphate
- LOX
lipoxygenase
- LT
leukotriene
- OKY-1581
sodium (E)-3-[4-(3-pyridylmethyl)phenyl]- 2-methylacrylate
- PAF
platelet-activating factor
- PAH
p-aminohippuric acid
- PG
prostaglandin
- Tx
thromboxane
References
- BORMAN R.A., JEWELL R., HILLIER K. Investigation of the effects of platelet-activating factor (PAF) on ion transport and prostaglandin synthesis in human colonic mucosa in vitro. Br. J. Pharmacol. 1998;123:231–236. doi: 10.1038/sj.bjp.0701602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASELLAS F., PAPO M., GUARNER F., ANTOLÍN M., SEGURA R.M., ARMENGOL J.R., MALAGELADA J.-R. Effects of thromboxane synthase inhibition on in vivo release of inflammatory mediators in chronic ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1995;7:221–226. [PubMed] [Google Scholar]
- CRAVEN P.A., DERUBERTIS F.R. Patterns of prostaglandin synthesis and degradation in isolated superficial and proliferative colonic epithelial cells compared to residual colon. Prostaglandins. 1983;26:583–604. doi: 10.1016/0090-6980(83)90196-x. [DOI] [PubMed] [Google Scholar]
- DENIZOT Y., CHAUSSADE S., NATHAN N., COLOMBEL J.F., BOSSANT M.-J., CHEROUKI N., BENVENISTE J., COUTURIER D. PAF-acether and acetylhydrolase in stool of patients with Crohn's disease. Dig. Dis. Sci. 1992;37:432–437. doi: 10.1007/BF01307739. [DOI] [PubMed] [Google Scholar]
- DIENER M., BRIDGES R.J., KNOBLOCH S.F., RUMMEL W. Neuronally mediated and direct effects of prostaglandins on ion transport in rat colon descendens. Naunyn-Scmiedeberg's Arch. Pharmacol. 1988;337:74–78. doi: 10.1007/BF00169480. [DOI] [PubMed] [Google Scholar]
- ELIAKIM R., KARMELI F., RAZIN E., RACHMILEWITZ D. Role of platelet-activating factor in ulcerative colitis. Gastroenterology. 1988;95:1167–1172. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN N., RAMWELL P.W. OKY-1581, a potential selective thromboxane synthetase inhibitor. Eur. J. Pharmacol. 1981;69:533–534. doi: 10.1016/0014-2999(81)90464-7. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., KROEGEL C., BARNES P.J. Platelet activating factor stimulates cyclo-oxygenase activity in guinea pig eosinophils. Concerted biosynthesis of thromboxane A2 and E-series prostaglandins. J. Immunol. 1990;144:3489–3497. [PubMed] [Google Scholar]
- HIBI S., OKAMOTO Y., TAGAMI K., NUMATA H., KOBAYASHI N., SHINODA M., KAWAHARA T., MURAKAMI M., OKETANI K., INOUE T., SHIBATA H., YAMATSU I. Novel dual inhibitors of 5-lipoxygenase and thromboxane A2 synthetase: synthesis and structure-activity relationships of 3-pyridylmethyl-substituted 2-amino-6-hydroxybenzothiazole derivatives. J. Med. Chem. 1994;37:3062–3070. doi: 10.1021/jm00045a011. [DOI] [PubMed] [Google Scholar]
- HIROHASHI T., SUZUKI H., SUGIYAMA Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3) J. Biol. Chem. 1999;274:15181–15185. doi: 10.1074/jbc.274.21.15181. [DOI] [PubMed] [Google Scholar]
- HYUN C.S., BINDER H.J. Mechanism of leukotriene D4 stimulation of Cl− secretion in rat distal colon in vitro. Am. J. Physiol. 1993;265:G467–G473. doi: 10.1152/ajpgi.1993.265.3.G467. [DOI] [PubMed] [Google Scholar]
- IKARI A., SAKAI H., SATO T., TAKEGUCHI N. Thromboxane A2 receptor linked with the Ca2+ pathway in rat colonic crypt cells. Biochem. Biophys. Res. Commun. 1999;258:708–712. doi: 10.1006/bbrc.1999.0674. [DOI] [PubMed] [Google Scholar]
- JACQUEMIN E., HAGENBUCH B., STIEGER B., WOLKOFF A.W., MEIER P.J. Expression cloning of a rat liver Na+-independent organic anion transporter. Proc. Natl. Acad. Sci. U.S.A. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALD B., OLAISON G., SJÖDAHL R., TAGESSON C. Novel aspect of Crohn's disease: increased content of platelet-activating factor in ileal and colonic mucosa. Digestion. 1990;46:199–204. doi: 10.1159/000200346. [DOI] [PubMed] [Google Scholar]
- KOUZUKI H., SUZUKI H., ITO K., OHASHI R., SUGIYAMA Y. Contribution of organic anion transporting polypeptide to uptake of its possible substrates into rat hepatocytes. J. Pharmacol. Exp. Ther. 1999;288:627–634. [PubMed] [Google Scholar]
- KULLAK-UBLICK G.-A., HAGENBUCH B., STIEGER B., WOLKOFF A.W., MEIER P.J. Functional characterization of the basolateral rat liver organic anion transporting polypeptide. Hepatology. 1994;20:411–416. [PubMed] [Google Scholar]
- LONGO W.E., CARTER J.D., CHANDEL B., NIEHOFF M., STANDEVEN J., DESHPANDE Y., VERNAVA A.M., POLITES G., KULKARNI A.D., KAMINSKI D.L. Evidence for a colonic PAF receptor. J. Surg. Res. 1995;58:12–18. doi: 10.1006/jsre.1995.1003. [DOI] [PubMed] [Google Scholar]
- MEENAN J., GROOL T.A., HOMMES D.W., DIJKHUIZEN S., TEN KATE F.J., WOOD M., WHITTAKER M., TYTGAT G.N., VAN DEVENTER S.J. Lexipafant (BB-882), a platelet activating factor receptor antagonist, ameliorates mucosal inflammation in an animal model of colitis. Eur. J. Gastroenterol. Hepatol. 1996;8:569–573. doi: 10.1097/00042737-199606000-00014. [DOI] [PubMed] [Google Scholar]
- NASSIF A., LONGO W.E., MAZUSKI J.E., VERNAVA A.M., KAMINSKI D.L. Role of cytokines and platelet-activating factor in inflammatory bowel disease: implications for therapy. Dis. Colon Rectum. 1996;39:217–223. doi: 10.1007/BF02068079. [DOI] [PubMed] [Google Scholar]
- OKETANI K., INOUE T., MURAKAMI M. Effect of E3040, an inhibitor of 5-lipoxygenase and thromboxane synthase, on rat bowel damage induced by lipopolysaccharide. Eur. J. Pharmacol. 2001a;427:159–166. doi: 10.1016/s0014-2999(01)01234-1. [DOI] [PubMed] [Google Scholar]
- OKETANI K., NAGAKURA N., HARADA K., INOUE T. In vitro effects of E3040, a dual inhibitor of 5-lipoxygenase and thromboxane A2 synthetase, on eicosanoid production. Eur. J. Pharmacol. 2001b;422:209–216. doi: 10.1016/s0014-2999(01)01022-6. [DOI] [PubMed] [Google Scholar]
- RAMPTON D.S., COLLINS C.E. Thromboxanes in inflammatory bowel disease – pathogenic and therapeutic implications. Aliment. Pharmacol. Ther. 1993;7:357–367. [PubMed] [Google Scholar]
- SAKAI H., DIENER M., GARTMANN V., TAKEGUCHI N. Eicosanoid-mediated Cl− secretion induced by the antitumor drug, irinotecan (CPT-11), in the rat colon. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:309–314. doi: 10.1007/BF00233252. [DOI] [PubMed] [Google Scholar]
- SAKAI H., SATO T., HAMADA N., YASUE M., IKARI A., KAKINOKI B., TAKEGUCHI N. Thromboxane A2, released by the anti-tumor drug irinotecan, is a novel stimulator of Cl− secretion in isolated rat colon. J. Physiol. (Lond.) 1997;505:133–144. doi: 10.1111/j.1469-7793.1997.133bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBHANI I., HOCHLAF S., DENIZOT Y., VISSUZAINE C., RENE E., BENVENISTE J., LEWIN M.M.J., MIGNON M. Raised concentrations of platelet activating factor in colonic mucosa of Crohn's disease patients. Gut. 1992;33:1220–1225. doi: 10.1136/gut.33.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIYAMA D., KUSUHARA H., SHITARA Y., ABE T., MEIER P.J., SEKINE T., ENDOU H., SUZUKI H., SUGIYAMA Y. Characterization of the efflux transport of 17β-estradiol-D-17β-glucuronide from the brain across the blood-brain barrier. J. Pharmacol. Exp. Ther. 2001;298:316–322. [PubMed] [Google Scholar]
- SUZUKI T., SAKAI H., IKARI A., TAKEGUCHI N. Inhibition of thromboxane A2-induced Cl− secretion by antidiarrhea drug loperamide in isolated rat colon. J. Pharmacol. Exp. Ther. 2000a;295:233–238. [PubMed] [Google Scholar]
- SUZUKI T., SAKAI H., TAKEGUCHI N. Thromboxane A2-mediated Cl− secretion induced by platelet-activating factor in isolated rat colon. Eur. J. Pharmacol. 2000b;400:293–303. doi: 10.1016/s0014-2999(00)00405-2. [DOI] [PubMed] [Google Scholar]
- TAKENAKA O., HORIE T., SUZUKI H., SUGIYAMA Y. Different biliary excretion systems for glucuronide and sulphate of a model compound; study using Eisai hyperbilirubinemic rats. J. Pharmacol. Exp. Ther. 1995;274:1362–1369. [PubMed] [Google Scholar]
- TAKENAKA O., HORIE T., SUZUKI H., SUGIYAMA Y. Carrier-mediated active transport of the glucuronide and sulphate of 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole (E3040) into rat liver; quantitative comparison of permeability in isolated hepatocytes, perfused liver and liver in vivo. J. Pharmacol. Exp. Ther. 1997;280:948–958. [PubMed] [Google Scholar]
- TANIGUCHI T., TSUKADA H., NAKAMURA H., KODAMA M., FUKUDA K., TOMINAGA M., SEINO Y. Effects of a thromboxane A2 receptor antagonist in an animal model of inflammatory bowel disease. Digestion. 1997;58:476–478. doi: 10.1159/000201486. [DOI] [PubMed] [Google Scholar]
- TOZAKI H., FUJITA T., ODORIBA T., TERABE A., SUZUKI T., TANAKA C., OKABE S., MURANISHI S., YAMAMOTO A. Colon-specific delivery of R68070, a new thromboxane synthase inhibitor, using chitosan capsules: therapeutic effects against 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. Life Sci. 1999;64:1155–1162. doi: 10.1016/s0024-3205(99)00044-2. [DOI] [PubMed] [Google Scholar]
- VILASECA J., SALAS A., GUARNER F., RODRIGUEZ R., MALAGELADA J.-R. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990;98:269–277. doi: 10.1016/0016-5085(90)90814-h. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L. Release of platelet-activating factor (PAF) and accelerated healing induced by a PAF antagonist in an animal model of chronic colitis. Can. J. Physiol. Pharmacol. 1988;66:422–425. doi: 10.1139/y88-071. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., BRAQUET P., IBBOTSON G.C., MACNAUGHTON W.K., CIRINO G. Assessment of the role of platelet-activating factor in an animal model of inflammatory bowel disease. J. Lipid Mediat. 1989;1:13–23. [PubMed] [Google Scholar]
- WARDLE T.D., HALL L., TURNBERG L.A. Platelet activating factor: release from colonic mucosa in patients with ulcerative colitis and its effect on colonic secretion. Gut. 1996;38:355–361. doi: 10.1136/gut.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA H., KOTAKI H., ITOH T., SAWADA Y., IGA T. Effect of anti-inflammatory bowel disease drug, E3040, on urate transport in rat renal brush border membrane vesicles. Eur. J. Pharmacol. 2000;406:45–48. doi: 10.1016/s0014-2999(00)00626-9. [DOI] [PubMed] [Google Scholar]


