Abstract
Distension-sensitive vagal afferent fibres from the guinea-pig oesophagus were recorded extracellularly in vitro. Most recorded units were spontaneously active firing at 3.2±0.3 Hz (n=41, N=41) and had low thresholds (less than 1 mm) to circumferential stretch. Dynamic and adapted phases of stretch-evoked firing, as well as a silent period were linearly dependent on the amplitude of stretch.
High K+ (7–12 mM) Krebs solution dose-dependently increased both spontaneous and stretch-evoked firing and reduced the duration of the silent period.
Charybdotoxin (ChTX, 100 nM) slightly increased spontaneous and stretch-evoked firing and decreased the silent period, while neither iberiotoxin (100 nM) nor apamin (0.5 μM) had significant effects. ω-Conotoxin GVIA (0.5 μM) did not significantly affect firing of vagal mechanoreceptors.
In the majority of single units, 4-aminopyridine (4-AP) concentration-dependently (EC50∼28 μM) increased spontaneous firing, strongly reduced the silent period but did not affect stretch (3 mm)-induced firing. Firing evoked by 1–2 mm was increased by 4-AP.
α-Dendrotoxin (DnTX, 300 nM) and DnTX K (30 nM) slightly increased spontaneous and stretch-evoked firing. There was no additive effect on spontaneous firing when ChTX and DnTX K were applied simultaneously.
Barium (100 μM) increased stretch-induced firing, probably due to an increase in intramural tension. Glibenclamide (10 μM) had no effect on spontaneous or stretch-induced firing.
The results indicate that voltage-gated 4-AP- and dendrotoxin-sensitive K+ channels are the main type of K+ channels that influence excitability of vagal mechano-sensitive endings of the guinea-pig oesophagus. They were involved in control of spontaneous firing and in stretch-induced firing evoked by moderate stretch, but none of the K+ channels appeared to be involved in adaptation to maintained stretch by their slowly adapting vagal mechanoreceptors.
Keywords: Afferents, oesophagus, vagus nerve, potassium channels
Introduction
The most diverse group of ion channels – K+ channels – are currently structurally divided into three major classes: (1) one pore domain Shaker-related channels with six transmembrane segments comprising voltage-gated K+ channels (Kv), Ca2+-activated Slo, Ca2+-regulated small conductance K+ channels (SK) etc; (2) one pore domain inward rectifiers K+ channels (Kir) with two transmembrane segments; (3) largest structural class of two pore domains with four transmembrane segments comprising TWIK, TASK, TRAAK channels etc (Bargmann, 1998; Jan & Jan, 1997; Lesage & Lazdunski, 2000). A variety of K+ channels are involved in modulation of excitability of vagal afferent neurones (Christian et al., 1994; Cordoba-Rodriguez et al., 1999; Glazebrook et al., 2002; Hay & Kunze, 1994a, b). However most of the data about a role of K+ channels in influencing excitability has been obtained from studies on cell bodies in the nodose and petrosal ganglia and only few studies have been carried out on sensory endings (McAlexander & Undem, 2000). Nodose ganglion neurones are a heterogeneous population innervating many visceral organs in the upper part of the body. From the studies on the nodose ganglion cell bodies, some distinct groups of neurones express different combinations of K+ channels. For example, it has been shown that Ca2+-dependent K+ channels with large single conductance (BK or maxi-K+ channels) and rapidly inactivating (transient) IA current were found only in restricted subpopulations of C-type nodose neurones (Christian et al., 1994; Ducreux & Puizillout, 1995).
It has been speculated that the same receptors and ionic channels are present on both nodose ganglion cell bodies and in their peripheral endings (Harper, 1991; Higashi et al., 1982). It has been demonstrated recently that the same type of K+ channels are present not only in the mechanically-sensitive endings of vagal afferents innervating the guinea-pig trachea and bronchus but also along their peripheral axons (McAlexander & Undem, 2000). However rapid adaptation of vagal mechanoreceptors in the trachea does not appear to be related to the adaptation observed at cell bodies (McAlexander et al., 1999). This suggests that it may be advantageous to study the role of ion channels directly in their peripheral endings, rather than relying on extrapolation of findings made in heterogeneous population of nodose ganglion nerve cell bodies.
Recently we have shown that specialized intraganglionic laminar endings (IGLEs) are the mechano-transduction sites of vagal mechanoreceptors in the guinea-pig upper gut (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al., 2001). We speculated that K+ channels might be involved in either the adaptation of vagal mechanoreceptors or setting their silent periods after mechanical stimulation (Zagorodnyuk et al., 2001). In addition, we have found that the excitability of vagal mechanically-sensitive endings in the guinea-pig oesophagus was increased in Ca2+-free Krebs solution, suggesting an involvement of Ca2+ dependent K+ channels (Zagorodnyuk et al., 2002). The aim of this study was to investigate which K+ channels may influence excitability and adaptation of vagal mechanically-sensitive endings of guinea-pig oesophagus. Preliminary accounts of this study have appeared in abstract form (Zagorodnyuk et al., 2002).
Methods
Extracellular recording
Adult guinea-pigs of both sexes (total N=41), weighing between 240 and 350 g, were killed humanely by stunning and exsanguination, in a manner approved by the Animal Welfare Committee of the Flinders University of South Australia. Method of extracellular recordings from vagal mechanically-sensitive afferents in guinea-pig oesophagus has been described previously (Zagorodnyuk & Brookes, 2000). Briefly, the distal oesophagus was opened up into a flat sheet and the mucosa removed leaving 10 mm long preparation which was washed with Krebs solution (mM: NaCl 118; KCl 4.75; NaH2PO4 1.0; NaHCO3 25; MgSO4 1.2; CaCl2 2.5; glucose 11; bubbled with 95% O2–5% CO2). The fine vagal nerve branches (originating from right vagus nerve), which innervated the distal region of the oesophagus, were dissected free of connective tissue and the preparation was placed in an organ bath with a volume of 5 mls. One edge of the preparation was fixed by pins. An array of hooks was used to attach the other edge of the preparation to an isometric force transducer (DSC no. 46-1001-01, Kistler-Morse, Redmond, WA, U.S.A.) mounted on a microprocessor controlled tissue stretcher (Brookes et al., 1999). This allowed the recording of circumferential tension (tension recorded from the circular muscle layer) developed during the stretch. The preparation was initially stretched to take up the slack, to a resting tension of 0.5–1 mN and at least 120 min of equilibration was allowed before experiments started. During the experiment, oesophageal preparations were stretched by 1–3 mm at 5 mm s−1 and held for 10 s, while monitoring circumferential tension. The empty diameter of the oesophagus in situ is about 2–2.5 mm (i.e. with a circumference of 6–8 mm). When the oesophagus was opened up and stretched to give minimal tension (0.5–1 mN) the circumference ranged from 7–9 mm. The maximum stretch of 3 mm thus represented an increase in diameter of about 50–60% of the empty oesophagus. Although no measurements of oesophagal diameter with a bolus of food have been performed in guinea-pig in vivo, stretch used in this study is likely to be well within physiological distension.
The dissected vagal trunk and a separate strand of connective tissue strand were pulled into a second small chamber (1 ml volume) under a partition made from a cover slip, and sealed in position with silicon grease (Ajax Chemicals, Australia). The Krebs solution in the small chamber was removed and replaced with paraffin oil and extracellular recordings were made with two platinum electrodes. Signals were amplified differentially (DAM 80, WPI, U.S.A.) and recorded at 20 kHz with a MacLab 8sp attached to a Macintosh G4 computer (Apple, Cupertino, CA, U.S.A.) using Chart 3.6.5. software (AD Instruments, Sydney, Australia). Single units were discriminated by spike amplitude and duration using Spike Histogram software (AD Instruments, Sydney, Australia).
To analyse firing rate of vagal mechanically-sensitive afferents the spontaneous firing rate within 10 s before the stretch was averaged, the dynamic phase of firing averaged within 3 s from the onset of the stretch and the adapted phase of firing during the last 3 s of the stretch were calculated. In preliminary experiments we found that the duration of the silent period remained constant over long periods (2–3 h) if intervals of 2–3 min were allowed between stretches. With shorter intervals (10–30 s) the silent period was significantly shorter, due to decreases in stretch-evoked firing (n=5, N=5). For this reason we used 3 min intervals between stretches in all studies.
Drugs
α-Dendrotoxin and dendrotoxin K were obtained from Alomone Labs (Jerusalem, Israel) while charybdotoxin and iberiotoxin were obtained from two sources: Alomone labs (Jerusalem, Israel) and Auspep (Melbourne, Australia). Apamin, 4-aminopyridine, glibenclamide, barium chloride were from Sigma. Different concentrations of 4-AP and high K+ Krebs solution were applied cumulatively when dose response relationship was studied. Each single dose was applied for 10–15 min until maximal effect was seen. Neurotoxins such as charybdotoxin, iberiotoxin, apamin, α-dendrotoxin, dendrotoxin K and ω-conotoxin GVIA were re-circulated for 30 min in 20 ml volume of final concentrations.
Data analysis
Results are expressed throughout as means±standard error of the mean, with n referring to the number of units and N to the number of animals. Statistical analysis was performed by Student's two-tail t-test for paired or unpaired data or by repeated measures one-way analysis of variance (ANOVA) with Dunnett's or Tukey's comparison tests using Prism 3 software (GraphPad Software, Inc., San Diego, CA, U.S.A.). Differences were considered significant if P<0.05.
Results
General properties: oesophageal firing rate and silent period
Most mechanically-sensitive units were spontaneously active, firing at 3.2±0.3 Hz (n=41, N=41) and had low thresholds (less than 1 mm) to circumferential stretch. Responses of vagal mechanically-sensitive endings to stretch (1–3 mm at 5 mm s−1, held for 10 s) could be readily separated into three components: (1) a dynamic phase of firing – during the initial peak in firing rate (with maximum around 1–3 s) following the onset of stretch; (2) an adapted phase of firing – firing remained elevated, compared to resting spontaneous discharge, for the duration of a maintained stretch (10 s); (3) a silent period (duration range from 0.5 s to 14 s) which followed removal of stretch (Figure 1a). There was a linear dependence between the dynamic phase of firing and the amplitude of stretch (r2=0.98) as well as between the adapted phase of firing and the amplitude of stretch (r2=0.94). The duration of the silent period was also linearly dependent on the amplitude of stretch (r2=0.97) (Figure 1b,c,d). The mean silent period was significantly different at three different amplitudes of stretch being 1.8±0.2 s (n=41, N=41) at 1 mm stretch, 3.5±0.3 s (n=41, N=41) at 2 mm and at 3 mm stretch, 6.6±0.6 s (n=41, N=41, P<0.01, one-way ANOVA, repeated measures, Tukey's multiple comparison test). This was probably due to significantly higher dynamic and adapted firing rates with larger stretch amplitudes (see Figure 1b,d). It is worth mentioning that the duration of silent period was in inverse linear relationship with spontaneous firing rate (r2=0.48 for 1 mm stretch) (not shown).
Figure 1.

Typical recording from mechanically-sensitive oesophageal vagal afferents and dependence of firing rate and silent period on stretch. (a) Typical recording from mechanically-sensitive oesophageal vagal afferents during 2 mm (at 5 mm s−1 for 10 s) circumferential stretch. (b) Averaged data of stretch (1–3 mm)-evoked firing. (c) Linear dependence of the dynamic phase of firing to amplitude of stretch. (d) Linear dependence of the duration of the silent period to amplitude of stretch. (e) Complex relationship between the duration of the silent period and the duration of the stretch (solid line). Simple modelled curve (scaled) based on numbers of action potentials, exponentially weighted from the time of the removal of stretch to preceding 20 s, during different durations of stretch (dashed line) (see text). Each value is the mean of 41 experiments for (b), (c) and (d) and seven preparations for (e). In (b), * and #=significant difference from control for dynamic and adapted phase of firing, respectively. In (c), *=significant difference from spontaneous firing; in (d), *=significant difference from the duration of spontaneous interspike intervals; in (e), *=significant difference from the duration of silent period during 10 s stretch, P<0.05.
When the amplitude of stretch was kept constant (3 mm) the duration of the silent period showed a complex relationship to stretch duration (n=7, N=6) when repeated at 3 min intervals. The longest silent period was typically seen with a 10 s stretch and the duration was significantly reduced with either shorter or longer stretches (Figure 1e). This appeared to be proportional to the number of action potentials weighted by their proximity to the removal of stretch. The number of action potentials per second was measured for the 20 s preceding the removal of stretch, during stretches of 0.5, 1, 3, 10, 30, 60, 90 and 120 s duration. The frequency of firing of action potentials was weighted by applying a reverse exponential decay from the time of the removal of stretch to the preceding 20 s. This ensures that both spontaneous, dynamic and adapted phases of stretch-induced firing are taken into account. The calculated curve representing the weighted frequency of action potentials closely matched the experimental data on duration of silent period for different stretch duration (Figure 1e). This suggests that the duration of the silent period is most likely related to the number of action potentials corrected for the duration of stretch.
The effect of high K+ Krebs solution was studied on oesophageal afferents in order to evaluate how dynamic and adapted phases of firing and silent period were affected by depolarization of nerve terminals. High K+ (7–12 mM, N=6) Krebs solution increased both spontaneous and stretch-evoked firing and reduced the silent period in a concentration-dependent manner (Figure 2). Maximum effect was seen with 12 mM (K+]o (the highest concentration applied) where spontaneous firing increased by 3.7±0.4 Hz (n=8, N=6, P<0.001); the dynamic phase increased by 5.5±0.7 Hz (n=8, N=6, P<0.001) and adapted phase of firing increased by 4.9±0.6 Hz (n=8, N=6, P<0.001) during 2 mm stretch. At the same time, the silent period was reduced by 52±4% at 2 mm stretch (n=8, N=6, P<0.001) (Figure 2). Intramural tension was not changed significantly by high K+ (7–12 mM) Krebs solution indicating that the effects seen were attributed to an increase in excitability of mechano-sensitive nerve endings. If K+ channels contribute physiologically to the control of excitability of these endings by being open at resting level of membrane potential, then blockade of K+ channels by specific blockers would result in depolarization similar to that evoked by raised [K+]o.
Figure 2.

Effect of high K+ Krebs solution on the firing and silent period of oesophageal afferents. (a, b) High K+ (7–12 mM, N=6) Krebs solution dose-dependently increased both spontaneous and stretch (2, 3 mm)-evoked firing and reduced the silent period (c). Each value in (a), (b) and (c) is the mean from eight units in six preparations. #, *, @=Significant difference from control for 7 mM K+, 10 mM K+ and 12 mM K+, respectively, P<0.05.
Because of cost, neurotoxins that selectively block particular types of K+ channels were applied by re-perfusion at their final concentration in 20 ml of Krebs solution for 30 min. We tested whether or not re-perfusion changed the spontaneous and stretch-induced firing of vagal afferents. Only spontaneous firing was slightly but significantly affected showing an increase of 0.3±0.1 Hz (n=9, N=6, P<0.01), while stretch-induced firing was not affected (Table 1). This small increase, due to re-perfusion itself, should be borne in mind in interpreting the effects of drug on spontaneous firing.
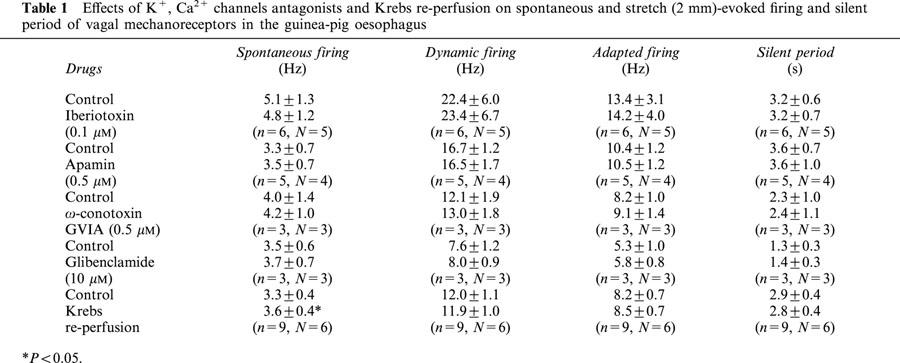
Table 1.
Effects of K+, Ca2+ channels antagonists and Krebs re-perfusion on spontaneous and stretch (2 mm)-evoked firing and silent period of vagal mechanoreceptors in the guinea-pig oesophagus
Effect of blockade of Ca2+-dependent K+ channels on firing rate and silent period
The effect of charbybdotoxin (ChTX), which blocks Ca2+-dependent K+ channels, in particular BK and some Ca2+-dependent K+ channels with an intermediate single conductance (IK) (Garcia et al., 1995; Grissmer et al., 1994; McManus, 1991) was studied. ChTX (100 nM for 30 min re-perfusion) increased spontaneous firing by 0.9±0.2 Hz (n=11, N=6, P<0.001). This increase in spontaneous firing evoked by ChTX was also significantly different (P<0.05) from the small increase seen in control Krebs re-perfusion experiments. Stretch (1–3 mm)-evoked dynamic and adapted phases of firing increased by 0.8–3.2 Hz and 1–2.8 Hz, respectively, and the silent period was significantly decreased by 17–25% (Figure 3). For example, during 2 mm stretch the dynamic phase increased by 1.9±0.4 Hz (n=11, N=6, P<0.001) and the adapted phase increased by 1±0.1 Hz (n=11, N=6, P<0.001). The rate of adaptation of stretch-induced firing measured relative to the peak dynamic response during 10 s stretch was not affected by ChTX (100 nM, n=11, N=6) (Figure 3). The silent period was significantly decreased by 17±4% (n=11, N=6, P<0.01) at 2 mm stretch (Figure 3). Basal and stretch-evoked intramural tension were not changed by ChTX (100 nM).
Figure 3.
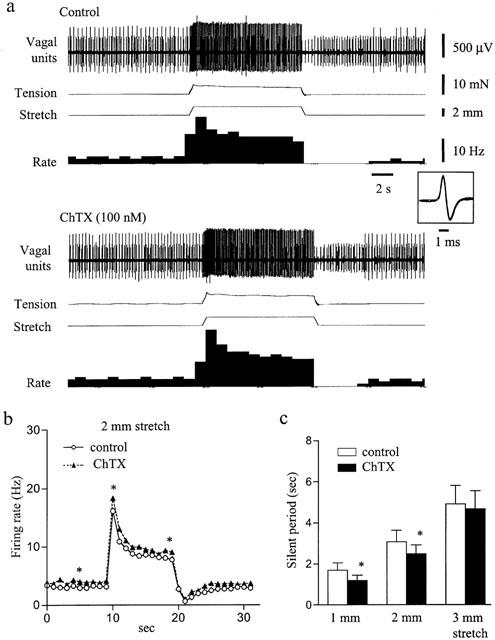
Effect of charybdotoxin (ChTX) on firing rate and silent period. (a) Typical recording of spontaneous and stretch (2 mm)-evoked firing in control and after 30 min of ChTX (100 nM). Note that in control and after ChTX firing rate of discriminated single unit (with big amplitude of action potentials, see inset with 10 superimposed action potentials on faster time scale) is shown. (b) Averaged data (from 11 units in six preparations) of effect of ChTX (100 nM) on firing evoked by 2 mm stretch. (c) Reduction in the duration of the silent period by ChTX (100 nM). Each value in (c) is the mean from 11 units in six preparations. *Significant difference from control, P<0.05.
Because ChTX can inhibit some voltage-dependent K+ channels as well as Ca2+-dependent K+ channels (Garcia et al., 1995; Grissmer et al., 1994), we tested the effect of a more selective BK channels blocker, iberiotoxin (Garcia et al., 1991). Iberiotoxin from two separate suppliers (100 nM, n=6, N=5) had no significant effects on spontaneous or stretch-induced firing (Table 1). Similarly, the selective SK channels blocker, apamin (Garcia et al., 1991) (0.5 μM, n=5, N=4), had no significant effects on spontaneous or stretch-evoked firing (Table 1).
Recently it was shown that the slow after hyperpolarization (AHP) following action potentials in nodose ganglion cell bodies, which is mediated by activation of Ca2+-dependent K+ channels, can be blocked by the N-type Ca2+ channels blocker, ω-conotoxin GVIA (Cordoba-Rodriguez et al., 1999). We therefore studied the effect of ω-conotoxin GVIA on the firing rate and silent period of vagal mechanoreceptors. ω-Conotoxin GVIA (0.5 μM, n=3, N=3) did not significantly affect either spontaneous or stretch (1–3 mm)-evoked firing, or the silent period following removal of stretch (Table 1).
Effect 4-AP and dendrotoxins on firing rate and silent period
The relatively non-selective blocker of voltage-gated K+ channels (Kv), 4-aminopyridine (4-AP) in most mechano-sensitive single units tested (six out of 10, N=10) potently increased spontaneous firing in a concentration-dependent manner. The threshold concentration of 4-AP that evoked significant increase in spontaneous firing rate, was 10 μM and EC50 was 27.7 μM (95% confidence limits were 20.1–38.1 μM, n=6, N=6) (Figures 4 and 5). The stretch-induced firing evoked by 1–2 mm stretch was also significantly increased by low (10–30 μM) concentrations of 4-AP. For example, during 2 mm stretch the dynamic and adapted phases of firing were increased by 0.8±0.7 Hz (n=6, N=6, NS) and by 1.4±0.5 Hz (n=6, N=6, P<0.05) at 10 μM of 4-AP and by 2±0.9 Hz (n=5, N=5, NS) and by 2.7±0.7 Hz (n=5, N=5, P<0.05) at 30 μM of 4-AP, respectively. However the firing rate evoked by 3 mm stretch was not significantly affected by 4-AP at any concentrations tested (3 μM–1 mM) (Figures 4 and 5), suggesting that the increase in excitability reached a ceiling. Another group of four units (out of 10) behaved very differently being only weakly affected by 4-AP (3 μM–1 mM). Thus two distinct groups of distension-sensitive units in the guinea-pig oesophagus with high and low sensitivity to 4-AP could be distinguished (Figure 5a). For example, in units with high sensitivity, spontaneous firing was increased by 4-AP (100 μM) by 6.4±0.6 Hz (n=6, N=6, P<0.0001) and the duration of the silent period was dramatically reduced by 80–87% during 1–3 mm stretch (n=6, N=6, P<0.05) (Figure 5). In units with low sensitivity, 4-AP, starting at concentration of 30 μM, only slightly increased spontaneous firing and did not affect stretch-induced firing. 4-AP (100 μM) increased spontaneous firing by 1.2±0.1 Hz (n=4, N=4, P<0.05) (Figure 5a) and reduced the silent period (by 27–30%, n=4, N=4, P<0.05) during 1–3 mm stretch in units with low sensitivity to 4-AP (not shown). The rate of adaptation of stretch-induced firing measured relative to the peak dynamic response was not significantly changed by 4-AP (n=10, N=10) in either high sensitivity units or low sensitivity units (Figures 4 and 5). When mechanical sensitivity of recorded units was abolished by severing the axon of the mechanically-sensitive endings distal to the recording point, 4-AP (1 mM) evoked (in three out of six units) activation of firing in otherwise silent axons. Overall basal and stretch-evoked intramural tensions were not affected by 4-AP, although at the highest dose (1 mM) asynchronous twitch contractions of oesophageal skeletal muscle fibres were evoked.
Figure 4.

Effect of low dose of 4-aminopyridine (4-AP) on the oesophageal spontaneous and stretch-evoked firing. (a) 4-AP (10 μM) increased both spontaneous and stretch (2 mm)-evoked firing. (b, c) Averaged data of the effect of 4-AP (10 μM) on spontaneous and stretch-evoked firing. Spontaneous firing rate was significantly enhanced in both cases, but stretch-evoked firing was significantly greater for 2 mm stretch but not for 3 mm stretch. Each value in (b) and (c) is the mean from six units in six preparations. *Significant difference from control, P<0.05.
Figure 5.

Concentration-dependent increase of spontaneous firing by 4-AP and effect of high dose of 4-AP on stretch-evoked firing and silent period. (a) Concentration-dependent increase in spontaneous firing of units with high sensitivity to 4-AP (n=4–6, N=6) and small increase in spontaneous firing in units with low sensitivity to 4-AP (n=4, N=4). (b) Averaged data (from six units in six preparations) of the effect of 4-AP (100 μM) on the spontaneous and stretch (3 mm)-evoked firing (b) and silent period (c) in units with high sensitivity to 4-AP. Despite a large increase in spontaneous firing rate, 4-AP (100 μM) did not increase stretch-induced firing evoked by a large amplitude (3 mm) stretch. *Significant difference from control, P<0.05.
It is well established that dendrotoxins act as selective voltage-gated K+ channels blockers. Studies with cloned K+ channels showed that α-dendrotoxin (α-DnTX) blocks voltage-gated Kv1.1, Kv1.2 and Kv1.6 channels (Harvey, 2001) while DnTX K selectively and more potently blocks voltage-gated Kv1.1 channels (Robertson et al., 1996). α-Dendrotoxin (300 nM) significantly increased spontaneous activity by 1.8±0.7 Hz (n=4, N=3, P<0.01). This increase in spontaneous firing evoked by α-DnTX was also significantly different (P<0.01) from the small increase seen in control Krebs re-perfusion experiments. The silent period was decreased significantly by 24–47% (Figure 6). Stretch (1–2 mm)-evoked firing was also significantly increased by α-DnTX. For example during 2 mm stretch, dynamic phase of firing was increased by 1.2±0.3 Hz (n=4, N=3, P<0.05) and adapted phase by 1.2±0.3 Hz (n=4, N=3, P<0.05) while the silent period was decreased by 35±8% (n=4, N=3, P<0.05). At the same time, neither dynamic (>2±2%, n=4, N=3, NS) nor adapted firing (>5±2%, n=4, N=3, NS) evoked by 3 mm stretch was signficantly affected by α-DnTX. There were no significant changes in the rate of adaptation of stretch-induced firing measured relative to the peak dynamic response (Figure 6). α-DntX (300 nM) did not affect intramural tension (n=4, N=3).
Figure 6.

Effect of α-dendrotoxin (α-DnTX, 300 nM) on spontaneous and stretch-evoked firing and silent period. Averaged data of the effect of α-DnTX (300 nM) on spontaneous and stretch (2, 3 mm)-evoked firing (a, b) and silent period (c). Each value in (a), (b) and (c) is the mean from four units in three preparations. *Significant difference from control, P<0.05.
The selective voltage-gated Kv1.1 channels blocker, DnTX K (30 nM) also increased excitability of vagal mechanoreceptors. Application of DnTX K (30 nM) evoked an increase in spontaneous rate by 0.6±0.2 Hz (n=6, N=4) but this did not reach significance compared with re-perfusion of Krebs solution as a control experiment. Stretch-evoked firing was slightly, but significantly, increased (by 8–17%) while the silent period was slightly decreased by 11–25% (n=6, N=4, NS). For example during 2 mm stretch, dynamic phase firing was increased by 0.9±0.3 Hz (n=6, N=4) and adapted firing by 0.6±0.1 Hz (n=6, N=4) (data not shown). DnTX K (30 nM) did not affect the rate of adaptation of stretch-induced firing and basal or stretch-evoked intramural tension (n=6, N=4).
Thus the overall effects of dendrotoxins were similar to those of low doses of 4-aminopyridine and also similar to the effect of ChTX (100 nM). Because ChTX, in addition to blockade of BK and IK channels, is capable of inhibiting voltage-dependent Kv 1.2 and Kv 1.3 channels (Garcia et al., 1995; Grissmer et al., 1994; Hay & Kunze, 1994a), we studied the effect of combined application of ChTX and DnTX K on spontaneous activity. Combined application of ChTX (100 nM) and DnTX K (30 nM) increased spontaneous firing rate by 0.8±0.2 Hz (n=3, N=3). There was no significant difference between the increase in spontaneous firing evoked by ChTX (100 nM) alone or DnTX K (30 nM) alone or during combined application of both peptides (NS, one way Anova, Tukey's multiple comparison test). These data suggest that excitatory effect of ChTX on vagal mechanoreceptors may have been due to its effect on voltage-gated, dendrotoxin sensitive K+ channels rather than on Ca2+-dependent K+ channels.
Effect of blockade of inward rectifier K+ channels on firing rate and silent period
It has been established that Ba2+ at relatively low concentrations (100 μM) selectively blocks inward rectifier K+ channels (Kir) (Doan & Kunze, 1999). Ba2+ (100 μM) did not affect the spontaneous firing rate (before: 4.2±0.6 Hz, after Ba2+: 4.6±0.5 Hz, n=4, N=4, NS) but did significantly increase stretch (1–3 mm)-induced firing by 17–23%. For example, Ba2+ (100 μM) increased dynamic (by 2.9±0.5 Hz, n=4, N=4, P<0.01) and adapted phases (by 1.8±0.6 Hz, n=4, N=4, P<0.01) of firing evoked by 2 mm stretch (Figure 7) while the silent period was significantly increased by Ba2+ by 21±7% (n=4, N=4, P<0.05). However this effect of Ba2+ could be explained by its effect on intramural tension. Ba2+ (100 μM) did not significantly change basal tension (before: 0.37±0.22 mN, after Ba2+: 0.69±0.30 mN, n=4, N=4, NS) but significantly increased intramural tension evoked by 1–3 mm stretch. For example, Ba2+ (100 μM for 20 min) increased dynamic (by 23±6%, n=4, N=4, P<0.01) and adapted tension (by 19±7%, n=4, N=4, P<0.01) evoked by 2 mm stretch (Figure 7). All effects of Ba2+ (100 μM) on firing rate, silent period and intramural tension were washable after half an hour. The selective blocker of ATP-dependent inward rectifier K+ channels (KATP channels), glibenclamide (Allard & Lazdunski, 1993) (10 μM, n=3, N=3), had no effect on either spontaneous or stretch-induced firing in guinea-pig vagal mechanoreceptors (Table 1).
Figure 7.

Effect of Ba2+ on spontaneous and stretch-evoked firing and intramural tension. (a) Typical recording of spontaneous and stretch (2 mm)-evoked firing in control and after 20 min of action of Ba2+ (100 μM). (b) Averaged data of the effect of Ba2+ (100 μM) on firing (b) and intramural tension (c) evoked by 2 mm stretch. Note that the increase in firing evoked by Ba2+ is paralleled by an increase in intramural tension during the stretch. Each value in (b) and (c) is the mean from four units in four preparations. *Significant difference from control, P<0.05.
Discussion
The data provide evidence that of the several types of K+ channels studied, 4-AP- and dendrotoxin-sensitive K+ channels are the main type of potassium channels which influence the excitability of vagal mechanically-sensitive endings of the guinea-pig oesophagus. Because excitability of mechanically-sensitive vagal afferents was increased dramatically by relatively low dose of 4-AP and by α-DnTX and DnTX K, our data strongly suggest that voltage-gated Kv1.1, Kv1.2 or Kv1.6 channels are present on their endings. At least some portion of these channels must be open under the resting conditions (basal tension ∼0.5–1 mN) as inhibition of these channels induced a dramatic increase in spontaneous firing in the majority of preparations. Recently, mRNAs for voltage-gated potassium channel subunits, Kv1.1, Kv1.2 and Kv1.6 were identified by RT–PCR from nodose ganglia and expression of the corresponding proteins was demonstrated in the majority of neurones of the rat nodose ganglia (Glazebrook et al., 2002).
One of the interesting results of this study was that 4-AP did not significantly affect dynamic or adapted firing evoked by the strongest (3 mm) stretch used despite dramatically increasing spontaneous firing and enhancing responses to smaller stretches. This was in contrast with the increase of excitability induced by high K+ Krebs solution, where spontaneous, dynamic and adapted phases of firing evoked by 1–3 mm stretch were all increased evenly. One possible explanation is that 4-AP- and α-DnTX act on at least two different voltage-dependent K+ channels with low and high voltage activation thresholds. In addition, different sensitivity to these blockers may be involved. In dorsal root ganglion neurones two types voltage-dependent K+ channels have been demonstrated: a low voltage, partially inactivating current and a high threshold non-inactivating current. α-DnTX (100 nM) selectively blocked the low voltage activated current but not the high threshold K+ current (Matteson & Blaustein, 1997). Another potential explanation for uneven effect of 4-AP at different stretches is that 4-AP- and dendrotoxin-sensitive K+ channels may be present on both the receptive fields of mechanically sensitive endings (IGLEs) and on their parent axons. In this case, during strongest stretch (3 mm), action potentials generated in the parent axon by 4-AP could collide with action potentials generated in IGLEs, resulting in less potentiation of 3 mm stretch-induced firing. Recently it has been shown that 4-AP (100 μM–1 mM) caused pronounced action potential discharge in parent axons of vagal afferents to the trachea after cutting off the mechanically sensitive endings in the guinea-pig trachea and bronchus (McAlexander & Undem, 2000). In our experiments, after cutting off mechanically-sensitive endings, 4-AP (1 mM) evoked firing in three out of six axons demonstrating the presence of 4-AP-sensitive K+ channels on axons.
4-AP (1 mM) evoked a depolarization of approximately 10 mV when applied to retrogradely labelled guinea-pig airway nodose ganglion neurones (McAlexander & Undem, 2000). Conversely, α-DnTX (50 nM) increased excitability without consistent changes in resting membrane potential of rat nodose ganglion neurones (Glazebrook et al., 2002). In that study, the α-DnTX-sensitive current was only a small component of the 4-AP (5 mM) sensitive K+ current and at least two or more 4-AP-sensitive K+ channels were proposed (Glazebrook et al., 2002). Similarly, studies with native K+ channels showed that α-DnTX even at the highest dose (1 μM) blocks only three out of five voltage-gated K+ channels in human peripheral myelinated axons (Reid et al., 1999). These data readily explain our finding that 4-AP was more efficacious than dendrotoxins in increasing spontaneous firing. This suggests that 4-AP-sensitive voltage-gated K+ channels other than Kv1.1, Kv1.2 and Kv1.6 are also present on these nerve terminals.
Nodose ganglion neurones are a heterogeneous population, as they innervate many visceral organs and tissues in the upper part of the body. Studies on nodose ganglion cell bodies revealed considerable heterogeneity in distribution of K+ channels. BK and rapidly inactivating (transient) IA current were found only in restricted subpopulations of C-type nodose neurones, while the voltage-gated slowly inactivating K+ current (‘delayed rectifier') was ubiquitous (Christian et al., 1994). The IA current was present only in 40–60% of guinea-pig and rabbit nodose ganglion neurones (Christian et al., 1994; Ducreux & Puizillout, 1995). This heterogeneity has been hypothesized to reflect differing functional roles and targets of subpopulations of nodose neurones (Christian et al., 1994). However our data show that the same functional classes of neurones (mechanically-sensitive neurones that project to the oesophagus) may have remarkable variation in the types and/or density of voltage-gated K+ channels: there were two distinct groups of mechanically-sensitive afferents – with high and low sensitivity to 4-AP. In some of the A cells of the rat nodose ganglion a D-current (voltage-gated, slowly inactivating K+ current) was inhibited by low doses of 4-AP (1–30 μM) and α-DnTX (10 nM). In other neurones, the transient IA current was insensitive to 4-AP below 100 μM (Stansfeld et al., 1986). However, recently, in rat nodose neurones, α-DnTX (10 nM)-sensitive fast-activating current with slow inactivation (similar to the D-current) was found in the majority of A and C type rat nodose neurones (Glazebrook et al., 2002).
We have found recently that excitability of vagal mechanically-sensitive endings in the guinea-pig oesophagus was increased in Ca2+-free Krebs solution (spontaneous and stretch-evoked firing was increased while silent period was decreased) suggesting an involvement of Ca2+ dependent K+ channels (Zagorodnyuk et al., 2002). However, ChTX, which is widely used as a blocker of BK and IK channels, had only minor excitatory effects on the excitability of these endings. In addition, its action may be partly due to its inhibition of Kv channels since combined application of ChTX and DnTX K did not result in additional effects. In dorsal root ganglion neurones, low voltage activated K+ channels were selectively blocked by either α-DnTX (100 nM) or ChTX (200 nM) (Matteson & Blaustein, 1997). The present data revealed that the duration of the silent period showed a complex relationship with the duration of the stretch. However, simple modelling, which takes into account the duration of the stretch and thus the time interval between action potentials and the beginning of the silent period, suggested that the numbers of action potentials, exponentially weighted from the time of the removal of stretch, were a good predictor of the duration of the silent period. We suggest that this relation is due to the dynamic of Ca2+ entering nerve terminals during action potentials. Increase in intracellular Ca2+ will enhance the open probability of Ca2+ dependent K+ channels resulting in a substantial silent period. The pronounced increase in intracellular Ca2+ after a short burst of action potentials (short stretch duration) is expected to be less efficiently buffered, leading to a relatively long silent period. However, the accumulation of intracellular Ca2+ during long stretch duration would improve the efficiency of Ca2+ buffering mechanisms, leading to a decrease in [Ca2+]i, and consequent shortening of the duration of the silent period. Potassium channels blockers, iberiotoxin and apamin, did not have effects on either firing rate or silent period, and present data suggest that neither BK nor apamin-sensitive SK channels are present in mechanically-sensitive vagal endings. It is unlikely that the lack of the effect of iberiotoxin and apamin is due to their inability to reach the nerve terminals during the 30 min application time since their molecular weight is smaller than that of ChTX and dendrotoxins. In addition, in our preliminary experiments, the BK potassium channel opener NS 1619 (50 μM) did not affect spontaneous or stretch-induced firing of vagal tension receptors in the guinea-pig oesophagus (n=2, N=2). Previously it was found that BK channels were present only in 16 out of 36 of the guinea-pig nodose neurones (Christian et al., 1994). In the rat nodose ganglion neurones, pharmacologically distinct IK channels have been identified which were insensitive to tetraethylammonium, apamin and ChTX (Hay & Kunze, 1994b). It is possible that IK type Ca2+ dependent K+ channels may contribute to the silent period and increased excitability seen in Ca2+-free Krebs solution; a selective blocker is currently unavailable to test this hypothesis.
Most mechanically-sensitive afferents of the guinea-pig oesophagus and stomach have multiple receptive fields (IGLEs). It seems likely that action potential generated in one receptive field (IGLE) invades other IGLEs and resets their spike generating mechanism, leading to coordinated adapted firing during maintained stretch where one spike-generating site entrains the others (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al., 2001). In addition, the temporary depression of spontaneous firing following stretch (i.e. silent period) suggests a temporary reduction in excitability in all transduction sites following bursts of spikes. Alternatively, the silent period could be partly due to recovery from inactivation of stretch-activated channels after being suddenly unloaded by rapid removal of stretch. Previously it was shown that in 20% of C-type neurones in the guinea-pig nodose ganglion, action potentials were followed by a slow AHP that is due to activation of Ca2+-dependent K+ channels and which is blocked completely by the N-type Ca2+ channels blocker, ω-conotoxin GVIA (Cordoba-Rodriguez et al., 1999; Undem & Weinreich, 1993). In our experiments ω-conotoxin GVIA (0.5 μM) did not affect excitability of vagal mechanically-sensitive afferents. These different results emphasize that extrapolation from nodose ganglion cell bodies to their peripheral endings may not always be appropriate. As for the other toxins discussed above, the lack of the effect of ω-conotoxin GVIA is unlikely to be due to its inability to reach the nerve terminals during the 30 min application.
It is well established that Ba2+ at relatively low concentration (100 μM) is a selective blocker of Kir channels while glibenclamide is a selective blocker of KATP channels (Allard & Lazdunski, 1993; Doan & Kunze, 1999). We did not find any effect of inward rectifier K+ channels blockers (Ba+ and glibenclamide) on the spontaneous activity of vagal mechanoreceptors in guinea-pig oesophagus. The small increase in stretch-evoked firing by Ba2+ could be readily explained by a proportional increase in stretch-evoked intramural tension. These data agree with recent data showing no evidence for presence of Kir channels in rat cell bodies of C-type nodose ganglion neurones (Doan & Kunze, 1999).
It is worth mentioning that the duration of the silent period was a good indicator of the level of excitability in the vagal oesophageal afferents. Spontaneous activity (that reflects excitability of nerve endings) was in inverse linear relationship with the duration of the silent period in control and during application of different drugs at the same amplitude of stretch. The duration of silent period was linearly related to the stretch-induced firing. Thus, changes in spontaneous or stretch-induced firing had an opposite effect on the duration of the silent period. High K+ Krebs solution, ChTX and dendrotoxins increased the spontaneous firing rate and simultaneously decreased the duration of the silent period despite an increase in stretch-evoked firing for the same stretch amplitude. 4-AP in high doses induced large increase in spontaneous firing, with simultaneous dramatic decrease in the duration of the silent period, while having no effect on stretch (3 mm)-induced firing. The dramatic reduction in the duration of the silent period with an increase in spontaneous firing is probably due to faster recovery from the reduced excitability of the mechanotransduction sites. Conversely, Ba2+ increased only stretch-evoked firing (having no effect on spontaneous firing), with consequent increase in the duration of the silent period.
None of the K+ channels studies, including 4-AP- and dendrotoxin-sensitive K+ channels, influenced the adaptation of vagal mechanoreceptors to maintained stretch since none of the K+ channels blockers studied were able to significantly change the rate of adaptation of stretch-induced firing measured relative to the peak dynamic response during 10 s stretches. It seems likely that adaptation in firing may be due to either intrinsic properties of stretch-activated channels themselves or to mechanical changes in the coupling of IGLEs to the surrounding connective tissue sheath (Zagorodnyuk et al., 2001). In this regard, the mechanism of adaptation of oesophageal vagal mechanoreceptors is probably similar to that of tracheal and bronchial vagal stretch receptors where visco-elastic elements within the airway tissue play a crucial role in their adaptation to mechanical stimulation (Davenport et al., 1981; McAlexander et al., 1999).
In conclusion, the data provide evidence that of the several types of K+ channels studied the voltage-gated 4-AP- and dendrotoxin-sensitive K+ channels are the main type of K+ channels that influence excitability of vagal mechanically-sensitive endings of the guinea-pig oesophagus. The results indicate that voltage-gated 4-AP- and dendrotoxin-sensitive K+ channels are involved in control of spontaneous firing and in stretch-induced firing evoked by moderate stretch.
Acknowledgments
This study was funded by DK58986 from the National Institute of Health (U.S.A.). S.J.H. Brookes was supported by a senior research fellowship of the NH&MRC of Australia.
Abbreviations
- AHP
after hyperpolarization
- 4-AP
4-aminopyridine
- BK
large conductance K+ channels
- ChTX
charybdotoxin
- DnTX
dendrotoxin
- IGLEs
intraganglionic laminar endings
- IK
intermediate conductance K+ channels
- Kir
inward rectifier K+ channels
- Kv
voltage-gated K+ channels
- SK
small conductance K+ channels
References
- ALLARD B., LAZDUNSKI M. Pharmacological properties of ATP-sensitive K+ channels in mammalian skeletal muscle cells. Eur. J. Pharmacol. 1993;236:419–426. doi: 10.1016/0014-2999(93)90480-6. [DOI] [PubMed] [Google Scholar]
- BARGMANN C.I. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- BROOKES S.J.H., CHEN B.N., COSTA M., HUMPHREYS C.M.S. Initiation of peristalsis by circumferential stretch in flat sheets of guinea-pig ileum. J. Physiol. 1999;516.2:528–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIAN E.P., TOGO J., NAPER K.E. Guinea pig visceral C-fiber neurons are diverse with respect to the K+ currents involved in action-potential repolarization. J. Neurophysiol. 1994;71:561–574. doi: 10.1152/jn.1994.71.2.561. [DOI] [PubMed] [Google Scholar]
- CORDOBA-RODRIGUEZ R., MOORE K.A., KAO J.P., WEINREICH D. Calcium regulation of a slow post-spike hyperpolarization in vagal afferent neurons. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7650–7657. doi: 10.1073/pnas.96.14.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT P.W., SANT'AMBROGIO F.B., SANT'AMBROGIO G. Adaptation of tracheal stretch receptors. Respir. Physiol. 1981;44:339–349. doi: 10.1016/0034-5687(81)90028-1. [DOI] [PubMed] [Google Scholar]
- DOAN T.N., KUNZE D.L. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J. Physiol. 1999;514:125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCREUX C., PUIZILLOUT J.J. A-current modifies the spike of C-type neurones in the rabbit nodose ganglion. J. Physiol. 1995;486:439–451. doi: 10.1113/jphysiol.1995.sp020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA M.L., GALVEZ A., GARCIA-CALVO, KING V.G., VAZQUEZ J., KACZOROWSKI G.J. Use of toxins to study potassium channels. J. Bioenerg. Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., KNAUS H.G., MUNUJOS P., SLAUGHTER R.S., KACZOROWSKI G.J. Charybdotoxin and its effects on potassium channels. Am. J. Physiol. 1995;269:C1–C10. doi: 10.1152/ajpcell.1995.269.1.C1. [DOI] [PubMed] [Google Scholar]
- GLAZEBROOK P.A., RAMIREZ A.N., SCHILD J.H., SHIEH C.C., DOAN T., WIBLE B.A., KUNZE D.L. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J. Physiol. 2002;541:467–482. doi: 10.1113/jphysiol.2001.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISSMER S., NGUYEN A.N., AIYAR J., HANSON D.C., MATHER R.J., GUTMAN G.A., KARMILOWICZ M.J., AUPERIN D.D., CHANDY K.G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- HARPER A.A. Similarities between some properties of the soma and sensory receptors of primary afferent neurones. Exp. Physiol. 1991;76:369–377. doi: 10.1113/expphysiol.1991.sp003504. [DOI] [PubMed] [Google Scholar]
- HARVEY A.L. Twenty years of dendrotoxins. Toxicon. 2001;39:15–26. doi: 10.1016/s0041-0101(00)00162-8. [DOI] [PubMed] [Google Scholar]
- HAY M., KUNZE D.L. Calcium-activated potassium channels in rat visceral sensory afferents. Brain Res. 1994a;639:333–336. doi: 10.1016/0006-8993(94)91749-3. [DOI] [PubMed] [Google Scholar]
- HAY M., KUNZE D.L. An intermediate conductance calcium-activated potassium channel in rat visceral sensory afferent neurons. Neurosci. Lett. 1994b;167:179–182. doi: 10.1016/0304-3940(94)91056-1. [DOI] [PubMed] [Google Scholar]
- HIGASHI H., UEDA N., NISHI S., GALLAGHER J.P., SHINNICK-GALLAGHER P. Chemoreceptors for serotonin (5-HT), acetylcholine (ACh), bradykinin (BK), histamine (H) and gamma-aminobutyric acid (GABA) on rabbit visceral afferent neurons. Brain Res. Bull. 1982;8:23–32. doi: 10.1016/0361-9230(82)90023-5. [DOI] [PubMed] [Google Scholar]
- JAN L.Y., JAN Y.N. Voltage-gated and inwardly rectifying potassium channels. J. Physiol. 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESAGE F., LAZDUNSKI M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- MATTESON D.R., BLAUSTEIN M.P. Scorpion toxin block of the early K+ current (IKf) in rat dorsal root ganglion neurones. J. Physiol. 1997;503:285–301. doi: 10.1111/j.1469-7793.1997.285bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCALEXANDER M.A., MYERS A.C., UNDEM B.J. Adaptation of guinea-pig vagal airway afferent neurones to mechanical stimulation. J. Physiol. 1999;1:239–247. doi: 10.1111/j.1469-7793.1999.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCALEXANDER M.A., UNDEM B.J. Potassium channel blockade induces action potential generation in guinea-pig airway vagal afferent neurones. J. Auton. Nerv. Syst. 2000;78:158–164. doi: 10.1016/s0165-1838(99)00075-2. [DOI] [PubMed] [Google Scholar]
- MCMANUS O.B. Calcium-activated potassium channels: regulation by calcium. J. Bioenerg. Biomembr. 1991;23:537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- REID G., SCHOLZ A., BOSTOCK H., VOGEL W. Human axons contain at least five types of voltage-dependent potassium channel. J. Physiol. 1999;518:681–696. doi: 10.1111/j.1469-7793.1999.0681p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON B., OWEN D., STOW J., BUTLER C., NEWLAND C. Novel effects of dendrotoxin homologues on subtypes of mammalian Kv1 potassium channels expressed in Xenopus oocytes. FEBS Lett. 1996;383:26–30. doi: 10.1016/0014-5793(96)00211-6. [DOI] [PubMed] [Google Scholar]
- STANSFELD C.E., MARSH S.J., HALLIWELL J.V., BROWN D.A. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurones by blocking a slowly inactivating outward current. Neurosci. Lett. 1986;64:299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- UNDEM B.J., WEINREICH D. Electrophysiologial properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. J. Auton. Nerv. Syst. 1993;44:17–33. doi: 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- ZAGORODNYUK V.P., BROOKES S.J.H. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAGORODNYUK V.P., CHEN B.N., BROOKES S.J.H. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAGORODNYUK V.P., CHEN B.M., COSTA M., BROOKES S.J.H. Calcium- and voltage-dependent potassium channels control excitability of vagal mechanoreceptors in guinea-pig oesophagus. Proc. Au. Neurosci. Soc. 2002;13:52P. [Google Scholar]


