Abstract
We used spinal microdialysis in awake rats to investigate whether the repeated withdrawal with naloxone during continuous spinal infusion of morphine would lead to a progressively greater spinal glutamate release and a more pronounced intrathecal tolerance.
Rats received lumbar intrathecal (IT) infusion of morphine (IT-M: 20 nmol μl−1 h−1) or saline (IT-S: 1 μl h−1) continuously for 3 days. Both groups were further subdivided to receive intraperitoneal (i.p.) injection of naloxone (IP-N: 0.6 mg kg−1) or saline (IP-S: 3 ml kg−1) every 24 h after the beginning of IT infusion. Daily thermal escape latencies, withdrawal signs, the resting basal release of spinal amino acids before IP injection and the release immediately after the injection (evoked) were measured.
Rats receiving IT morphine showed a maximum increase in thermal escape latency on day 1, after which this value declined, with the fastest decline observed in IT morphine+IP naloxone group. On day 1, no significant difference was observed among groups in the resting basal release of amino acids. Rats in IT morphine+i.p. naloxone group displayed a progressive increase in this value. The release was not significantly altered in other groups.
For the IT-M+IP-N group, basal resting dialysate concentrations of Glu, Asp and Tau rose steadily over the 3-day infusion interval. No change in basal resting release was noted for any other treatment.
Evoked release (after i.p. naloxone) in IT-M animals displayed a progressive increase over the three repeated exposures. Evoked release did not change significantly in other treatment groups.
The degree of precipitated withdrawal significantly correlated with the increase in glutamate acutely evoked by i.p. injection.
The present results show that periodic transient withdrawal of spinal opiate agonist activity leads to a progressive increase in glutamate outflow and withdrawal signs, in a manner consistent with an enhanced development of spinal tolerance.
Keywords: Morphine, tolerance, amino acid, withdrawal, spinal, antagonism
Introduction
Intrathecal co-administration of an NMDA antagonist with morphine prevents the reduction of analgesic activity otherwise observed with repeated opiate exposure alone (Gutstein & Trujillo, 1993; Mao et al., 1994; Dunbar & Yaksh, 1996), while intrathecal NMDA antagonism will attenuate precipitated withdrawal behaviours evoked by naloxone injections in morphine-tolerant animals (Gutstein & Trujillo, 1993; Mao et al., 1994; Dunbar & Yaksh, 1996). Importantly, acute delivery of the NMDA antagonist after the onset of spinal tolerance will reduce the magnitude of withdrawal signs but will not reverse the opiate tolerance (Dunbar & Yaksh, 1996; Jhamandas et al., 1996; Dunbar & Pulai, 1998), suggesting the importance of an ongoing glutamate release during opiate exposure and during withdrawal.
As glutamate receptor activation must occur during tolerance development and in withdrawal (as evidenced by the actions of NMDA antagonists), we hypothesized that there is a corresponding increase in glutamate release during tolerance development and during opiate withdrawal. In rats rendered tolerant to systemic or to spinally delivered morphine, precipitated withdrawal will increase significant glutamate release in the locus coeruleus (Aghajanian et al., 1994) and from the spinal cord (Jhamandas et al., 1996), respectively. However, spinal glutamate release during the development phase of tolerance displayed little change in resting levels over the interval of morphine infusion (Jhamandas et al., 1996).
Given the role of glutamate receptor activation in tolerance development, the above work led us to hypothesize that periodic withdrawal of opiates would yield corresponding intervals of increased glutamate release, and the increased release would lead to augmented tolerance development. This hypothesis is counterintuitive, as tolerance and dependence are considered to be related to the duration and magnitude of opiate exposure with systemic (Cox et al., 1975; Yano & Takemori, 1977; Bhargava, 1978) and intrathecal opiates (Stevens & Yaksh. 1989a, b, c). Reducing occupancy would therefore be expected to diminish tolerance. We demonstrated that periodic blockade of opiate receptor occupancy by daily naloxone injections in rats chronically infused with morphine resulted in an exaggerated rate of tolerance development (Ibuki et al., 1997). This suggested that periodic cycling of opiate receptor activation does indeed lead to an enhanced development of tolerance.
From these results, we now hypothesize that corresponding to the augmented tolerance associated with daily transient receptor antagonism with naloxone under continuous morphine infusion, there will be a progressive increase in the spinal release of excitatory amino acids. Accordingly, the specific aims of this study were to confirm the previous finding that repeated transient naloxone antagonism exaggerates tolerance development and to extend these observations according to the several hypotheses outlined above by (1) observing the effect of continuous intrathecal (IT) morphine infusion or continuous morphine infusion with daily naloxone antagonism on daily resting spinal amino acid release; (2) measuring the acute changes in spinal amino acid release from the daily resting release evoked by daily naloxone treatment; and (3) assessing the correlation between acute changes in spinal amino acid release and the magnitude of withdrawal behaviour.
Methods
All experiments were performed according to protocols reviewed and approved by the Institutional Animal Care Committee of the University of California at San Diego.
Animals
Male Sprague Dawley rats weighing 300–350 g were housed on a 12-h light/dark cycle and given free access to food and water before and following the surgery and daily treatments. On the last day of the study, rats were sacrificed by pentobarbital injection.
Experimental design
Animals were randomly assigned to one of four groups at the time of surgery to receive the following treatment:
IT-M+IP-N: intrathecal (IT) morphine (20 nmol h−1) with daily intraperitoneal (i.p.) injection of naloxone (0.6 mg kg−1) (n=8);
IT-M+IP-S: IT morphine (20 nmol h−1) with daily i.p. injection of saline (3 ml kg−1) (n=6);
IT-S+IP-N: IT saline (1 μl h−1) with daily i.p. injection of naloxone (0.6 mg kg−1) (n=8); or,
IT-S+IP-S: IT saline (1 μl h−1) with daily i.p. injection of saline (3 ml kg−1) (n=6).
On each day of infusion (1 to 3), the thermal escape latency was assessed. The dialysis catheter was then connected. After an initial 30 min washout, samples for resting basal amino acid release were taken. Accordingly, testing of thermal escape latency preceded resting basal sample collection by approximately 30–40 min. The rat was then given the i.p. treatment (naloxone or saline), and additional samples were collected following a 7-min stabilization period to measure the spinal amino acid release evoked by the i.p. injection. Withdrawal signs were observed and quantified during the microdialysis period. In our previous study, naloxone 0.6 mg kg−1 i.p. was shown to produce approximately 2 h of reversal (Ibuki et al., 1997).
Animal preparation
Rats were prepared with a chronically implanted spinal loop dialysis catheter to measure release from the intrathecal space and a catheter connected to an osmotic minipump to deliver morphine.
Catheter preparation
For intrathecal infusion, stretched PE10 catheters were prepared as previously described and connected to an osmotic minipump (Model 2002: 1 μl h−1; Alza, Palo Alto, CA, U.S.A.) filled with morphine sulphate solution or saline (0.9%) immediately prior to implantation, (Dunbar & Yaksh, 1996; Jhamandas et al., 1996; Ibuki et al., 1997). This pump is designed to deliver the drug at a constant rate of 1 μl h−1 for 7 days, after an initial activation period in the animal of approximately 4 h.
For intrathecal dialysis, the intrathecal loop microdialysis catheter was constructed of hollow cuprophan fibers (Filtral, AH 69-HF), as previously described (Marsala et al., 1995). Briefly, an 18-cm fibre was covered with epoxy to block permeability except for a 4-cm portion at the central part of the fibre (active dialysis site). After the epoxy had dried, a thin copper wire was inserted through the catheter to stiffen it for implantation, and the catheter was then folded so that the active site formed a loop. The two ends of the microdialysis loop catheter were glued to a 4-cm long PE 10 tube and fused to one another.
Implantation surgery
As previously described (Jhamandas et al., 1996), rats were anaesthetized with halothane (1–2% in room air) and placed in a stereotaxic head holder. A midline incision was made and the atlanto-occipital membrane exposed. The microdialysis catheter was inserted through a small slit in the dura and arachnoid membrane. The catheter was then passed intrathecally to a depth of 8.5 cm, to the level of the lumbar intrathecal space. This was followed by insertion of the intrathecal catheter for drug delivery. The two free ends of the microdialysis catheter (PE 10 tube) were externalized percutaneously on the top of the head and plugged with stainless steel pins. The intrathecal catheter was also externalized through the skin at the top of the skull. The minipump was then attached to the catheter and implanted subcutaneously in a pocket on the upper neck and the wound sutured. After recovery from anaesthesia, the rat was returned to its cage. Animals showing signs of a neurological disorder were euthanized with an overdose of barbiturate.
Spinal amino acid release
Microdialysis procedures
On days 1, 2 and 3 following implantation surgery, spinal microdialysis was performed to assess amino acid release immediately before (resting basal release) and after (evoked release) an i.p. injection of naloxone or saline in animals receiving continuous IT infusion of morphine or saline. First, the two ends of the microdialysis catheter were connected to PE 50 tubing to create inflow and outflow connections. The rat was placed in a clear Plexiglas chamber and dialysis was initiated. Artificial cerebrospinal fluid (ACSF) consisting of (mM) Na+ 151.1, K+ 2.6, Mg2+ 0.9, Ca2+ 1.3, Cl− 122.7, HCO3− 21, HPO4 2.5, and dextrose 3.5, was used as the perfusion solution and infused at a rate of 10 μl min−1. ACSF was bubbled with 95% O2/5% CO2 and the final pH was adjusted to 7.2 prior to use. After connection, a 30-min washout period was completed and two baseline samples were collected (10-min collection time for each sample). Then, each animal received an i.p. injection of either naloxone or saline, and a 7-min stabilization period was followed by two sample collections (10-min collection time for each sample). Samples were collected on ice and frozen at −20°C until analysis by high performance liquid chromatography (HPLC). Following the final sample collection, the inflow and outflow tubes were disconnected and the rat was returned to its cage. On day 3, the animals were sacrificed with an overdose of barbiturate following the final sample collection.
Amino acid analysis
Dialysis samples were analysed for different amino acids by phenyl- isothiocynate derivatization procedure (Jhamandas et al., 1996) using a Waters HPLC equipped with a reverse phase C-18 column (3.9×300 mm, 4 mm particle) and u.v. detector (Waters). Methionine sulphone was added to each sample as an internal standard. External standards (40, 400 and 4000 pmol of authentic amino acids) were run at the beginning and end of each sample group. Peak heights were normalized to the methionine sulphone peak and then quantified based on a linear relationship between peak height and the amounts of corresponding standards. Sensitivity was 5–10 pmol per injection.
Behavioral testing
Antinociceptive testing
The assessment of nociceptive thresholds was performed using a 52.5°C hot plate. Hot-plate latency was measured as the time interval between the placement of the feet on the surface of the copper plate and a response of one of the following: licking of either hind paw, jumping off the hot plate, or extreme agitation or vocalization. A cut-off time in the absence of a response was set at 60 s.
Opioid withdrawal testing
Following the i.p. injection of saline or naloxone, during the microdialysis period, rats were assessed for withdrawal signs: allodynia (light touch, air puff), spontaneous vocalization, abnormal body posture, ejaculation, urination, diarrhoea, exophthalmus, piloerection, head shakes, hind limb extension, chewing and licking response, and tremor. The withdrawal signs were quantified using a rating scale of 1 to 3 (mild, moderate and severe) on a standardized score sheet (Milne et al., 1985; Cridland et al., 1991; Jhamandas et al., 1996). Each withdrawal sign was scored separately and the total score for a rat was then calculated by adding the score of each component.
Data expression and statistics
Based on the proposed hypotheses, spinal amino acid release was analysed in two ways. First, baseline spinal release for each animal prior to the i.p. injection of saline or naloxone was expressed as a per cent of the baseline release of that animal observed on day 1. Secondly, the release during each period after i.p. naloxone or saline was expressed as a percentage of pre injection baseline for that day. Statistical analysis of antinociceptive testing was done with a one-way ANOVA across groups, as appropriate. For comparisons of single groups over time (e.g. days 1, 2 and 3), a one-way repeated measures ANOVA was accomplished. If the one-way ANOVA was significant, a Dunnett's t-test was carried out for post hoc analysis. For presentation purposes, group data are presented in a ranked order with adjacent pairs being shown as statistically significant (P<0.05) with ‘<' or not different with ‘='. Examination of the covariance between behaviour and the release of various amino acids was accomplished using linear regression analysis, plotting the behavioural score during withdrawal versus the release for each individual animal and the regression calculated using STATVIEW. A P-value of <0.05 was considered significant.
Results
Development of opioid antinociceptive tolerance
Continuous IT saline infusion had no effect on hot plate response latency over the 3-day interval, (P>0.05) (Figure 1). Intraperitoneal injection of naloxone in the saline-infused rats did not alter the ongoing baseline hot plate response latency over the time course of 3 days as compared to i.p. injection of saline (Figure 1, P>0.05).
Figure 1.
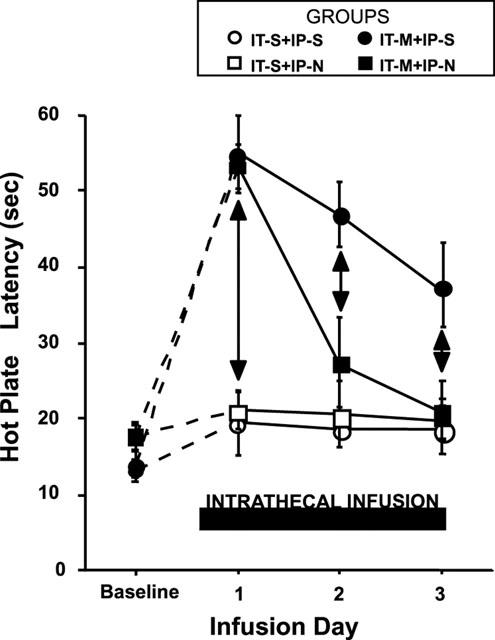
Daily changes in hot plate response latency in rats treated with continuous intrathecal (IT) infusion of morphine (M) (20 nmol h−1) or saline (S) and daily intraperitoneal (i.p.) injections of naloxone (N) (0.6 mg kg−1) or saline (S). (IT-M+IP-N, rats with continuous IT infusion of morphine for 3 days and daily i.p. injection of naloxone; IT-M+IP-S, rats with continuous IT infusion of morphine for 3 days and daily i.p. injection of saline; IT-S+IP-N, rats with continuous IT infusion of saline and daily i.p. injection of naloxone; IT-S+IP-S, rats with continuous IT infusion of saline and daily i.p. injection of saline). Each test was performed prior to the daily injection of naloxone or saline. Each line represents the mean±s.e.mean of six rats. Both IT-M+IP-N and IT-M+IP-S, the withdrawal latency on day 1 was significantly increased from the baseline and then declined. The development of tolerance was more enhanced in IT-M+IP-N compared with IT-M+IP-S. One-way repeated measures ANOVA (P<0.05). Vertical arrows indicate differences of P<0.05.
In contrast to the intrathecal infusion of saline, rats receiving continuous IT morphine infusion showed a significant elevation of their hot plate response latency on day 1 to an almost maximum latency (Figure 1). Thus, the rank order of thermal escape latency measured 24 h after the initiation of spinal infusion (day 1) and prior to the injection of i.p. naloxone or saline was: IT-M+IP-N=IT-M+IP-S >IT-S+IP-N=IT-S+IP-S=pre-infusion baseline (one-way ANOVA; P<0.05).
In all morphine-infused animals, a daily progressive reduction was observed in antinociceptive activity over the ensuing days (Figure 1). The magnitude of the decline for the morphine infused rats differed for rats treated daily with i.p. naloxone (group IT-M+IP-N) or saline (IT-M+IP-S). Thus, on days 2 and 3 of infusion, the ordering of the thermal escape latency measured 24 h after the preceding i.p. injection was: day 2: IT-M+IP-S>IT-M+IP-N=IT-S+IT-N=IT-S+IP-S=pre-infusion baseline (one-way ANOVA; P<0.05) and, day 3: IT-M+IP-S>IT-M+IT-N=IT-S+IP-N=IT-S+IP-S=pre-infusion baseline, respectively (one-way ANOVA; P<0.05). Thus, periodic transient naloxone antagonism enhanced the loss of antinociceptive activity of continuously infused morphine, as measured daily 24 h after the preceding i.p. injection of naloxone.
Spinal amino acid release
As shown in Table 1, no significant difference was observed in the resting basal release of glutamate (Glu), aspartate (Asp), taurine (Tau), serine (Ser), glycine (Gly) or glutamine (Gln) among all groups of rats on day 1 prior to the i.p. delivery of naloxone or saline. Citrulline levels were typically at or below detectable limits and the analysis of these data were not pursued.
Table 1.
Resting amino acid release on day 1*
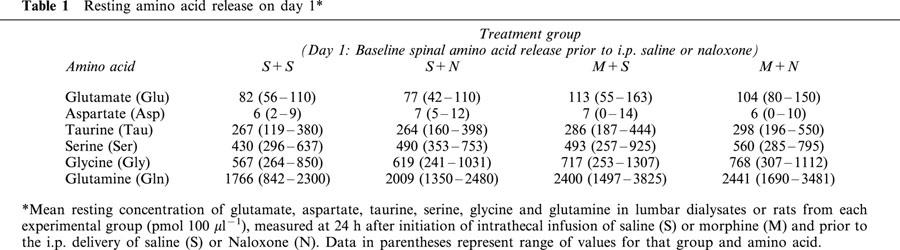
Resting basal spinal amino acid release
For the IT-M+IP-S, IT-S+IP-N and IT-S+IP-S groups, the baseline release (prior to i.p. saline or naloxone) of Glu, Asp and Tau did not change significantly over the 3 infusion days (one-way ANOVA, repeated measures, P>0.05). In contrast, in the IT-M+IP-N group, baseline concentrations of Glu, Asp and Tau rose steadily over the 3-day infusion interval, and this increase was significant on day 3 (one-way ANOVA, repeated measures, P<0.05; Dunnett's t-test, P<0.05 as compared to day 1). Figure 2 shows the daily spinal Glu, Asp, Tau and Ser release expressed as a percentage increase from day 1 resting levels. The dialysate concentrations of other amino acids, including Ser, Gly and Gln, were not altered.
Figure 2.
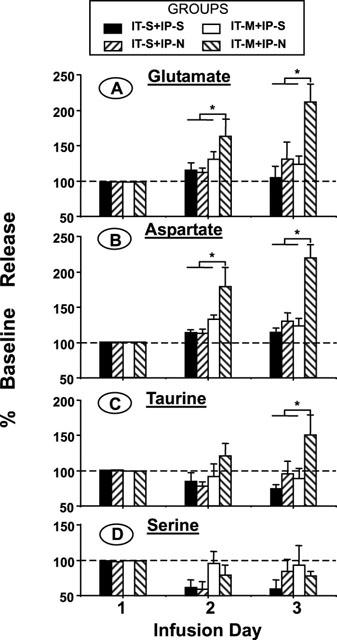
Daily release of spinal amino acids expressed as a percentage of day 1 resting basal levels (prior to i.p. naloxone or saline) in four groups of rats (as described in Figure 1). Samples were collected by microdialysis prior to the daily intraperitoneal injection of saline or naloxone. Daily basal spinal glutamate release increased modestly in rats with continuous morphine infusion and daily saline injections. The release was significantly elevated in rats treated with morphine infusion and daily naloxone injections on days 2 and 3. The baseline spinal release of taurine in IT-M+IP-N group was also increased significantly on day 3. Each line represents the mean±s.e.mean of six rats. *P<0.05 versus release in other groups on that day, one way ANOVA, Dunnett's t-test post hoc comparison).
Evoked spinal amino acid release after IP saline or Naloxone injection
In rats continuously infused with IT morphine, a progressive daily increase in the percentage increase in the dialysate concentrations of Glu, Asp and Tau was observed after i.p. naloxone (IT-M+IP-N group: One-way ANOVA, repeated measures, P<0.01; Dunnett's t-test, P<0.05 as compared to day 1). Figure 3 shows the post i.p. injection release expressed as a percentage increase from the respective daily baseline release following i.p. saline or naloxone on day 1, 2, 3 for Glu, Asp and Tau. As indicated, after naloxone infusion, a 2.5 fold or greater increase was observed on day 3. There were no changes in Ser dialysate concentrations in this group.
Figure 3.
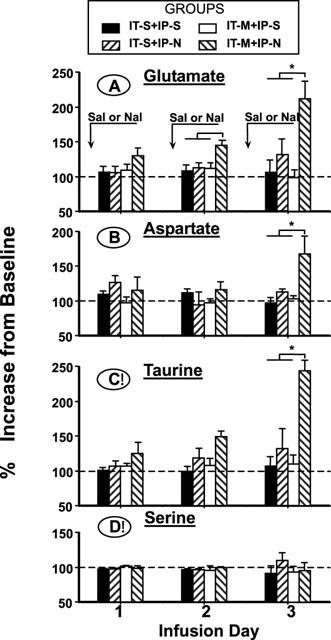
Daily spinal glutamate, aspartate, taurine and serine release in the four groups measured concurrently after the injection of intraperitoneal (i.p.) naloxone (0.6 mg kg−1) or saline (S). Release is expressed as the percentage of the respective daily resting, prior to i.p. naloxone or saline in rats undergoing continuous intrathecal (IT) infusion of morphine (M) (20 nmol h−1) or saline (S). The increases in glutamate, aspartate and taurine from the resting release were significant on day 3 in rats with IT morphine infusion and daily i.p. naloxone injections. There was no significant change in the release of spinal serine in any group. Each line represents the mean±s.e.mean of six rats. *P<0.01 versus release in other groups on that day, one way ANOVA Dunnett's t-test comparison.
In rats receiving continuous IT saline infusion and daily naloxone or saline injections or IT morphine infusion and daily i.p. saline (IT-S+IP-N and IT-S+IP-S and IT-M+IP-S) no statistically significant changes were observed in the release of any spinal amino acid (one-way ANOVA, P>0.10).
Behaviour
The withdrawal scores reported in this study, shown in Figure 4 represent naloxone-induced behaviour (allodynia to light touch or air puff, abnormal posture and spontaneous vocalization) and autonomic hyperactivity (urination, diarrhoea, ejaculation, piloerection, and exopthalmus). Rats receiving continuous IT saline infusion and daily i.p. naloxone (IT-S+IP-N) or i.p. saline (IT-S+IP-S) injection showed no sign of withdrawal over a period of 3 days. The group mean±s.e.mean withdrawal index score on day 3 was IT-S+IP-N=0.6±0.1; IT-S+IP-S=0.5±0.1, out of a possible maximum score of 30 which was not different from day 1 (two one-way ANOVA repeated measures, P>0.05).
Figure 4.
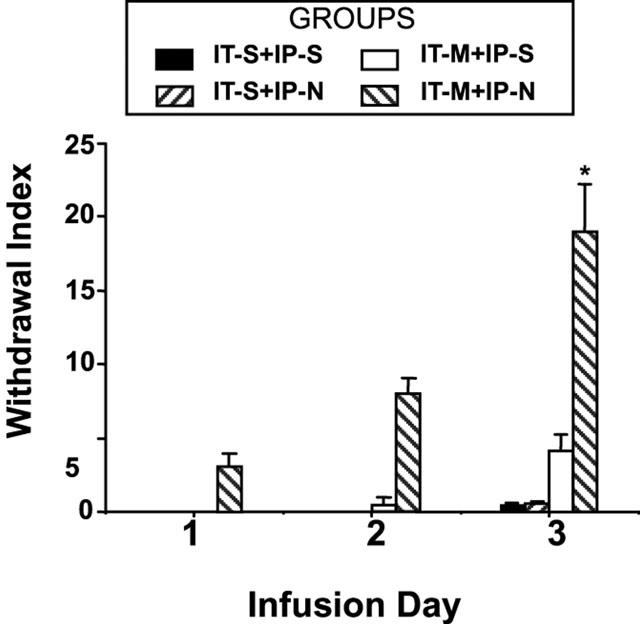
Figure shows the daily changes of the prominence of the precipitated withdrawal, as reflected by the withdrawal index.
Rats with continuous morphine infusion and daily saline injections (IT-M+IP-S) also showed few signs of withdrawal. The group mean±s.e.mean withdrawal index score on day 3 was 4.2±1.1.
All rats with continuous morphine infusion and naloxone injections (IT-M+IP-N) showed behaviours indicative of significant withdrawal on days 2 and 3, with the highest scores on day 3. The group mean±s.e.mean withdrawal index score on days 1, 2 and 3 for the IT-M+IP-N group were: day 1=3.1±0.9; day 2 = 8±1.1 and day 3=19±3.2 (one-way ANOVA, repeated measures; P<0.05; Dunnett's t-test, P<0.05 as compared to day 1).
Correlation between spinal amino acid release and withdrawal behaviour
Regression lines were plotted for the relationship between the increase in Glu, Asp, Tau, and Ser from daily resting release, and the total withdrawal scores for each animal from all four treatment groups. Table 2 and Figure 5 (for Glu and Ser) represents the results. As shown in these graphs and table, significant correlation was observed between the withdrawal scores and the increase from the resting release of Glu and Tau on days 1, 2 and 3 (P<0.05) and for Asp on day 3 (P<0.05) following drug injection. As shown in the R2 value, the withdrawal signs displayed the most significant correlation with the increase in the release of glutamate on day 3. For serine, no significant correlation was present.
Table 2.
Correlation between changes in the release of amino acids and withdrawal scores*
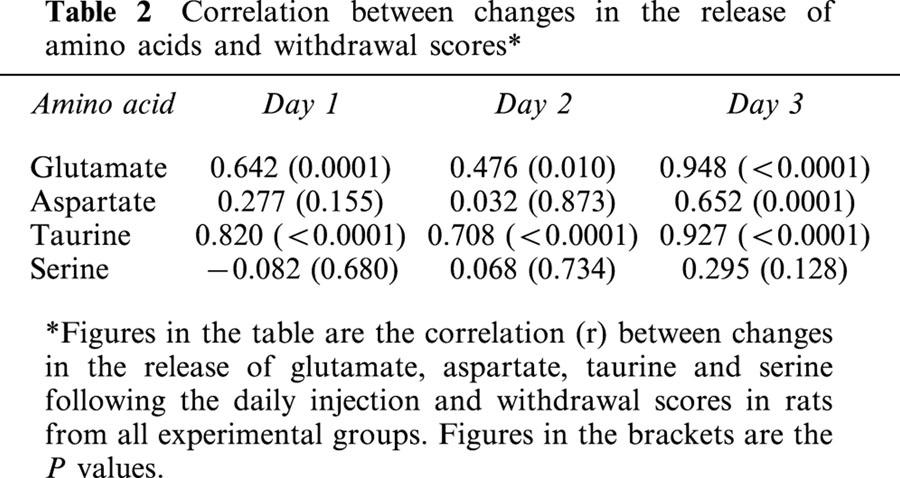
Figure 5.
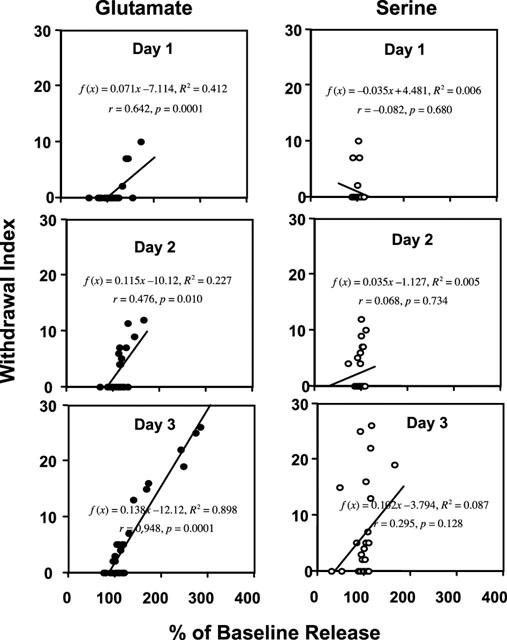
Figure shows the regression and correlation (r) between changes in the release of glutamate and serine following the daily injection and withdrawal scores in rats from the four experimental treatment groups (see text for further detail).
Discussion
Spinal NMDA receptors appear to play two distinct roles in the sequelae associated with chronic spinal opiate delivery. First, co-administration of NMDA receptor antagonists with morphine reduces the loss of effect observed with continued or repeated exposure of the system to the agonist (morphine). Importantly, as reviewed, the acute delivery of NMDA antagonists in a morphine tolerant animal does not ‘reverse' tolerance. Secondly, naloxone in a spinal morphine tolerant rat evokes withdrawal and hyperalgesia, which are reversed by spinal administration of NMDA antagonist (Mao et al., 1994; Dunbar & Yaksh, 1996). These results led to the hypothesis that glutamate is released during tolerance and withdrawal, and that the subsequent activation of the NMDA receptor is a causal linkage in the observed effects. In subsequent work, however, we observed that over the time course of spinal tolerance, there was no increase in spinal excitatory amino acid (EAA: glutamate and aspartate) concentration in the dialysate, but that with a bolus injection of i.p. naloxone, there was an increase in spinal EAA release that corresponded to the magnitude and time course of the withdrawal behaviour (Jhamandas et al., 1996). These observations thus suggested that if the NMDA receptor were playing a causal role in the tolerance process, then the resting EAA release might provide sufficient receptor occupancy to initiate the progressive changes that led to loss of opiate agonist action. These observations led to the next series of studies in which it was reasoned that an enhanced glutamate release might augment tolerance development. In behavioural studies, we then demonstrated that periodic naloxone reversal will initiate an enhanced tolerance to the antinociceptive effects of continuously infused intrathecal morphine (Ibuki et al., 1997). These observations then led to the present studies in which we sought to determine if the repeated delivery of naloxone would lead to an enhanced spinal EAA release. We reasoned that if the magnitude of tolerance was augmented by periodic delivery of naloxone that the delivery of naloxone should lead to progressively greater degrees of spinal EAA release and enhanced withdrawal signs.
The present studies indeed demonstrated that with repeated withdrawal, there was a more rapid loss of opioid antinociception; there was an attendant increase in spinal EAA release with each repetition of the naloxone exposure and the magnitude of the attendant withdrawal signs rose over the course of the naloxone repetitions.
Spinal microdialysis and amino acid release
Loop dialysis catheter systems have been extensively employed to assess the concentration of dialyzable substances in the lumbar intrathecal space of amino acids and prostanoids. Peripheral stimuli such as the injection of irritant into the paw (Malmberg & Yaksh, 1992a, b; Malmberg & Yaksh, 1995), inflammation of the knee joint (Yang et al., 1996a) or central stimulus, such as intrathecal kainate or nicotine (Yang et al., 1996b; Khan et al., 1996) have been shown to enhance the release of glutamate, aspartate and taurine, but not that of other amino acids, such as serine, glycine or asparagine. Importantly, this release is stable over intervals as long as 7–10 days (Marsala et al., 1995). In the present experiments, an increase in daily resting and naloxone-evoked release was observed with glutamate, aspartate and taurine. In contrast, similar changes were not observed with any of the other amino acids, including serine or glutamine. This differential effect upon dialysate concentrations of the amino acids confirms that the effects were not the result of a nonspecific change in collectability or dialysis membrane function. Glutamate and aspartate are considered to be principal excitatory neurotransmitters (Mayer & Westbrook, 1987), and taurine has inhibitory effects during excitation (Lehman et al., 1984). Increased release of taurine may be an early protection mechanism against excitability and neurotoxicity, as previously reported (Menendez et al., 1989). The spinal release of serine and glutamine did not change following naloxone injection, a finding which presumably reflects their roles in metabolic and not transmitter processes (Thanki et al., 1983).
Tolerance and spinal amino acid release
In the IT morphine-infused rat receiving daily i.p. naloxone (IT-M+IP-N), we identified three distinct effects: (i) i.p. naloxone evoked an increase in spinal EAA release in animals receiving continuous IT morphine infusion, (ii) this evoked increase was increased over the 3-day infusion interval, and (iii) there was an unexpected persistent and progressive increase over the 3-day infusion intervals in the baseline release of glutamate, aspartate and taurine.
Notably, there was a progressive enhancement of daily baseline glutamate, aspartate and taurine release, as assessed prior to the injection of naloxone in animals receiving continuous IT morphine. This increase was not observed in morphine-infused animals receiving i.p. saline or in animals receiving i.p. naloxone and IT saline infusions. Moreover, this effect was related to the withdrawal induced by naloxone in opiate-tolerant rats. While naloxone acutely increases spinal excitatory amino acid (EAA) release in morphine tolerant animals (Jhamandas et al., 1996), the effects noted 24 h after naloxone dosing do not reflect a persistence of the antagonist. Naloxone was injected at a dose previously observed to produce significant reversal for approximately 2 h (Ibuki et al., 1997). This is consistent with the known half-life of naloxone in rat brain and plasma, which is approximately 0.4 h (Misra et al., 1976). Accordingly, by pharmacokinetic and behavioural criteria in morphine-infused rats, the effects noted 24 h after naloxone dosing does not reflect a residual presence of naloxone.
The observation that the naloxone-evoked spinal release of glutamate, aspartate and taurine in morphine-tolerant animals increased daily is consistent with the observation that an increased duration of opiate exposure leads to a more dependent state. In the present study, we did not examine the effects of naloxone on day 3 in rats receiving IT morphine and daily i.p. saline. In a previous study, we showed that the delivery of naloxone given only once after 4 days of IT morphine infusion resulted in an absolute increase in spinal glutamate baseline release, which was 3 fold that of pre-intrathecal infusion glutamate baseline release (Jhamandas et al., 1996). In contrast, in the present study, with only 3 days of infusion and daily i.p. naloxone, the evoked Glu, Asp and Tau release was approximately 5–6 fold that of pre-infusion release. Note that basal glutamate release on day 3 with daily naloxone was increased approximately 2.2 fold as compared to day 1, and naloxone-evoked release as compared to day-3 basal release was increased approximately 2.5 fold. These results are consistent with the greater dependence in daily naloxone-treated, continuously morphine infused rats.
Mechanisms underlying repeated spinal withdrawal and enhanced tolerance
The relationship between glutamate release and opiate receptor tolerance is complex and likely reflects several components. One possible cascade involves activation of cellular kinases, particularly PKC. Mu opiate agonists through Phospholipase C increase neuronal IP3 (Tsu & Wong, 1996), increasing [Ca2+]i through intracellular storage sites. Ca2+ activates PKC which can phosphorylate the NMDA receptor or a related protein. NMDA receptor phosphorylation increases the functionality of the NMDA ionophore which serves to increase calcium flux (Cerne et al., 1993). The importance of this linkage to opioid tolerance is suggested by the observation from several laboratories that intrathecal PKC inhibitors, dose-dependently and stereospecifically reverse or attenuate the tolerance induced by chronic spinal opiate exposure (Mayer et al., 1995; Narita et al., 1996; Granados-Soto et al., 2000). Previous work has shown that increased NMDA receptor activity can lead to downstream events which include an increase in the spinal release of excitatory transmitters including substance P, glutamate and prostaglandins (Liu et al., 1997; Yaksh et al., 2001) and that such increased spinal receptor activity can initiate prominent states of facilitated nociceptive processing (hyperalgesia) (Yaksh et al., 1999). This cascade suggests the presence of a positive feed back that would arise from the enhanced PKC expression and activity that arises during the above noted opiate withdrawal. Accordingly, increased glutamate release during withdrawal of opioid receptor occupancy would serve to antagonize the antinociceptive effect of IT morphine (Srivastava et al., 1995) and additionally enhance opiate receptor phosphorylation to further enhance the decrease in μ receptor function.
Clinical implications
Regardless of the specific mechanism by which chronic opiate exposure leads to changes in glutamate release, there are several issues related to the withdrawal-evoked spinal glutamate release that should be considered. First, periodic dosing leads to enhanced spinal glutamate release. Whether this alone enhances the rate of tolerance development is not certain, but such periodic withdrawal leads to augmented glutamate release, which in turn results in a sensitization of spinal nociceptive processing. Such hyperalgesia is routinely observed in preclinical models, wherein bolus IT morphine dosing is performed (Mao et al., 1994; Dunbar & Pulai, 1998). Such considerations might equally apply to the human pain patient. This would represent an important rationale for development of adjuvant drug approaches targeting the NMDA receptor. Secondly, while we have investigated morphine, NMDA receptor antagonism exerts similar effects upon other analgesics that act spinally through G-coupled proteins, such as the alpha 2 agonists (Dunbar & Yaksh, 1997). Thirdly, it is important to note that our experiments employed a precipitated withdrawal as opposed to an abstinence paradigm. In the former, there is an abrupt withdrawal of opiate receptor occupancy, and in the latter, there is a progressive reduction in opiate receptor occupancy over a time course that parallels the half-life of the drug. In this regard, the present findings raise the issue of precipitated withdrawal in opiate-tolerant patients. Such rapid withdrawal under an anaesthetic regimen has been reported (Kienbaum et al., 1998). Our work indicates that this withdrawal leads to enhanced spinal glutamate release. Such increased release at the spinal level may account in part for the florid physiological (cardiovascular and motor reflex) signs observed with precipitated withdrawal in humans and animals. Of particular importance, we would suggest that the significant release of glutamate generated by such precipitated withdrawal may have long term deleterious consequences (Nakamura et al., 1994; Hirata et al., 1997). The safety factor for such an approach may be dependent upon the ability of the anaesthetic regimen to suppress glutamate release or otherwise protect the neuraxis from the consequences of excessive activation of glutamate receptors, e.g. as with the concurrent use of glutamate ionophore antagonists. Additional research is clearly needed.
Acknowledgments
We thank Alan Moore and Steve Rossi, Ph.D. for their analysis of the amino acids and Takero Yamane, M.D. for his graphic works. This research was supported by grants from DA02110 (T.L. Yaksh) and a Shimamura fellowship (T. Ibuki). Some of this work was presented at the Society for Neuroscience 26th Annual Meeting (Washington, D.C., U.S.A.), 19 November 1996.
Abbreviations
- IT
intrathecal
- NMDA
N-methyl-D-aspartate
References
- AGHAJANIAN G.K., KOGAN J.H., MOGHADDAM B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- BHARGAVA H.N. Quantitation of morphine tolerance induced by pellet implantation in the rat. J Pharm Pharmacol. 1978;30:133–135. doi: 10.1111/j.2042-7158.1978.tb13183.x. [DOI] [PubMed] [Google Scholar]
- CERNE R., RUSIN K.I., RANDIC M. Enhancement of the N-methyl-D-aspartate response in spinal dorsal horn neurons by cAMP-dependent protein kinase. Neurosci. Lett. 1993;161:124–128. doi: 10.1016/0304-3940(93)90275-p. [DOI] [PubMed] [Google Scholar]
- COX B.M., GINSBURG M., WILLIS J. The offset of morphine tolerance in rats and mice. Br. J. Pharmacol. 1975;53:383–391. doi: 10.1111/j.1476-5381.1975.tb07374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIDLAND R.A., SUTAK M., JHAMANDAS K. Characteristics of precipitated withdrawal from spinal morphine: changes in [Met5]enkephalin levels. Eur. J. Pharmacol. 1991;203:93–103. doi: 10.1016/0014-2999(91)90795-r. [DOI] [PubMed] [Google Scholar]
- DUNBAR S.A., PULAI IJ. Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J. Pharm. Exp. Ther. 1998;284:678–686. [PubMed] [Google Scholar]
- DUNBAR S.A., YAKSH T.L. Concurrent spinal infusion of MK801 blocks spinal tolerance and dependence induced by chronic intrathecal morphine in the rat. Anesthesiology. 1996;84:1177–1188. doi: 10.1097/00000542-199605000-00020. [DOI] [PubMed] [Google Scholar]
- DUNBAR S.A., YAKSH T.L. Spinal infusion of N-methyl-D-aspartate antagonist MK801 induces hypersensitivity to the spinal alpha-2 agonist ST91 in the rat. J. Pharmacol. Exper. Ther. 1997;281:1219–1225. [PubMed] [Google Scholar]
- GRANADOS-SOTO V., KALCHEVA I., HUA X.-Y., NEWTON A., YAKSH T.L. Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain. 2000;85:395–404. doi: 10.1016/S0304-3959(99)00281-X. [DOI] [PubMed] [Google Scholar]
- GUTSTEIN H.B., TRUJILLO K.A. MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res. 1993;626:332–334. doi: 10.1016/0006-8993(93)90597-g. [DOI] [PubMed] [Google Scholar]
- HIRATA A., NAKAMURA R., KWAK S., NAGATA N., KAMAKURA K. AMPA receptor-mediated slow neuronal death in the rat spinal cord induced by long-term blockade of glutamate transporters with THA. Brain Res. 1997;771:37–44. doi: 10.1016/s0006-8993(97)00709-9. [DOI] [PubMed] [Google Scholar]
- IBUKI T., DUNBAR S., YAKSH T.L. Effect of transient naloxone antagonism on tolerance development in rats receiving continuous spinal morphine infusion. Pain. 1997;70:125–132. doi: 10.1016/s0304-3959(96)03283-6. [DOI] [PubMed] [Google Scholar]
- JHAMANDAS K.H., MARSALA M., IBUKI T., YAKSH T.L. Spinal amino acid release and precipitated withdrawal in rats chronically infused with spinal morphine. J. Neurosci. 1996;16:1–9. doi: 10.1523/JNEUROSCI.16-08-02758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN I.M., MARSALA M., PRINTZ M.P., TAYLOR P., YAKSH T.L. Intrathecal nicotinic agonist-elicited release of excitatory amino acids as measured by in vivo spinal microdialysis in rats. J. Pharm. Exper. Ther. 1996;278:97–106. [PubMed] [Google Scholar]
- KIENBAUM P., THURAUF N., MICHEL M.C., SCHERBAUM N., GASTPAR M., PETERS J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88:1154–1161. doi: 10.1097/00000542-199805000-00004. [DOI] [PubMed] [Google Scholar]
- LEHMAN A., HAGBERG H., HAMBERGER A. A role for taurine in the maintenance of homeostasis in the central nervous system during hyperexcitation. Neurosci. Lett. 1984;52:341–346. doi: 10.1016/0304-3940(84)90185-x. [DOI] [PubMed] [Google Scholar]
- LIU H., MANTYH P.W., BASBAUM A.I. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. The effect of morphine on formalin-evoked behavior and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in conscious rats. Br. J. Pharmacol. 1992a;114:1069–1075. doi: 10.1111/j.1476-5381.1995.tb13315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992b;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: A microdialysis study in unanesthetized rats. J. Neurosci. 1995;15:2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAO J., PRICE D.D., MAYER D.J. Thermal hyperalgesia in association with development of morphine tolerance in rats: role of excitatory amino acid receptors and protein kinase. J. Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSALA M., MALMBERG A.B., YAKSH T.L. The spinal loop dialysis catheter: characterization of use in the unanesthetized rat. J. Neurosci. Methods. 1995;62:43–53. doi: 10.1016/0165-0270(95)00053-4. [DOI] [PubMed] [Google Scholar]
- MAYER D.J., MAO J., PRICE D.D. The association of neuropathic pain, morphine tolerance and dependence, and the translocation of protein kinase C. Nida Research Monograph. 1995;147:269–298. [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog. Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- MENENDEZ N., HERRERAS O., SOLIS J.M., HERRANZ A.S., MARTIN DEL RIO R. Extracellular taurine increases in rat hippocampus evoked by specific glutamate receptor activation is related to excitatory potency of glutamate agonist. Neurosci. Lett. 1989;102:64–69. doi: 10.1016/0304-3940(89)90308-x. [DOI] [PubMed] [Google Scholar]
- MILNE B., CERVENKO F.W., JHAMANDAS K., SUTAK M. Intrathecal clonidine: analgesia and effect on opiate withdrawal in the rat. Anesthesiology. 1985;62:34–38. [PubMed] [Google Scholar]
- MISRA A.L., PONTANI R.B., VADLAMANI N.L., MULE S.J. Physiological disposition and biotransformation of [allyl-1′,3′-14C naloxone in the rat and some comparative observations on nalorphine. J. Pharm. Exp. Ther. 1976;196:257–268. [PubMed] [Google Scholar]
- NAKAMURA R.., KAMAKURA K., KWAK S. Late-onset selective neuronal damage in the rat spinal cord induced by continuous intrathecal administration of AMPA. Brain Res. 1994;654:279–285. doi: 10.1016/0006-8993(94)90490-1. [DOI] [PubMed] [Google Scholar]
- NARITA M., MIZOGUCHI H., KAMPINE J.P., TSENG L.F. Role of protein kinase C in desensitization of spinal delta-opioid-mediated antinociception in the mouse. Br. J. Pharmacol. 1996;118:1829–1835. doi: 10.1111/j.1476-5381.1996.tb15610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRIVASTAVA R.K., GOMBAR K.K., KAUR A.H., KHOSLA P. Attenuation of morphine-induced antinociception by L-glutamic acid at the spinal site in rats. Can. J. Anaesth. 1995;42:541–546. doi: 10.1007/BF03011695. [DOI] [PubMed] [Google Scholar]
- STEVENS C.W., YAKSH T.L. Magnitude of opioid dependence after continuous intrathecal infusion of μ- and δ-selective opioids in the rat. Eur. J. Pharm. 1989a;166:467–472. doi: 10.1016/0014-2999(89)90360-9. [DOI] [PubMed] [Google Scholar]
- STEVENS C.W., YAKSH T.L. Time course characteristics of tolerance development to continuously infused antinociceptive agents in rat spinal cord. J. Pharm. Exp. Ther. 1989b;251:216–223. [PubMed] [Google Scholar]
- STEVENS C.W., YAKSH T.L. Potency of infused spinal antinociceptive agents is inversely related to magnitude of tolerance after continuous infusion. J. Pharm. Exp. Ther. 1989c;250:1–8. [PubMed] [Google Scholar]
- THANKI C.M., SUGDEN S.R., THOMAS A.J., BRADFORD H.F. In vivo release from cerebral cortex of 14C glutamate synthesized from U-14C glutamine. J. Neurochem. 1983;41:611–617. doi: 10.1111/j.1471-4159.1983.tb04785.x. [DOI] [PubMed] [Google Scholar]
- TSU R.C., WONG Y.H. Gi-mediated stimulation of type II adenylyl cyclase is augmented by Gq-coupled receptor activation and phorbol ester treatment. J. Neurosci. 1996;16:1317–1323. doi: 10.1523/JNEUROSCI.16-04-01317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAKSH T.L., DIRIG D.M., CONWAY C.M., SVENSSON C., LUO Z.D., ISAKSON P.C. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J. Neurosci. 2001;21:5847–5853. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAKSH T.L., HUA X.Y., KALCHEVA I., NOZAKI-TAGUCHI N., MARSALA M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG L.C., MARSALA M., YAKSH T.L. Characterization of time course of spinal amino acids, citrulline and PGE2 release after carrageenan/kaolin-induced knee joint inflammation: a chronic microdialysis study. Pain. 1996a;67:345–354. doi: 10.1016/0304-3959(96)03106-5. [DOI] [PubMed] [Google Scholar]
- YANG L.C., MARSALA M., YAKSH T.L. Effect of spinal kainic acid receptor activation on spinal amino acid and prostaglandin E2 release in rat. Neuroscience. 1996b;75:453–461. doi: 10.1016/0306-4522(96)00294-1. [DOI] [PubMed] [Google Scholar]
- YANO I., TAKEMORI A.E. Inhibition by naloxone of tolerance and dependence in mice treated acutely and chronically with morphine. Res. Comm. Chem. Path. Pharmacol. 1977;16:721–734. [PubMed] [Google Scholar]
- ZHANG L., YU Y., MACKIN S., WEIGHT F.F., UHL G.R., WANG J.B. Differential mu opiate receptor phosphorylation and desensitization induced by agonists and phorbol esters. J. Biol. Chem. 1996;271:11449–11454. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]


