Abstract
This review focuses on the dysfunction of the intrinsic brain renin–angiotensin system (RAS) in the pathogenesis of hypertension. Hyperactivity of the brain RAS plays a critical role in mediating hypertension in both humans and animal models of hypertension, including the spontaneously hypertensive rat (SHR). The specific mechanisms by which increased brain RAS activity results in hypertension are not well understood but include increases in sympathetic vasomotor tone and impaired arterial baroreflex function. We discuss the contribution of endogenous angiotensin (Ang) II actions on presympathetic vasomotor rostral ventrolateral medulla neurons to enhance sympathetic activity and maintain hypertension. In addition, we discuss Ang II-induced attenuation of afferent baroreceptor feedback within the nucleus tractus solitarius and its relevance to the development of hypertension. We also outline the cellular and molecular mechanisms of Ang II signal transduction that may be critical for the initiation and establishment of hypertension. In particular, we present evidence for a phosphoinositide-3-kinase-dependent signaling pathway that appears to contribute to hypertension in the SHR, possibly via augmented Ang II-induced increases in neuronal firing rate and enhanced transcriptional noradrenaline neuromodulation. Finally, we outline future directions in utilizing our understanding of the brain RAS dysfunction in hypertension for the development of improved therapeutic intervention in hypertension.
Keywords: Brain renin–angiotensin system, angiotensin receptor signal transduction, spontaneously hypertensive rat, sympathetic activity, Baroreflex, phosphoinositide-3-kinase, noradrenaline neuromodulation
Introduction
Essential hypertension is one of the most prevalent cardiovascular disorders, afflicting approximately 15% of the population. It is a major risk factor for other cardiovascular diseases such as peripheral vascular disease and ischemic heart disease, as well as stroke and end-stage renal disease, thereby resulting in considerable morbidity and mortality. The etiology of this disease is multifactorial, resulting from the interaction of a number of genetic and environmental factors. Despite decades of research efforts, the specific mechanisms involved in mediating the elevation in blood pressure (BP) that characterizes hypertension are poorly understood. BP is regulated by sympathetic and parasympathetic nerve activity, circulating hormones and local autoregulatory mechanisms that interact to control cardiac output and vascular resistance. In addition, BP is modulated by the central integration of afferent neural and humoral inputs from the periphery. An increasing number of studies have demonstrated a critical role for the central nervous system in the development and maintenance of hypertension. In particular, increases in sympathetic nerve activity and alterations in arterial baroreflex function appear to contribute to the pathogenesis of this disease.
The development of hypertension in various animal models of hypertension, such as the spontaneously hypertensive rat (SHR), the renin transgenic (TGR mRen2) rat, the Dahl salt-sensitive rat and the deoxycorticosterone acetate (DOCA)-salt rat, is associated with increases in sympathetic activity (Takeda & Bunag, 1980; Arribas et al., 1996; Cabassi et al., 2002; Leenen et al., 2002). Increased sympathetic nerve activity would cause an elevation in BP via a direct vasoconstriction, and by increasing the force and rate of contraction of the heart. In addition, renal sympathetic nerve activity causes renin secretion that activates the systemic renin–angiotensin system (RAS) leading to angiotensin (Ang) II-induced vasoconstriction and antinatriuresis. Sustained increases in sympathetic outflow would also contribute to elevation of BP by causing trophic effects on vascular smooth muscle leading to increases in vascular resistance and enhanced responses to vasoconstrictor stimuli.
Alteration in arterial baroreflex function has also been implicated in the development of hypertension. The arterial baroreflex responds to changes in BP detected by carotid sinus and aortic arch baroreceptors, by modulating parasympathetic and sympathetic nerve activity and, hence, heart rate and vascular tone. This minimizes fluctuations in BP and maintains it close to a particular set point. In response to a static increase in BP, the baroreflex rapidly resets towards a higher pressure (Andresen & Yang, 1989). In hypertensive conditions, resetting of the operational point of the arterial baroreflex may therefore contribute to maintaining an increased BP rather than opposing it. In SHR, Lyon hypertensive rats, TGR mRen2 rats, DOCA-salt rats and Dahl salt-sensitive rats, the gain of the baroreflex, a measure of the baroreceptor reflex sensitivity, is decreased compared to normotensive controls resulting in blunted baroreflex control of heart rate and sympathetic nerve activity (Miyajima & Bunag, 1986; Hayashi et al., 1988; Nakamura et al., 1988; Lantelme et al., 1998; Borgonio et al., 2001). In many of these models, the impairment in baroreflex function precedes the development of hypertension and may therefore contribute to the development and maintenance of hyper-tension.
Similar to animal models of hypertension, primary human hypertension is also associated with increases in sympathetic activity and blunted arterial baroreflexes. Patients in the early phase of hypertension exhibit elevated plasma noradrenaline (NA) levels (Goldstein, 1981) and increased cardiac and renal NA spillover (Esler et al., 1988). While noncentral mechanisms such as facilitation of NA release (Floras, 1992) and impairment of neuronal NA reuptake (Rumantir et al., 2000) contribute to increased NA spillover, centrally mediated increases in sympathetic nerve outflow appear to play an important role. Microneurograph recordings of the peroneal nerve (Anderson et al., 1989; Grassi et al., 1998) and single-unit recordings (Grassi et al., 1998) in hypertensive patients indicate increased sympathetic nerve discharge, supporting the concept of a central neural mechanism for sympathetic activation in hypertension. In addition, in untreated hypertensive patients, spectral analysis of heart rate variability reveals an enhanced low-frequency component (0.1 Hz), which is considered a marker of sympathetic activity (Guzzetti et al., 1988). Furthermore, the efficacy of centrally acting antihypertensive agents such as clonidine also implicates a central mechanism in primary human hypertension. In both borderline hypertensives and hypertensive patients, the baroreflex control of heart rate and muscle sympathetic nerve activity is blunted and the gain of the baroreflex is decreased (Matsukawa et al., 1991a 1991b). While these studies implicate central mechanisms of sympathetic activation in the pathogenesis of hypertension, particular areas/nuclei involved are yet to be identified.
In hypertensive animals, functional changes within the central nervous system have been detected largely in hypothalamic and medullary areas that modulate sympathetic outflow (Colombari et al., 2001; De Wardener, 2001). A large number of neurotransmitters and neuromodulators contribute to regulating sympathetic outflow. Our research efforts have focused on a neuropeptide, Ang II, as increased expression and activity of components of the intrinsic brain RAS play a key role in many forms of experimental hypertension including the SHR, the TGR mRen2 rat, the Dahl salt-sensitive rat, the DOCA-salt rat and renal hypertensive rats (Itaya et al., 1986; Berecek et al., 1987; Huang & Leenen, 1998; Fontes et al., 2000; De Wardener, 2001; Kagiyama et al., 2001; Park & Leenen, 2001). Some of these models, including the SHR, exhibit both a hyperactive endocrine RAS, characterized by elevated circulating Ang II, as well as a hyperactive tissue RAS, characterized by elevated tissue Ang II. Within the brain, Ang II contributes to cardiovascular regulation via its action at various hypothalamic and medullary areas to enhance sympathetic outflow, blunt the sensitivity of the baroreflex and stimulate secretion of vasopressin (Culman et al., 1995; Averill & Diz, 2000; McKinley et al., 2001; Dampney et al., 2002). This article provides a brief overview of the brain RAS with emphasis on recent developments in this field. We then critically review recent studies that have advanced our understanding of the dysfunction of the brain RAS that underlies altered central BP regulation during the development and maintenance of hypertension. We will also discuss the cellular and molecular basis of Ang II signal transduction that may contribute to hypertension. Finally, we outline future directions that one must consider in advancing the field of central BP regulation from the experimental to the therapeutic level.
Altered regulation of the brain RAS in hypertension
Overview of the brain RAS
The RAS is an enzymatic cascade by which angiotensinogen is cleaved by renin and then by angiotensin converting enzyme (ACE) to produce Ang II, and subsequently cleaved by aminopeptidases to form other Angs. The brain expresses genes that encode all components of the RAS including angiotensin type I (AT1) and type II (AT2) receptors (Lenkei et al., 1997; Phillips & Sumners, 1998). A notable difference between the brain RAS and the systemic RAS is that the renin transcript predominantly expressed in the brain contains an alternate first exon (Lee-Kirsch et al., 1999; Clausmeyer et al., 2000; Sinn & Sigmund, 2000). This transcript encodes a truncated renin isoform that is predicted to be intracellular as it lacks the prefragment that targets to the secretory pathway. Recently, a receptor for prorenin/renin that appears to increase the efficiency of angiotensinogen cleavage to Ang I has been identified (Nguyen et al., 2002). It is highly expressed in tissues in which an intrinsic RAS has been described, including the brain, and may play an important physiological role in these tissues where angiotensinogen and renin concentrations are much lower than in plasma. Although Ang II actions are the best characterized, a role for Ang III (Ang 2–8) in cardiovascular regulation is emerging and it has been suggested that conversion of Ang II to Ang III is required for central Ang II actions (Reaux et al., 2001). Irrespective of the ligand (Ang II or Ang III), almost all the central actions on BP regulation and fluid homeostasis are mediated by the AT1 receptor (Llorens-Cortes & Mendelsohn, 2002). Accordingly, within the brain, high densities of the AT1 receptor are distributed in cardiovascular regulatory areas including the lamina terminalis, that is, the subfornical organ (SFO), median preoptic nucleus (MnPO) and organum vasculosum laminae terminalis, the paraventricular nucleus (PVN), lateral parabrachial nucleus, ventrolateral medulla and the nucleus tractus solitarius (NTS) (Obermuller et al., 1991; Tsutsumi & Saveedra, 1991; Phillips et al., 1993; Allen et al., 1999). In humans, a single gene on chromosome 3 encodes the AT1 receptor (Curnow et al., 1992) whereas two AT1 receptor subtypes, AT1A and AT1B, encoded by distinct genes on different chromosomes have been identified in rodents (Murphy et al., 1991; Elton et al., 1992; Sandberg et al., 1992; Sasamura et al., 1992; Lewis et al., 1993). The AT1A receptor is the predominantly expressed subtype in central cardiovascular regulatory areas in rodents (Lenkei et al., 1995), contributes to maintaining resting BP, and mediates pressor responses to central administration of Ang II (Davisson et al., 2000; Li et al., 2003). Although the AT2 receptor is moderately expressed in certain nuclei involved in cardiovascular regulation such as the locus coeruleus, lateral septum and the medial amygdala (Song et al., 1992; Lenkei et al., 1997), its contribution to BP regulation in these areas remains elusive. However, functional evidence implicates a role for an AT2 receptor-mediated contribution to baroreflex regulation (Lin et al., 1997; 2001; Luoh & Chan, 1998). Vasopressin release elicited by central administration of Ang II is mediated mainly by AT1 receptors, but is also mediated by AT2 receptors (Hogarty et al., 1992; Veltmar et al., 1992). An increase in endogenous Ang III also causes vasopressin release but the receptor that mediates this effect has not yet been identified (Zini et al., 1996). A receptor that is pharmacologically distinct from either AT1 or AT2 receptors appears to mediate Ang 1–7 actions on BP regulation, which in most cases oppose Ang II actions (Fontes et al., 1994; Couto et al., 2002). Thus, the broad function of the brain RAS in cardiovascular regulation is fairly well understood but its contribution to BP regulation in different areas/nuclei, and its altered regulation in hypertension is still a focus of much investigation.
Dysfunction of the brain RAS is implicated in the development of hypertension
The SHR has been the most widely used animal model for investigation of brain RAS dysfunction in hypertension. Increases in brain angiotensinogen expression precede the development of hypertension in SHR (Tamura et al., 1996), especially in the preoptic area where increases in angiotensinogen were apparent in 4-week-old SHR and increased with age (Shibata et al., 1993). In addition, renin-like activity in the anterior hypothalamus and NTS was higher in SHR compared to WKY rats during the development of hypertension (Ruiz et al., 1990). Ang II content and turnover within the hypothalamus, and Ang II immunoreactivity within the PVN and supraoptic nucleus, is also increased in adult SHR compared to their normotensive WKY control rats (Weyhenmeyer & Phillips, 1982; Ganten et al., 1983; Hermann et al., 1984; Phillips & Kimura, 1988). SHR also exhibit increased density of Ang II binding sites within the MnPO, SFO, PVN and NTS, and AT1A receptor mRNA within the preoptic area compared to WKY rats (Gutkind et al., 1988; Komatus et al., 1996). Furthermore, a recent study demonstrated increased cellular AT1 receptor density within the RVLM in SHR versus WKY rats (Hu et al., 2002). Pressor responses to intracerebroventricular (i.c.v.) injection of Ang II, or microinjections of Ang II within the preoptic area, NTS and RVLM, and depressor effects within the CVLM, are also correspondingly greater in SHR versus WKY rats, indicating increased receptor density and/or sensitivity in the SHR (Casto & Phillips, 1985; Matsuda et al., 1987; Wright et al., 1987; Muratani et al., 1991; Zhu et al., 1998). Thus, it can be concluded from these studies that a hyperactivity of the brain RAS precedes and/or parallels the development of hypertension in the SHR.
Studies utilizing pharmacological agents have provided evidence that the hyperactivity of the brain RAS mediates hypertension. Blockade of activity of the brain RAS utilizing injections of ACE inhibitors such as captopril, or an AT1 receptor antagonist, losartan, into the lateral ventricle, decreases BP in SHR and TGR mRen2 rats as well as other models of hypertension, but does not affect BP in their normotensive controls (Hutchinson et al., 1980; Faber & Brody, 1984; Itaya et al., 1986; Berecek et al., 1987; Teruya et al., 1995; Szczepanska-Sadowska et al., 1998; Park & Leenen, 2001). Furthermore, microinjections of losartan into the anterior hypothalamic area also result in a decrease in BP in SHR (Yang et al., 1992). The depressor effect following inhibition of brain RAS activity in hypertensive rats appears to be mainly due to a decrease in sympathetic activity (Berecek et al., 1987; Huang & Leenen, 1998). These studies indicate that a hyperactivity of the brain RAS mediates hypertension in SHR and other hypertensive models. The role of the brain RAS in maintaining BP in normotensive rats is less clear as there are discrepancies in the literature, with some reports suggesting a tonic role for Ang peptides in maintaining resting BP (Ito & Sved, 1996; Tagawa et al., 1999; Fontes et al., 2000).
Antisense gene targeting of components of the RAS within the brain has provided further support for the view that a hyperactive brain RAS mediates hypertension. Inhibition of brain RAS activity by i.c.v. administration of antisense oligonucleotides to angiotensinogen or AT1 receptor mRNA resulted in a short-term decrease in BP in SHR, whereas it had no effect in WKY rats (Wielbo et al., 1995; Kagiyama et al., 1999). Adeno-associated virus vector-mediated delivery of AT1 receptor antisense oligonucleotides, either i.c.v. or directly into the hypothalamus, extended the magnitude and duration of the depressor effect up to 9 weeks in SHR (Phillips et al., 1997). In addition, cross-breeding of transgenic rats that exhibit decreased glial angiotensinogen concentrations with TGR mRen2 rats results in a marked reduction in brain Ang content and BP possibly via a vasopressinergic mechanism (Schinke et al., 1999). Although studies employing PVN lesions or GABAergic blockade have implicated this nucleus in mediating hypertension via sympathetic activation (Ciriello et al., 1984; Takeda et al., 1991; Allen, 2002), antisense oligonucleotide inhibition of angiotensinogen mRNA within the PVN did not affect BP in SHR up to 24 h after injection despite decreased plasma catecholamine and vasopressin levels (Kagiyama et al., 1999). This suggests that Ang II within the PVN contributes to maintaining resting sympathetic activity and vasopressin release consistent with inhibition of Ang II effects mediated by parvocellular and magnocellular subdivisions of the PVN, but does not contribute significantly to maintaining hypertension in adult SHR. In TGR mRen2 rats, AT1 receptor antisense oligonucleotide administration into the PVN did not significantly alter BP, but abolished salt-induced exacerbation of hypertension in these rats (Li et al., 1996). Overall, these findings have confirmed that hyperactivity of the brain RAS contributes to maintaining hypertension. Overactivity of the RAS within the PVN alone does not account for this effect but contributes to salt-induced exacerbation of hypertension.
Studies employing gene transfer or transgenic techniques to overexpress RAS components in a tissue-specific or cell type-specific manner in normotensive rats or mice have provided further insight into brain RAS dysfunction in hypertension, and a means to distinguish the functional contributions of the brain RAS from those of the systemic RAS. In normotensive rats, brain-specific in vivo gene transfer of the human ACE gene utilizing the hemagglutinating virus of Japan complexed with liposomes led to the widespread distribution of the transgene including expression in hypothalamic nuclei and in the ventral medulla (Nakamura et al., 1999). These rats exhibited elevated central Ang II levels and developed hypertension. In mice, overexpression of both the human angiotensinogen and the human renin genes under the control of either a glial fibrillary acidic protein promoter or a synapsin I promoter resulted in glia- or neuron-specific expression of the transgenes respectively (Morimoto et al., 2002). Both glia- and neuron-targeted double transgenic mice developed moderate hypertension, indicating that increased conversion of angiotensinogen to its active peptides within either glia or neurons results in hypertension. In the glia-targeted transgenic mice, AT1 receptor activation and sympathetic activity but not vasopressin mechanisms mediated the hypertensive effect. Neuron-specific overexpression of the AT1A receptor driven by a neuron-specific enolase promoter resulted in expression of the AT1A transgene in neurons within cardiovascular as well as noncardiovascular regulatory areas in the brain with lower levels of expression in the adrenal medulla (Lazartigues et al., 2002). Resting BP was normal in the AT1A transgenic mice, indicating that an increase in AT1A receptors by itself does not result in hypertension. In these transgenic mice, central AT1 receptor blockade decreased BP whereas it did not affect BP in nontransgenic mice, indicating that AT1 receptors contribute to resting BP only in mice with overexpression of AT1A receptors. This study therefore suggests that an increase in the production of Ang II is required for the development of hypertension. Alternatively, effective baroreceptor buffering may prevent the development of hypertension in the AT1A transgenic mice. While these studies were able to limit expression of the transgene to a particular cell type within the CNS, further studies that limit transgene expression to particular areas/nuclei would be required to dissect the contribution of the brain RAS in different cardiovascular regulatory areas in the development of hypertension.
The precise mechanisms by which increased brain RAS activity results in hypertension is not yet known, but appear to involve increased sympathetic vasomotor tone. AT1 receptors are associated with presympathetic vasomotor neurons in the RVLM (Dampney et al., 2002), the major source of excitatory supraspinal input to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord. Bilateral microinjections of nonselective peptide Ang II receptor antagonists, (Sar1, Thr8)Ang II (sarthran) or (Sar1, Ile8)Ang II (sarile), into the RVLM result in profound decreases in sympathetic nerve activity and BP in normotensive rats (Ito & Sved, 1996; Tagawa et al., 1999). However, Lin et al. (1997), (2001) did not observe a decrease in BP in response to sarile microinjections into the RVLM for reasons that are unclear but may relate to differences in experimental conditions. Similar to findings in normotensive rats, microinjections of sarthran in SHR decreased BP to levels expected with total autonomic blockade (Ito et al., 2002). The depressor effect of sarthran does not appear to be mediated by AT1, AT2 or Ang 1–7 receptors (Hirooka et al., 1997; Ito & Sved, 2000; Potts et al., 2000), raising questions regarding the endogenous ligand and receptor(s) that mediate this action. In anesthetized normotensive rats, AT1 receptor blockade within the RVLM does not significantly alter sympathetic nerve activity or BP (Averill et al., 1994; Fontes et al., 1994; Potts et al., 2000). However, a minor role for AT1 receptors in contributing to resting activity of RVLM neurons is unmasked following blockade of tonic GABAergic inhibition (Tagawa et al., 2000). In contrast, in anesthetized SHR, bilateral RVLM microinjections of an AT1 receptor antagonist, candersartan, resulted in large decreases in BP and sympathetic nerve activity that were not observed in their normotensive controls (Allen, 2001). A similar decrease in BP was elicited by microinjections of valsartan, another AT1 receptor antagonist, into the RVLM in SHR, but not WKY rats (Ito et al., 2002). Furthermore, in conscious TGR mRen2 rats, bilateral RVLM microinjections of an AT1 receptor antagonist resulted in a decrease in BP (Fontes et al., 2000). Thus, in contrast to normotensive rats, AT1 receptor stimulation within the RVLM contributes to the enhanced tonic sympathoexcitatory activity of RVLM vasomotor neurons in hypertensive rats. This finding is consistent with a study that demonstrated an upregulation of AT1 receptors within the RVLM in SHR (Hu et al., 2002). The input to AT1 receptors in the RVLM appears to be from the PVN as disinhibition of the PVN resulted in an augmented decrease in BP in SHR compared to WKY rats, and this effect was eliminated by AT1 receptor blockade within the RVLM (Ito et al., 2002). In SHR, RVLM vasomotor neurons are subject to decreased tonic GABAergic inhibition and enhanced excitatory amino-acid input compared to normotensive controls (Smith & Barron, 1990; Ito et al., 2000). Combined blockade of RVLM ionotropic excitatory amino-acid receptors and AT1 receptors in SHR resulted in an additive effect (Ito et al., 2002), suggesting actions via independent mechanisms. Alternatively, a single mechanism that augments both excitatory amino-acid input and AT1 receptor stimulation of RVLM neurons and results in sympathoexcitation and hypertension cannot be ruled out.
There is functional evidence for the presence of Ang 1–7 receptors in the RVLM as RVLM microinjections of Ang 1–7 increase BP (Fontes et al., 1994). Conversely, blockade of endogenous Ang 1–7 actions within the RVLM resulted in a decrease in BP in both anesthetized and conscious normotensive rats, suggesting a role for Ang 1–7 in maintaining BP (Fontes et al., 1994; 1997). In conscious TGR mRen2 rats, RVLM microinjections of an Ang 1–7 antagonist resulted in augmented decreases in BP compared to normotensive rats (Fontes et al., 2000). Thus, in TGR mRen2 rats, endogenous Ang 1–7 actions within the RVLM contribute to maintaining hypertension.
Increased brain RAS activity may also contribute to alterations in baroreflex function. Afferent baroreceptor input to the NTS inhibits sympathetic activity via a multisynaptic pathway involving an excitatory projection from the NTS to the CVLM, a subsequent inhibitory projection to the RVLM, and, lastly, an excitatory projection from the RVLM to sympathetic preganglionic neurons in the IML. Blockade of central AT1 receptors utilizing i.c.v. infusions of losartan facilitated baroreflex control of heart rate in both normotensive rats and SHR, whereas i.c.v. infusion of an Ang 1–7 antagonist blunted baroreflex sensitivity of normotensive rats, but did not significantly alter the depressed baroreflex sensitivity of SHR (Oliveira et al., 1996). This study suggests that stimulation of AT1 receptors and Ang 1–7 actions have opposing effects on the baroreflex response, causing a decrease or increase in baroreflex gain respectively. The blunted baroreflex sensitivity in SHR may relate in part to diminished central actions of Ang 1–7. Within the NTS, AT1 receptor blockade facilitates baroreflex control of heart rate and, conversely, AT1 receptor activation by exogenous Ang II depresses both sympathetic and vagal components of baroreflex-induced bradycardia, indicating that Ang II decreases baroreflex gain (Casto & Phillips, 1985; Campagnole-Santos et al., 1988; Kasparov et al., 1998; Matsumura et al., 1998; Boscan et al., 2001). Ang II-induced suppression of the baroreflex response was attenuated by inhibition of conversion of Ang II to Ang III, suggesting that part of the action of Ang II may result from conversion to Ang III (Luoh & Chan, 1998). While Ang II mediates its effects via AT1 receptors, it appears that Ang III acts on both AT1 and AT2 receptors to depress the baroreflex response (Luoh & Chan, 1998). SHR and TGR mRen2 rats exhibit high medullary Ang II content and increased Ang II binding densities within the NTS (Gutkind et al., 1988; Senanayake et al., 1994; Morishita et al., 1995). Baroreflex control of heart rate in these hypertensive models is blunted compared to control rats and may be reversed by AT1 receptor blockade within the NTS (Matsumura et al., 1998; Diz et al., 2002), indicating that increased AT1 receptor stimulation within the NTS results in blunting of the baroreflex in these hypertensive models. Ang 1–7 actions in the NTS also appear to contribute to baroreflex modulation exerting a tonic facilitatory role on the bradycardic component (Chaves et al., 2000). In SHR, sensitivity to Ang 1–7 within the NTS is decreased and may therefore contribute to the blunted baroreflex control in this model (Chaves et al., 2000). In addition, Colombari et al. (2001) have suggested that enhanced chemoreceptor afferent input to the commissural NTS in SHR contributes to enhanced RVLM vasomotor neuron activity and may therefore contribute to hypertension in this model.
In summary, studies utilizing different approaches and animal models have verified that a hyperactive brain RAS mediates hypertension. Increased brain RAS activity may result in the development and maintenance of hypertension via both AT1 receptor activation and endogenous Ang 1–7 actions on RVLM vasomotor neurons. In addition, AT1 receptor-mediated suppression of afferent baroreceptor feedback as well as a reduced sensitivity to Ang 1–7 within the NTS may also contribute to the development of hypertension.
Cellular and molecular basis for brain RAS dysfunction in hypertension
In spite of identification and characterization of mechanisms that contribute to dysfunction of the brain RAS in hypertension, little is known about the cellular and molecular basis of this dysfunction. Central AT1 receptor-mediated actions of Ang II are mediated by facilitation of excitatory transmission via catecholamines (Jenkins et al., 1996; Raizada et al., 1999), glutamate (Ferguson et al., 2001) and substance P (Paton & Kasparov, 1999; Diz et al., 2002), as well as enhanced inhibitory GABAergic neurotransmission (Paton et al., 2001). A number of studies have provided evidence that the pressor effects of Ang II are mediated by noradrenergic neurotransmission. AT1 receptors are localized on noradrenergic neurons in specific brain nuclei/areas (Yang et al., 1997). Central Ang II administration enhances NA release in the PVN in parallel to the pressor response (Stadler et al., 1992), and increases tyrosine hydroxylase (TH)mRNA and activity in the hypothalamus and brainstem (Yu et al., 1996). In addition, central catecholaminergic depletion or noradrenergic antagonist administration prevents the pressor response to central Ang II, indicating that the Ang II-induced pressor response is mediated by NA (Camacho & Phillips, 1981; Jones, 1984; Bellin et al., 1987). In hypertensive SHR, Ang II-induced increases in TH activity and mRNA within the hypothalamus and brainstem are enhanced compared to WKY rats (Yu et al., 1996). Enhanced Ang II-induced NA neuromodulation and alterations in AT1 signal transduction mechanisms that mediate the NA neuromodulation may therefore contribute to hypertension in the SHR.
AT1 receptor signal transduction and alterations in hypertension
We have utilized primary neuronal cultures derived from the hypothalamus and brainstem of newborn prehypertensive SHR and normotensive WKY rats to elucidate Ang II-mediated signal transduction pathways and identify differences that could contribute to the overactive brain RAS in the SHR. Similar to Ang receptor subtype expression in vivo, SHR neuronal cultures exhibit increased AT1 receptor gene expression and functional receptors compared to WKY cultures (Raizada et al., 1984; 1993). A large percentage of the AT1 receptors are localized on catecholaminergic neurons (Raizada et al., 1999). Ang II, via AT1 receptor-mediated signaling events, rapidly results in release of NA in these neuronal cultures, which we have termed an evoked response (Raizada et al., 1999). Persistent AT1 receptor stimulation results in increased activity and transcription of catecholamine synthesizing enzymes such as TH and dopamine β-hydroxylase (D-βH), and the NA transporter (NAT), that is, enhanced responses to Ang II (Raizada et al., 1999). These observations are consistent with Ang II-induced NA synthesis and release observed following central injections in vivo (Sumners & Phillips, 1983; Stadler et al., 1992; Yu et al., 1996). In SHR neuronal cultures, Ang II-induced NA release and increases in NAT and TH transcription, and TH activity are enhanced (Lu et al., 1996b; Raizada et al., 1996), consistent with augmented in vivo Ang II stimulation of TH activity and mRNA in adult (hypertensive) SHR (Yu et al., 1996). These neuronal cultures therefore provide a simple in vitro system in which we are able to mimic augmented Ang II actions on NA modulation in adult SHR, and preclude nonspecific influences that would confound results obtained from studies in the intact brain.
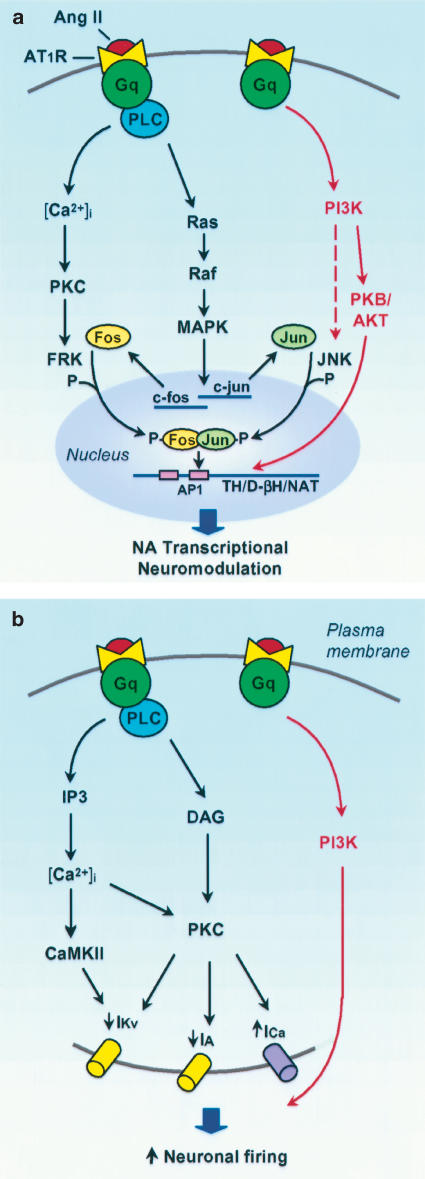
Similar to AT1 receptor signaling in peripheral tissue (Inagami & Eguchi, 2000; Touyz & Schiffrin, 2000; Sayeski & Bernstein, 2001), brain AT1 receptors appear to signal via multiple pathways. In neuronal cultures, Ang II, via the AT1 receptor, activates Gq, increases phospholipase C (PLC) activity and phosphoinositide (PI) hydrolysis, resulting in increased cytosolic free Ca2+ and subsequent activation of Ca2+-dependent enzymes such as protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CaMKII) (Raizada et al., 1999). Seltzer et al. (1995) reported an AT1 receptor-mediated increase in PI turnover in the median eminence providing support for this pathway in vivo. Neuronal AT1 receptors also signal via a PLC-dependent Ras–Raf-mitogen-activated protein (MAP) kinase pathway (Raizada et al., 1999). Activation of this pathway is essential for Ang II-induced increases in transcription of TH, D-βH and the NAT, and TH activity (Yang et al., 1996; Yang & Raizada, 1998; Figure 1a). Similar to Ang II, Ang III also increases TH activity and NA release via an AT1 receptor–PLC-dependent pathway in rat hypothalamic tissue (Rodriguez-Campos et al., 2000).
Figure 1.
AT1 receptor signal transduction pathways that result in transcriptional NA neuromodulation (a) and increased firing rate (b) in neuronal cultures. (a) AT1 receptor activation increases the transcription of TH, D-βH and the NAT via a PLC-dependent Ras–Raf–MAP kinase pathway. This induces Fos and Jun by increasing c-fos and c-jun gene expression. PKC and PI3K mediate FRK and JNK activity, respectively, which in turn phosphorylate Fos and Jun, resulting in transactivation of the AP1 transcription complex. Interaction of the AP1 complex with AP1 binding sites in the promotor regions of TH, D-βH and the NAT results in transcription of these genes. A PI3K–PKB/Akt pathway (highlighted in red) uniquely mediates the augmented transcriptional NA neuromodulation in SHR neurons. Increased AP1 nuclear binding in SHR neurons may be due to enhanced PI3K-mediated JNK activity, resulting in subsequent increases in Jun phosphorylation and AP1 binding. (b) AT1 receptor activation inhibits IKv and IA, and stimulates ICa resulting in increased neuronal firing rate via PLC-mediated activation of PKC. PLC also activates CaMKII, which contributes to IKv inhibition. An additional signaling pathway that utilizes PI3K (pathway highlighted in red) mediates the enhanced Ang II-induced neuronal firing response in SHR neurons. Ang, angiotensin; AP1, activating protein 1; AT1R, angiotensin type 1 receptor; [Ca2+]i, intracellular calcium; CaMKII, calcium/calmodulin-dependent protein kinase II; DAG, diacylglycerol; D-βH, dopamine β-hydroxylase; FRK, Fos-regulating kinase; IA, transient A type K+ current; ICa, total Ca2+ current; IKv, delayed rectifier K+ current; IP3, inositol 1,4,5-triphosphate; JNK, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; NA, noradrenaline; NAT, NA transporter; PI3K, phosphoinositide-3-kinase; PKB, protein kinase B; PKC, protein kinase C; PLC, phospholipase C; TH, tyrosine hydroxylase.
Furthermore, Ang II induces transcription factors such as Fos and Jun by increasing c-fos and c-jun gene expression respectively (Yang et al., 1996; Sumners et al., 2002), and increases activity of Fos-regulating kinase (FRK) and c-Jun NH2-terminal kinase (JNK) (Huang et al., 1998) which phosphorylate Fos and Jun prior to the formation of a fully active activating protein 1 (AP1) complex and translocation to the nucleus. In addition, AT1 receptor activation results in increased binding activity at AP1 binding sites (Lu et al., 1996a; Figure 1a). We have therefore proposed that Ang II-induced transcription of TH, D-βH and NAT results from the interaction of the active AP1 transcription complex with AP1 binding sites in the promotor regions of these genes (Raizada et al., 1999). The production of Fos and Jun is mediated by MAP kinase activity, whereas the activity of FRK and JNK is dependent on PKC and phosphoinositide-3-kinase (PI3K) respectively (Yang et al., 1996; Huang et al., 1998; Sumners et al., 2002). It therefore appears that diverse signaling pathways are involved in the regulation of Ang II-induced NA neuromodulation, and altered regulation of one or more of these pathways may result in the augmented NA neuromodulation observed in the SHR.
Despite augmented Ang II-mediated NA neuromodulation in SHR neuronal cultures, AT1 receptor-mediated regulation of the MAP kinase signaling pathway (Yang & Raizada, 1998) and induction of Fos and Jun are comparable in SHR and WKY neuronal cultures (Lu et al., 1996a). Similarly, i.c.v. injections of Ang II caused a comparable stimulation of MAP kinase phosphorylation in the hypothalamus and brainstem of adult WKY and SHR (Yang & Raizada, 1998). In addition, bilateral microinjection of a MAP kinase inhibitor into the RVLM caused a similar percentage decrease in BP in SHR and WKY rats (Seyedabadi et al., 2001). These studies indicate that the MAP kinase pathway contributes to NA neuromodulation and BP regulation to a similar extent in both strains. However, Ang II-mediated AT1 receptor phosphorylation is greater in SHR compared to WKY neurons and was demonstrated to be MAP kinase dependent (Yang et al., 1997). The reason for this discrepancy is not clear. Despite similar Ang II induction of Fos and Jun in WKY and SHR neuronal cultures, i.c.v. Ang II injections cause a greater increase in expression of Fos, Jun and Krox-24 in areas involved in cardiovascular homeostasis and osmoregulation such as the SFO, OVLT, PVN and the NTS, in hypertensive SHR compared to WKY rats (Blume et al., 1997). Whether enhanced Ang II-induced expression of transcription factors in the adult SHR relates to the enhanced transcriptional NA neuromodulation in response to Ang II in this model of hypertension is yet to be determined.
In SHR neurons, in addition to the Ras–Raf MAP kinase pathway, a MAP kinase-independent signaling pathway that involves PI3 K appears to modulate enhanced Ang II-mediated NA neuromodulation (Figure 1a, highlighted in red). Although AT1 receptor activation results in comparable (∼3.6-fold) stimulation of PI3 K activity in WKY and SHR neuronal cultures, its effect in SHR neurons is more persistent (Yang & Raizada, 1999). In WKY cultures PI3K activity returns to control values within 30 min following Ang II stimulation whereas in SHR cultures a two-fold stimulation in activity was detected even an hour after Ang II treatment. In addition, the rate of nuclear translocation of PI3 K is greater in SHR versus WKY neuronal cultures.
Consistent with Ang II stimulation of PI3 K activity in neuronal cultures, central Ang II injections in adult SHR caused more persistent increases in hypothalamic PI3K activity compared to WKY rats (Yang & Raizada, 1999). PI3K inhibition resulted in an ∼50% attenuation of Ang II-induced stimulation of TH and NAT mRNA and (3H)NA uptake in SHR neuronal cultures whereas it had no effect in WKY neurons (Yang & Raizada, 1999). Furthermore, combined inhibition of MAP kinase and PI3K activity abolished Ang II-induced increases in TH and NAT mRNA in the SHR cultures. Ang II causes a five-fold greater induction of nuclear AP1 binding activity in SHR versus WKY neurons. PI3 K inhibition decreased AP1 binding activity in nuclear fractions of SHR neurons to levels similar to that in WKY neurons, whereas it had no effect on AP1 binding activity in WKY neurons (Yang & Raizada, 1999). Enhanced AP1 binding activity in SHR neuronal nuclear fractions may possibly be due to enhanced PI3K-mediated JNK activity, which would increase phosphorylation of Jun and may therefore result in increased AP1 binding. Consistent with enhanced PI3K signaling in SHR neurons, Ang II also causes an enhanced and more persistent stimulation of protein kinase B (PKB/Akt) activity (Yang & Raizada, 1999; Figure 1a, highlighted in red), an established downstream kinase in the PI3K signaling pathway. AT1 receptor inhibition and PI3K inhibition abolished PKB/Akt stimulation, suggesting that the AT1 receptor–PI3K pathway mediates PKB/Akt simulation (Yang & Raizada, 1999). These studies indicate that AT1 receptor activation in SHR neurons causes enhanced PI3K-mediated AP1 binding activity and PKB/Akt activity, resulting in the augmented NA neuromodulation in SHR neurons. This PI3K pathway does not appear to mediate NA neuromodulation in WKY neurons, but has been implicated in Ang II-induced neuritogenesis (Yang et al., 2001).
Ang II-evoked NA release results from modulation of neuronal electrical activity via effects on membrane ion channels and their dependent currents. Ang II inhibits delayed rectifier K+ current (IKv) and transient A type K+ current (IA), stimulates total Ca2+ current, and results in increases in neuronal firing rate in neuronal cultures (Sumners et al., 2002; Figure 1b). These effects are mediated by the AT1 receptor as they occur in the presence of an AT2 receptor antagonist and are abolished by losartan, an AT1 receptor antagonist. These effects are consistent with the effects of Ang II on IA and neuronal firing in SFO, PVN and SON neurons as well as on neuronal firing rate in MnPO and RVLM neurons in brain slices or in situ (Felix & Schlegel, 1978; Suga & Suzuki, 1979; Tasker & Dudek, 1991; Yang et al., 1992; Armstrong, 1995; Ferguson & Li, 1996; Li & Ferguson, 1996; Bai & Renaud, 1998). PKC inhibition attenuates Ang II-induced decreases in IA and IKv and abolishes Ang II-evoked increases in total Ca2+ current, indicating that Ang II effects on these ion channel currents are either partly or wholly mediated by PKC (Sumners et al., 1996; Wang et al., 1997; Zhu et al., 1999). Studies utilizing anti-PKCα antibodies and PKCα antisense oligonucleotides implicate this isozyme in mediating Ang II-induced changes in IKv (Sumners et al., 2002).
Concurrent inhibition of PKC and CaMKII completely abolished Ang II effect on IKv, indicating that PKC and CaMKII mediate this effect (Zhu et al., 1999). Both PKC and CaMKII also mediate Ang II-induced increases in neuronal firing rate (Sun et al., 2002a,2002b).
In SHR cultures, AT1 receptor activation results in an augmented increase in neuronal firing rate (∼65%) compared to WKY neurons under the same conditions (Sun et al., 2002a, 2002b). This is consistent with enhanced Ang II-induced depolarization and firing of RVLM neurons in neonatal SHR compared to WKY rats (Matsuura et al., 2002). PKC and CaMKII inhibitors completely blocked Ang II-induced firing in WKY neuronal cultures whereas it only attenuated it by ∼50% in SHR cultures, suggesting that an additional signaling pathway mediates the enhanced Ang II neuronal firing response in SHR neurons. Inhibition of PI3K attenuated Ang II-mediated neuronal firing in SHR but not in WKY neurons, indicating that a signaling pathway utilizing PI3K may mediate the augmented Ang II-induced response in SHR neurons (Sun et al., 2002a, 2002b; Figure 1b, highlighted in red).
From the preceding discussion, it is apparent that both AT1 receptor-mediated NA neuromodulation and increases in neuronal firing rate are partly mediated by PI3K in SHR but not WKY neurons. It therefore appears that a signaling pathway involving PI3K may mediate the augmented Ang II-induced NA neuromodulation and neuronal firing in the SHR. PI3K-dependent NA neuromodulation and neuronal firing occurs in neurons from prehypertensive rats and is therefore due to an intrinsic difference in the SHR rather than a compensatory change in response to an increase in BP. The relevance of this pathway in BP regulation, and hence hypertension in this model, was demonstrated by Seyedabadi et al. (2001). Bilateral microinjections of a PI3K inhibitor into the RVLM of adult SHR resulted in an ∼35% decrease in BP, resulting in pressures similar to that in untreated or vehicle-treated WKY rats, but had no effect on BP in WKY rats. The decrease in BP in SHR was gradual (requiring hours), suggesting that PI3K mediates the elevated BP in SHR possibly via transcriptional or translational effects.
The PI3K inhibitor also blunted enhanced Ang II-induced pressor responses in SHR to levels comparable to responses in WKY rats. These studies therefore implicate a PI3K-dependent pathway in mediating the hypertension in SHR possibly via Ang II-evoked increases in neuronal firing and enhanced NA transcriptional neuromodulation.
Perspectives and future directions
In recent years, clear progress has been made in our understanding of the role of the brain RAS in the pathogenesis of hypertension. Genetic models of hypertension, such as the SHR, exhibit a hyperactive endocrine and tissue RAS, making it difficult to distinguish between the relative contributions of the two systems in functional studies. Earlier transgenic models have also been plagued by the same limitation. Recent advances in antisense strategies, in vivo gene transfer and transgenic techniques have enabled the development of models that exhibit brain- or nuclei/area-specific gain or loss of function. Studies utilizing these techniques have helped to clarify the role of specific components of the brain RAS in mediating hypertension. Progress has also been made in understanding the altered cellular and molecular basis for brain RAS dysfunction in hypertension. Studies have demonstrated that augmented Ang II-induced NA neuromodulation and hypertension is uniquely mediated by a PI3K-dependent signaling pathway. One of our challenges is to utilize our understanding of the brain RAS dysfunction in hypertension for the development of improved therapeutic intervention in hypertension. For example, the PI3K-mediated NA neuromodulation may present a potential target for brain/cell type-specific targeted genetic regulation using gene transfer techniques. Another challenge is to identify other genes whose expression is also uniquely linked to the development of hypertension. The development of high-throughput microarray gene profiling technology has enabled the assessment of the expression of a large number of genes within a single experiment. This technology provides tremendous potential for the elucidation of genetic regulation of signaling pathways and identification of target genes that are activated or repressed by the brain RAS. We are currently using this approach to identify genes that encode intracellular signaling proteins and target genes that are regulated by the brain RAS and contribute to the altered responsiveness to Ang II in hypertensive models (Francis et al., 2001; Veerasingham et al., 2002). The combination of microarray gene profiling with validation of the altered expression of identified genes, cellular and in vivo physiological approaches will further our understanding of brain RAS dysfunction in hypertension. Using this approach we have identified a decrease in central γ-adducin expression in models of hypertension that express a hyperactive brain RAS (Yang et al., 2002). Further studies will be required to determine whether this decrease in γ-adducin contributes to the development of hypertension in these models. The identification of genes that are uniquely linked to the development or maintenance of hypertension would provide novel targets of potential therapeutic value for the control, and possibly the cure of hypertension.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute Grant 33610. S.J.V. was supported by a fellowship from the Canadian Institutes of Health Research.
Abbreviations
- ACE
angiotensin converting enzyme
- Ang
angiotensin
- AP1
activating protein 1
- AT1 and AT2
angiotensin type 1 and type 2
- BP
blood pressure
- CaMKII
calcium/calmodulin-dependent protein kinase II
- D-βH
dopamine β-hydroxylase
- DOCA
deoxycorticosterone acetate
- FRK
Fos-regulating kinase
- IA
transient A type K+ current
- i.c.v.
intracerebroventricular
- IKv
delayed rectifier K+ current
- JNK
c-Jun NH2-terminal kinase
- MAP
mitogen-activated protein
- MnPO
median preoptic nucleus
- NA
noradrenaline
- NAT
noradrenaline transporter
- NTS
nucleus tractus solitarius
- PI
phosphoinositide
- PI3K
phosphoinositide-3-kinase
- PKB and PKC
protein kinase B and C
- PLC
phospholipase C
- PVN
paraventricular nucleus
- RAS
renin–angiotensin system
- SFO
subfornical organ
- SHR
spontaneously hypertensive rat(s)
- TGR mRen2
renin transgenic rat(s)
- TH
tyrosine hydroxylase
References
- ALLEN A.M. Blockade of angiotensin AT1 receptors in the rostral ventrolateral medulla of spontaneously hypertensive rats reduces blood pressure and sympathetic nerve discharge. J. Renin–Angiotensin–Aldosterone Syst. 2001;2:S120–S124. doi: 10.1177/14703203010020012101. [DOI] [PubMed] [Google Scholar]
- ALLEN A.M. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- ALLEN A.M., MACGREGOR D.P., MCKINLEY M.J., MENDELSOHN F.A. Angiotensin II receptors in the human brain. Regul. Pept. 1999;79:1–7. doi: 10.1016/s0167-0115(98)00138-4. [DOI] [PubMed] [Google Scholar]
- ANDERSON E.A., SINKEY C.A., LAWTON W.J., MARK A.L. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- ANDRESEN M.C., YANG M. Arterial baroreceptor resetting: contributions of chronic and acute processes. Clin. Exp. Pharmacol. Physiol. 1989;15 Suppl:19–30. doi: 10.1111/j.1440-1681.1989.tb02993.x. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG W.E. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog. Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- ARRIBAS S.M., ALONSO M.J., MARIN J., FERNANDES F., LLERGO J.L., SANCHEZ-FERRER C.F., SALAICES M. Noradrenergic transmission in the tail artery of hypertensive rats transgenic for the mouse renin gene Ren-2. J. Auton. Pharmacol. 1996;16:69–77. doi: 10.1111/j.1474-8673.1996.tb00414.x. [DOI] [PubMed] [Google Scholar]
- AVERILL D.B., DIZ D.I. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res. Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- AVERILL D.B., TSUCHIHASHI T., KHOSLA M.C., FERRARIO C.M. Losartan, non-peptide angiotensin II-type I (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II and L-glutamate in rostral ventrolateral medulla. Brain Res. 1994;665:175–180. doi: 10.1016/0006-8993(94)91344-7. [DOI] [PubMed] [Google Scholar]
- BAI D., RENAUD L.P. ANG II AT1 receptors induce depolarization and inward current in rat median preoptic neurons in vitro. Am. J. Physiol. 1998;275:R632–R639. doi: 10.1152/ajpregu.1998.275.2.R632. [DOI] [PubMed] [Google Scholar]
- BELLIN S.I., BHATNAGAR R.K., JOHNSON A.K. Periventricular noradrenergic systems are critical for angiotensin-induced drinking and blood pressure responses. Brain Res. 1987;403:105–112. doi: 10.1016/0006-8993(87)90128-4. [DOI] [PubMed] [Google Scholar]
- BERECEK K.H., KIRK K.A., NAGAHAMA S., OPARIL S. Sympathetic function in spontaneously hypertensive rats after chronic administration of captopril. Am. J. Physiol. 1987;252:H796–H806. doi: 10.1152/ajpheart.1987.252.4.H796. [DOI] [PubMed] [Google Scholar]
- BLUME A., LEBRUN C.J., HERDEGEN T., BRAVO R., LINZ W., MOLLENHOFF E., UNGER T. Increased brain transcription factor expression by angiotensin in genetic hypertension. Hypertension. 1997;29:592–598. doi: 10.1161/01.hyp.29.2.592. [DOI] [PubMed] [Google Scholar]
- BORGONIO A., PUMMER S., WITTE K., LEMMER B. Reduced baroreflex sensitivity and blunted endogenous nitric oxide synthesis precede the development of hypertension in TGR(mREN2)27 rats. Chronobiol. Int. 2001;18:215–226. doi: 10.1081/cbi-100103187. [DOI] [PubMed] [Google Scholar]
- BOSCAN P., ALLEN A.M, PATON J.F. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the nucleus of the solitary tract. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- CABASSI A., VINCI S., CANTONI A.M., QUARTIERI F., MOSCHINI L., CAVAZZINI S., CAVATORTA A., BORGHETTI A. Sympathetic activation in adipose tissue and skeletal muscle of hypertensive rats. Hypertension. 2002;39:656–661. doi: 10.1161/hy0202.103471. [DOI] [PubMed] [Google Scholar]
- CAMACHO A., PHILLIPS M.I. Separation of drinking and pressor responses to central angiotensin by monoamines. Am. J. Physiol. 1981;240:R106–R113. doi: 10.1152/ajpregu.1981.240.1.R106. [DOI] [PubMed] [Google Scholar]
- CAMPAGNOLE-SANTOS M.J., DIZ D.I., FERRARIO C.M. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension. 1988;11:I167–I171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- CASTO R., PHILLIPS M.I. Neuropeptide action in nucleus tractus solitarius: angiotensin specificity and hypertensive rats. Am. J. Physiol. 1985;249:R341–R347. doi: 10.1152/ajpregu.1985.249.3.R341. [DOI] [PubMed] [Google Scholar]
- CHAVES G.Z., CALIGIORNE S.M., SANTOS R.A., KHOSLA M.C., CAMPAGNOLE-SANTOS M.J. Modulation of the baroreflex control of heart rate by angiotensin (1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J. Hypertens. 2000;18:1841–1848. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- CIRIELLO J., KLINE R.L., ZHANG T.X., CAVERSON M.M. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res. 1984;310:355–359. doi: 10.1016/0006-8993(84)90159-8. [DOI] [PubMed] [Google Scholar]
- CLAUSMEYER S., REINECKE A., FARRENKOPF R., UNGER T., PETERS J. Tissue-specific expression of a rat renin transcript lacking the coding sequence for the prefragment and its stimulation by myocardial infarction. Endocrinology. 2000;141:2963–2970. doi: 10.1210/endo.141.8.7623. [DOI] [PubMed] [Google Scholar]
- COLOMBARI E., SATO M.A., CRAVO S.L., BERGAMACHI C.T., CAMPOS R.R., JR, LOPES O.U. Role of the medulla oblongata in hypertension. Hypertension. 2001;38:549–554. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- COUTO A.S., BALTATU O., SANTOS R.A., GANTEN D., BADER M., CAMPAGNOLE-SANTOS M.J. Differential effects of angiotensin II and angiotensin-(1–7) at the nucleus tractus solitarii of transgenic rats with low brain angiotensinogen. J. Hypertens. 2002;20:919–925. doi: 10.1097/00004872-200205000-00027. [DOI] [PubMed] [Google Scholar]
- CULMAN J., HOHLE S., QADRI F., EDLING O., BLUME A., LEBRUN C., UNGER T. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clin. Exp. Hypertens. 1995;17:281–293. doi: 10.3109/10641969509087071. [DOI] [PubMed] [Google Scholar]
- CURNOW K.M., PASCOE L., WHITE P.C. Genetic analysis of the human type-1 angiotensin II receptor. Mol. Endocrinol. 1992;6:1113–1118. doi: 10.1210/mend.6.7.1508224. [DOI] [PubMed] [Google Scholar]
- DAMPNEY R.A.L., FONTES M.A.P., HIROOKA Y., HORIUCHI J., POTTS P.D., TAGAWA T. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin. Exp. Pharm. Physiol. 2002;29:467–472. doi: 10.1046/j.1440-1681.2002.03658.x. [DOI] [PubMed] [Google Scholar]
- DAVISSON R.L., OLIVERIO M.I., COFFMAN T.M., SIGMUND C.D. Divergent functions of angiotensin II receptor isoforms in the brain. J. Clin. Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WARDENER H. The hypothalamus and hypertension. Physiol. Rev. 2001;81:1559–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- DIZ D.I., JESSUP J.A., WESTWOOD B.M., BOSCH S.M., VINSANT S., GALLAGHER P.E., AVERILL D.B. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin. Exp. Pharm. Physiol. 2002;29:473–482. doi: 10.1046/j.1440-1681.2002.03659.x. [DOI] [PubMed] [Google Scholar]
- ELTON T.S., STEPHAN C.C., TAYLOR G.R., KIMBALL M.G., MARTIN M.M., DURAND J.N., OPARIL S. Isolation of two distinct type I angiotensin II receptor genes. Biochem. Biophys. Res. Commun. 1992;184:1067–1073. doi: 10.1016/0006-291x(92)90700-u. [DOI] [PubMed] [Google Scholar]
- ESLER M.J.G., KORNER P., WILLETT I., DUDLEY F., HASKING G., ANDERSON W., LABERT G. The assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- FABER J.E., BRODY M.J. Central nervous system action of angiotensin during onset of renal hypertension in awake rats. Am. J. Physiol. 1984;247:H349–H360. doi: 10.1152/ajpheart.1984.247.3.H349. [DOI] [PubMed] [Google Scholar]
- FELIX D., SCHLEGEL W. Angiotensin receptive neurones in the subfornical organ. Structure–activity relations. Brain Res. 1978;149:107–116. doi: 10.1016/0006-8993(78)90591-7. [DOI] [PubMed] [Google Scholar]
- FERGUSON A.V., LI Z. Whole cell patch recordings from forebrain slices demonstrate angiotensin II inhibits potassium currents in subfornical organ neurons. Regul. Pept. 1996;66:55–58. doi: 10.1016/0167-0115(96)00049-3. [DOI] [PubMed] [Google Scholar]
- FERGUSON A.V., WASHBURN D.L., LATCHFORD K.J. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp. Biol. Med. 2001;226:85–96. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- FLORAS J. Epinephrine and the genesis of hypertension. Hypertension. 1992;19:1–18. doi: 10.1161/01.hyp.19.1.1. [DOI] [PubMed] [Google Scholar]
- FONTES M.A., PINGE M.C., NAVES V., CAMPAGNOLE-SANTOS M.J., LOPES O.U., KHOSLA M.C., SANTOS R.A. Cardiovascular effects produced by microinjection of angiotensins and angiotensin antagonists into the ventrolateral medulla of freely moving rats. Brain Res. 1997;750:305–310. doi: 10.1016/s0006-8993(96)01476-x. [DOI] [PubMed] [Google Scholar]
- FONTES M.A., SILVA L.C., CAMPAGNOLE-SANTOS M.J., KHOSLA M.C., GUERTZENSTEIN P.G., SANTOS R.A. Evidence that angiotensin-(1–7) plays a role in the central control of blood pressure at the ventro-lateral medulla acting through specific receptors. Brain Res. 1994;665:175–180. doi: 10.1016/0006-8993(94)91171-1. [DOI] [PubMed] [Google Scholar]
- FONTES M.A.P., BALTATU O., CALIGIORNE S.M., CAMPAGNOLE-SANTOS M.J., GANTEN D., BADER M., SANTOS R.A.S. Angiotensin peptides acting at rostral ventrolateral medulla contribute to hypertension of TGR(mREN2)27 rats. Physiol. Genom. 2000;2:137–142. doi: 10.1152/physiolgenomics.2000.2.3.137. [DOI] [PubMed] [Google Scholar]
- FRANCIS S.C., YANG H., VEERASINGHAM S., SUMNERS C., RAIZADA M.K.Differential gene expression profiling in SHR versus WKY brains Hypertension 200138496(Abstract) [Google Scholar]
- GANTEN D., HERMANN K., BAYER C., UNGER T., LANG R.E. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science. 1983;221:869–871. doi: 10.1126/science.6879184. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN D. Plasma norepinephrine in essential hypertension: a study of the studies. Hypertension. 1981;27:520–529. doi: 10.1161/01.hyp.3.1.48. [DOI] [PubMed] [Google Scholar]
- GRASSI G., CATTANEO B.M., SERAVALLE G., LANFRANCHI A., MANCIA G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- GUTKIND J.S., KURIHARA M., CASTREN E., SAAVEDRA J.M. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J. Hypertens. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- GUZZETTI S., PICCALUGA E., CASATI R., CERUTTI S., LOMBARDI F., PAGANI M., MALLIANI A. Sympathetic predominance in essential hypertension: a study employing spectral analysis of heart rate variability. J. Hypertens. 1988;6:711–717. doi: 10.1097/00004872-198809000-00004. [DOI] [PubMed] [Google Scholar]
- HAYASHI J., TAKEDA K., KAWASAKI S., NAKAMURA Y., OGURO M., NAKATA T., TANABE S., LEE L.C., SASAKI S., NAKAGAWA M. Central attenuation of baroreflex by angiotensin II in normotensive and spontaneously hypertensive rats. Am. J. Hypertens. 1988;1:15S–22S. doi: 10.1093/ajh/1.3.15s. [DOI] [PubMed] [Google Scholar]
- HERMANN K., MCDONALD W., UNGER T., LANG R.E., GANTEN D. Angiotensin biosynthesis and concentrations in brain of normotensive and hypertensive rats. J. Physiol. (Paris) 1984;79:471–480. [PubMed] [Google Scholar]
- HIROOKA Y., POTTS P.D., DAMPNEY R.A.L. Role of angiotensin II receptor subtypes in mediating the sympathoexcitatory effects of exogenous and endogenous angiotensin peptides in the rostral ventolateral medulla of the rabbit. Brain Res. 1997;772:107–114. doi: 10.1016/s0006-8993(97)00861-5. [DOI] [PubMed] [Google Scholar]
- HOGARTY D.C., SPEAKMAN E.A., PUIG V., PHILLIPS M.I. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992;586:289–294. doi: 10.1016/0006-8993(92)91638-u. [DOI] [PubMed] [Google Scholar]
- HU L., ZHU D.N., YU Z., WANG J.Q., SUN Z.J., YAO T. Expression of angiotensin II type 1 AT(1) receptor in the rostral ventrolateral medulla in rats. J. Appl. Physiol. 2002;92:2153–2161. doi: 10.1152/japplphysiol.00261.2001. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., LEENEN F.H. Both brain angiotensin II and ‘ouabain' contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension. 1998;32:1028–1033. doi: 10.1161/01.hyp.32.6.1028. [DOI] [PubMed] [Google Scholar]
- HUANG X.C., DENG T., SUMNERS C. Angiotensin II stimulates activation of Fos-regulating kinase and c-Jun NH2-terminal kinase in neuronal cultures from rat brain. Endocrinology. 1998;139:245–251. doi: 10.1210/endo.139.1.5686. [DOI] [PubMed] [Google Scholar]
- HUTCHINSON J.S., MENDELSOHN F.A., DOYLE A.E. Blood pressure responses of conscious normotensive and spontaneously hypertensive rats to intracerebroventricular and peripheral administration of captopril. Hypertension. 1980;2:546–550. doi: 10.1161/01.hyp.2.4.546. [DOI] [PubMed] [Google Scholar]
- INAGAMI T., EGUCHI S. Angiotensin II-mediated vascular smooth muscle cell growth signaling. Braz. J. Med. Biol. Res. 2000;33:619–624. doi: 10.1590/s0100-879x2000000600002. [DOI] [PubMed] [Google Scholar]
- ITAYA Y., SUZUKI H., MATSUKAWA S., KONDO K., SARUTA T. Central renin–angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am. J. Physiol. 1986;251:H261–H268. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- ITO S., KOMATSU K., TSUKAMOTO K., KANMATSUSE K., SVED A.F. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension. 2002;40:552–559. doi: 10.1161/01.hyp.0000033812.99089.92. [DOI] [PubMed] [Google Scholar]
- ITO S., KOMATSU K., TSUKAMOTO K., SVED A.F. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneous hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- ITO S., SVED A.F. Blockade of angiotensin receptors in rat rostral ventrolateral medulla removes excitatory vasomotor tone. Am. J. Physiol. 1996;270:R1317–R1323. doi: 10.1152/ajpregu.1996.270.6.R1317. [DOI] [PubMed] [Google Scholar]
- ITO S., SVED A.F. Pharmacological profile of depressor response elicited by sarthran in rat ventrolateral medulla. Am. J. Physiol. 2000;279:H2961–H2966. doi: 10.1152/ajpheart.2000.279.6.H2961. [DOI] [PubMed] [Google Scholar]
- JENKINS T.A., ALLEN A.M., CHAI S.Y., MACGREGOR D.P., PAXINOS G., MENDELSOHN F.A. Interactions of angiotensin II with central dopamine. Adv. Exp. Med. Biol. 1996;396:93–103. doi: 10.1007/978-1-4899-1376-0_10. [DOI] [PubMed] [Google Scholar]
- JONES D.L. Injections of phentolamine into the anterior hypothalamus-preoptic area of rats blocks both pressor and drinking responses produced by central administration of angiotensin II. Brain Res. Bull. 1984;13:127–133. doi: 10.1016/0361-9230(84)90014-5. [DOI] [PubMed] [Google Scholar]
- KAGIYAMA S., TSUCHIHASHI T., ABE L., MATSUMURA K., FUJISHIMA M. Antisense inhibition of angiotensinogen attenuates vasopressin release in the paraventricular hypothalamic nucleus of spontaneously hypertensive rats. Brain Res. 1999;829:120–124. doi: 10.1016/s0006-8993(99)01375-x. [DOI] [PubMed] [Google Scholar]
- KAGIYAMA S., VARELA A., PHILLIPS M.I., GALLI S.M. Antisense inhibition of brain rennin–angiotensin system decreased blood pressure in chronic 2-kidney, 1 clip hypertensive rats. Hypertension. 2001;37:371–375. doi: 10.1161/01.hyp.37.2.371. [DOI] [PubMed] [Google Scholar]
- KASPAROV S., BUTCHER J.W., PATON J.F.R. Angiotensin II receptors within the nucleus of the solitary tract mediate the developmental attenuation of the baroreceptor vagal reflex in pre-weaned rats. J. Auton. Nerv. Syst. 1998;74:160–168. doi: 10.1016/s0165-1838(98)00149-0. [DOI] [PubMed] [Google Scholar]
- KOMATUS C., SHIBATA K., FURUKAWA T. The developmental increase of the AT1A, but not the AT1B, receptor mRNA level at the preoptic area in spontaneously hypertensive rats. Life Sci. 1996;58:1109–1121. doi: 10.1016/0024-3205(96)00069-0. [DOI] [PubMed] [Google Scholar]
- LANTELME P., CERUTTI C., LO M., PAULTRE C.Z., DUCHER M. Mechanisms of spontaneous baroreflex impairment in lyon hypertensive rats. Am. J. Physiol. 1998;275:R920–R925. doi: 10.1152/ajpregu.1998.275.3.R920. [DOI] [PubMed] [Google Scholar]
- LAZARTIGUES E., DUNLAY S.M., LOIHL A.K., SINNAYAH P., LANG J.A., ESPELUND J.J., SIGMUND C.D., DAVISSON R.L. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cradiovascular sensitivity in transgenic mice. Circ. Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- LEE-KIRSCH M.A., GAUDET F., CARDOSO M.C., LINDPAINTNER K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ. Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- LEENEN F.H., RUZICKA M., HUANG B.S. The brain and salt-sensitive hypertension. Curr. Hypertens. Rep. 2002;4:129–135. doi: 10.1007/s11906-002-0037-y. [DOI] [PubMed] [Google Scholar]
- LENKEI Z., CORVOL P., LLORENS-CORTES C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res. Mol. Brain Res. 1995;30:53–60. doi: 10.1016/0169-328x(94)00272-g. [DOI] [PubMed] [Google Scholar]
- LENKEI Z., PALKOVITS M., CORVOL P., LLORENS-CORTES C. Expression of angiotensin II type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front. Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- LEWIS J.L., SERIKAWA T, WARNOCK D.G. Chromosomal localization of angiotensin II type 1 receptor isoforms in the rat. Biochem. Biophys. Res. Commun. 1993;194:677–682. doi: 10.1006/bbrc.1993.1875. [DOI] [PubMed] [Google Scholar]
- LI Z., FERGUSON A.V. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices. Modulation of IA by angiotensin II. Neuroscience. 1996;71:133–145. doi: 10.1016/0306-4522(95)00434-3. [DOI] [PubMed] [Google Scholar]
- LI Z., IWAI M., WU L., SHIUCHI T., JINNO T., CUI T.-X., HORIUCHI M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am. J. Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- LIN K.S., CHAN J.Y., CHAN S.H.H. Involvement of AT2 receptors at NRVL in tonic baroreflex suppression by endogenous angiotensins. Am. J. Physiol. 1997;272:H2204–H2210. doi: 10.1152/ajpheart.1997.272.5.H2204. [DOI] [PubMed] [Google Scholar]
- LIN K.S., CHAN S.H.H., CHAN J.Y.H. Tonic suppression of spontaneous baroreceptor reflex by endogenous angiotensins via AT2 subtype receptors at nucleus reticularis ventolateralis in the rat. Synapse. 2001;40:85–94. doi: 10.1002/1098-2396(200104)40:1<85::AID-SYN1029>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- LLORENS-CORTES C., MENDELSOHN F.A.O. Organisation and functional role of the brain angiotensin system. J. Renin–Angiotensin–Aldosterone Syst. 2002;3:S39–S48. doi: 10.3317/jraas.2002.029. [DOI] [PubMed] [Google Scholar]
- LU D., YANG H., RAIZADA M.K. Angiotensin II regulation of neuromodulation: downstream signaling mechanism from activation of mitogen-activated protein kinase. J. Cell. Biol. 1996a;135:1609–1617. doi: 10.1083/jcb.135.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU D., YU K., PADDY M.R., ROWLAND N.E., RAIZADA M.K. Regulation of norepinephrine transport system by angiotensin II in neuronal cultures of normotensive and spontaneously hypertensive rat brains. Endocrinology. 1996b;137:763–772. doi: 10.1210/endo.137.2.8593828. [DOI] [PubMed] [Google Scholar]
- LUOH H.F., CHAN S.H. Participation of AT1 and AT2 receptor subtypes in the tonic inhibitory modulation of baroreceptor reflex response by endogenous angiotensins at the nucleus tractus solitarii in the rat. Brain Res. 1998;782:73–82. doi: 10.1016/s0006-8993(97)01198-0. [DOI] [PubMed] [Google Scholar]
- MATSUDA T., SHIBATA K., ABE M., TOMONAGA M., FURUKAWA T. Potentiation of pressor response to angiotensin II at the preoptic area in spontaneously hypertensive rat. Life Sci. 1987;41:749–754. doi: 10.1016/0024-3205(87)90455-3. [DOI] [PubMed] [Google Scholar]
- MATSUKAWA T., GOTOH E., HASEGAWA O., MIYAJIMA E., SHIONOIRI H., TOCHIKUBO O., ISHII M. Reduced arterial baroreflex control of muscle sympathetic nerve activity in young borderline hypertensives. Funct. Neurol. 1991a;6:113–120. [PubMed] [Google Scholar]
- MATSUKAWA T., GOTOH E., HASEGAWA O., SHIONOIRI H., TOCHIKUBO O., ISHII M. Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J. Hypertens. 1991b;9:537–542. doi: 10.1097/00004872-199106000-00009. [DOI] [PubMed] [Google Scholar]
- MATSUMURA K., AVERILL D.B., FERRARIO C.M. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am. J. Physiol. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- MATSUURA T., KUMAGAI H., KAWAI A., ONIMARU H., IMAI M., OSHIMA N., SAKATA K., SARUTA T. Rostral ventrolateral medulla neurons of neonatal Wistar–Kyoto and spontaneouly hypertensive rats. Hypertension. 2002;40:560–565. doi: 10.1161/01.hyp.0000032043.64223.87. [DOI] [PubMed] [Google Scholar]
- MCKINLEY M.J., ALLEN A.M., MATHAI M.L., MAY C., MCALLEN R.M., OLDFIELD B.J., WEISINGER R.S. Brain angiotensin and body fluid homeostasis. Jpn. J. Physiol. 2001;51:281–289. doi: 10.2170/jjphysiol.51.281. [DOI] [PubMed] [Google Scholar]
- MIYAJIMA E., BUNAG R.D. Impaired sympathetic baroreflexes in prehypertensive Dahl hypertension-sensitive rats. Clin. Exp. Hypertens. A. 1986;8:1049–1061. doi: 10.3109/10641968609044085. [DOI] [PubMed] [Google Scholar]
- MORIMOTO S., CASSELL M.D., SIGMUND C.D. Glial- and neuronal-specific expression of the rennin–angiotensin system in brain alters blood pressure, water intake, and salt preference. J. Biol. Chem. 2002;277:33235–33241. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- MORISHITA R., HIGAKI J., NAKAMURA Y., AOKI M., YAMADA K., MORIGUCHI A., RAKUGI H., TOMITA N., TOMITA S., YU H.Effect of an antihypertensive drug on brain angiotensin II levels in renal and spontaneously hypertensive rats Clin. Exp. Pharmacol. Physiol. 199522665–669.et al [DOI] [PubMed] [Google Scholar]
- MURATANI H., AVERILL D.B., FERRARIO C.M. Effect of angiotensin II in ventrolateral medulla of spontaneously hypertensive rats. Am. J. Physiol. 1991;260:R977–R984. doi: 10.1152/ajpregu.1991.260.5.R977. [DOI] [PubMed] [Google Scholar]
- MURPHY T.J., ALEXANDER R.W., GRIENDLING K.K., RUNGE M.S., BERNSTEIN K.E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- NAKAMURA S., MORIGUCHI A., MORISHITA R., YAMADA K., NISHII T., TOMITA N., OHISHI M., KANEDA Y., HIGAKI J., OGIHARA T. Activation of the brain angiotensin system by in vivo human angiotensin-converting enzyme gene transfer in rats. Hypertension. 1999;34:302–308. doi: 10.1161/01.hyp.34.2.302. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y., TAKEDA K., NAKATA T., HAYASHI J., KAWASAKI S., LEE L.C., SASAKI S., NAKAGAWA M., IJICHI H. Central attenuation of aortic baroreceptor reflex in prehypertensive DOCA-salt-loaded rats. Hypertension. 1988;12:259–266. doi: 10.1161/01.hyp.12.3.259. [DOI] [PubMed] [Google Scholar]
- NGUYEN G., DELARUE F., BURCKLE C., BOUZHIR L., GILLER T., SRAER J.-D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBERMULLER N., UNGER T., CULMAN J., GOHLKE P., BOTTARI S.P. Distribution of angiotensin II receptor subtypes in rat brain nuclei. Neurosci. Lett. 1991;132:11–15. doi: 10.1016/0304-3940(91)90420-x. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA D.R., SANTOS R.A., SANTOS G.F., KHOSLA M., CAMPAGNOLE-SANTOS M.J. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension. 1996;27:1284–1290. doi: 10.1161/01.hyp.27.6.1284. [DOI] [PubMed] [Google Scholar]
- PARK C.G., LEENEN F.H. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J. Korean. Med. Sci. 2001;5:553–537. doi: 10.3346/jkms.2001.16.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON J.F., BOSCAN P., MURPHY D., KASPAROV S. Unravelling mechanisms of action of angiotensin II on cardiorespiratory function using in vivo gene transfer. Acta Physiol. Scand. 2001;173:127–137. doi: 10.1046/j.1365-201X.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- PATON J.F., KASPAROV S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii–a microinjection study in the rat. J. Physiol. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS M.I., KIMURA B. Brain angiotensin in the developing spontaneously hypertensive rat. J. Hypertens. 1988;6:607–612. doi: 10.1097/00004872-198808000-00002. [DOI] [PubMed] [Google Scholar]
- PHILLIPS M.I., MOHUCZY-DOMINIAK D., COFFEY M., GALLI S.M., KIMURA B., WU P., ZELLES T. Prolonged reduction of high blood pressure with an in vivo, nonpathogenic, adeno-associated viral vector delivery of AT1-R mRNA antisense. Hypertension. 1997;29:374–380. doi: 10.1161/01.hyp.29.1.374. [DOI] [PubMed] [Google Scholar]
- PHILLIPS M.I., SHEN L., RICHARDS E.M., RAIZADA M.K. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul. Pept. 1993;44:95–107. doi: 10.1016/0167-0115(93)90233-x. [DOI] [PubMed] [Google Scholar]
- PHILLIPS M.I., SUMNERS C. Angiotensin II in central nervous system physiology. Regul. Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- POTTS P.D., ALLEN A.M., HORIUCHI J., DAMPNEY R.A. Does angiotensin II have a significant tonic action on cardiovascular neurons in the rostral and caudal VLM. Am. J. Physiol. 2000;279:R1392–R1402. doi: 10.1152/ajpregu.2000.279.4.R1392. [DOI] [PubMed] [Google Scholar]
- RAIZADA M.K., LU D., TANG W., KURIAN P., SUMNERS C. Increased angiotensin II type-1 receptor gene expression in neuronal cultures from spontaneously hypertensive rats. Endocrinology. 1993;132:1715–1722. doi: 10.1210/endo.132.4.8462471. [DOI] [PubMed] [Google Scholar]
- RAIZADA M.K., LU D., YANG H., RICHARDS E.M., GELBAND C.H., SUMNERS C. Brain angiotensin receptor subtypes and their coupling to distinct signal transduction pathways. Adv. Mol. Cell. Endocrinol. 1999;9:75–101. [Google Scholar]
- RAIZADA M.K., LU D., YANG H., YU K. AT1-receptors and cellular actions of angiotensin II in neuronal cultures of stroke prone-spontaneously hypertensive rat brain. Adv. Exp. Med. Biol. 1996;396:71–78. doi: 10.1007/978-1-4899-1376-0_8. [DOI] [PubMed] [Google Scholar]
- RAIZADA M.K., MUTHER T.F., SUMNERS C. Increased angiotensin II receptors in neuronal cultures from hypertensive rat brain. Am. J. Physiol. 1984;247:C364–C372. doi: 10.1152/ajpcell.1984.247.5.C364. [DOI] [PubMed] [Google Scholar]
- REAUX A., FOURNIE-ZALUSKI M.C., LLOREN-CORTES C. Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol. Metab. 2001;12:157–162. doi: 10.1016/s1043-2760(01)00381-2. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-CAMPOS M., KADARIAN C., RODANO V., BIANCIOTTI L., FERNANDEZ B., VATTA M. AT1 receptor and phospholipase C are involved in angiotensin III modulation of hypothalamic noradrenergic transmission. Cell. Mol. Neurobiol. 2000;20:747–762. doi: 10.1023/a:1007059010571. [DOI] [PubMed] [Google Scholar]
- RUIZ P., BASSO N., CANNATA M.A., TAQUINI A.C. The rennin–angiotensin system in different stages of spontaneous hypertension in the rat (SHR) Clin. Exp. Hypertens. A. 1990;12:63–81. doi: 10.3109/10641969009074720. [DOI] [PubMed] [Google Scholar]
- RUMANTIR M.S., KAYE D.M., JENNINGS G.L., VAZ M., HASTINGS J.A., ESLER M.D. Phenotypic evidence of faulty neuronal norepinephrine reuptake in essential hypertension. Hypertension. 2000;36:824–829. doi: 10.1161/01.hyp.36.5.824. [DOI] [PubMed] [Google Scholar]
- SANDBERG K., JI H., CLARK A.J., SHAPIRA H., CATT K.J. Cloning and expression of a novel angiotensin II receptor subtype. J. Biol. Chem. 1992;267:9455–9458. [PubMed] [Google Scholar]
- SASAMURA H., HEIN L., KREIGER J.E., PRATT R.E., KOBILKA B.K., DZAU V.J. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem. Biophys. Res. Commun. 1992;185:253–259. doi: 10.1016/s0006-291x(05)80983-0. [DOI] [PubMed] [Google Scholar]
- SAYESKI P.P., BERNSTEIN K.E. Signal transduction mechanisms of the angiotensin II type AT(1)-receptor: looking beyond the heterotrimeric G protein paradigm. J. Renin–Angiotensin–Aldosterone Syst. 2001;2:4–10. doi: 10.3317/jraas.2001.007. [DOI] [PubMed] [Google Scholar]
- SCHINKE M., BALTATU O., BOHM M., PETERS J., RASCHER W., BRICCA G., LIPPOLDT A., GANTEN D., BADER M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc. Natl. Acad. Sci. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELTZER A.M., ZORAD S., SAAVEDRA J.M. Stimulation of angiotensin II AT1 receptors in rat median eminence increases phosphoinositide hydrolysis. Brain Res. 1995;705:24–30. doi: 10.1016/0006-8993(95)01100-5. [DOI] [PubMed] [Google Scholar]
- SENANAYAKE P.D., MORIGUCHI A., KUMAGAI H., GANTEN D., FERRARIO C.M., BROSNIHAN K.B. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- SEYEDABADI M., GOODCHILD A.K., PILOWSKY P.M. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38:1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- SHIBATA K., KOMATSU C., MISUMI Y., FURUKAWA T. Developmental differences of angiotensinogen mRNA in the preoptic area between spontaneously hypertensive and age-matched Wistar–Kyoto rats. Mol. Brain Res. 1993;19:115–120. doi: 10.1016/0169-328x(93)90155-i. [DOI] [PubMed] [Google Scholar]
- SINN P.L., SIGMUND C.D. Identification of three human renin mRNA isoforms from alternative tissue-specific transcriptional initiation. Physiol. Genom. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- SMITH J.K., BARRON K.W. GABAergic responses in ventrolateral medulla in spontaneously hypertensive rats. Am. J. Physiol. 1990;258:R450–R456. doi: 10.1152/ajpregu.1990.258.2.R450. [DOI] [PubMed] [Google Scholar]
- SONG K., ALLEN A.M., PAXINOS G., MENDELSOHN F.A.O. Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J. Comp. Neurol. 1992;316:467–484. doi: 10.1002/cne.903160407. [DOI] [PubMed] [Google Scholar]
- STADLER T., VELTMAR A., QADRI F., UNGER T. Angiotensin II evokes noradrenaline release from the paraventricular nucleus in conscious rats. Brain Res. 1992;569:117–122. doi: 10.1016/0006-8993(92)90377-l. [DOI] [PubMed] [Google Scholar]
- SUGA T., SUZUKI M. Effects of angiotensin II on the medullary neurons and their sensitivity to acetylcholine and catecholamines. Jpn. J. Pharmacol. 1979;29:541–552. doi: 10.1254/jjp.29.541. [DOI] [PubMed] [Google Scholar]
- SUMNERS C., FLEEGAL M., ZHU M. Angiotensin AT1 receptor signalling pathways in neurons. Clin. Exp. Pharm. Physiol. 2002;29:483–490. doi: 10.1046/j.1440-1681.2002.03660.x. [DOI] [PubMed] [Google Scholar]
- SUMNERS C., PHILLIPS M.I. Central injection of angiotensin II alters catecholamine activity in rat brain. Am. J. Physiol. 1983;244:R257–R263. doi: 10.1152/ajpregu.1983.244.2.R257. [DOI] [PubMed] [Google Scholar]
- SUMNERS C., ZHU M., GELBAND C.H., POSNER P. Angiotensin II type 1 receptor modulation of neuronal K+ and Ca2+ currents: intracellular mechanisms. Am. J. Physiol. 1996;271:C154–C163. doi: 10.1152/ajpcell.1996.271.1.C154. [DOI] [PubMed] [Google Scholar]
- SUN C., DU J., SUMNERS C., RAIZADA M.K.Phosphatidylinositol 3-kinase mediates enhanced chronotropic action of angiotensin II in the SHR brain neurons Hypertension 2002a. abstract (In press)
- SUN C., SUMNERS C., RAIZADA M.K. Chronotropic action of angiotensin II in neurons via protein kinase C and CaMKII. Hypertension. 2002b;39:562–566. doi: 10.1161/hy0202.103057. [DOI] [PubMed] [Google Scholar]
- SZCZEPANSKA-SADOWSKA E., PACZWA P., LON S., GANTEN D. Increased pressor function of central vasopressinergic system in hypertensive renin transgenic rats. J. Hypertens. 1998;16:1505–1514. doi: 10.1097/00004872-199816100-00016. [DOI] [PubMed] [Google Scholar]
- TAGAWA T., FONTES M.A.P., POTTS P.D., ALLEN A.M., DAMPNEY R.A.L. The physiological role of AT1 receptors in the ventrolateral medulla. Braz. J. Med. Biol. Res. 2000;33:643–652. doi: 10.1590/s0100-879x2000000600005. [DOI] [PubMed] [Google Scholar]
- TAGAWA T., HORIUCHI J., POTTS P.D., DAMPNEY R.A.L. Sympathoinhibition after angiotensin receptor blockade in the rostral ventrolateral medulla is independent of glutamate and GABA receptors. J. Auton. Nerv. Syst. 1999;77:21–30. doi: 10.1016/s0165-1838(99)00026-0. [DOI] [PubMed] [Google Scholar]
- TAKEDA K., BUNAG R.D. Augmented sympathetic nerve activity and pressor responsiveness in DOCA hypertensive rats. Hypertension. 1980;2:97–101. doi: 10.1161/01.hyp.2.1.97. [DOI] [PubMed] [Google Scholar]
- TAKEDA K., NAKATA T., TAKESAKO T., ITOH H., HIRATA M., KAWASAKI S., HAYASHI J., OGURO M., SASAKI S., NAKAGAWA M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991;543:296–300. doi: 10.1016/0006-8993(91)90040-3. [DOI] [PubMed] [Google Scholar]
- TAMURA K., UMEMURA S., NYUI N., YAMAKAWA T., YAMAGUCHI S., ISHIGAMI T., TANAKA S., TANIMOTO K., TAKAGI N., SEKIHARA H., MURAKAMI K., ISHII M. Tissue-specific regulation of angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension. 1996;27:1216–1223. doi: 10.1161/01.hyp.27.6.1216. [DOI] [PubMed] [Google Scholar]
- TASKER J.G., DUDEK F.E. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J. Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERUYA H., MURATANI H., TAKISHITA S., SESOKO S., MATAYOSHI R., FUKIYAMA K. Brain angiotensin II contributes to the development of hypertension in Dahl–Iwai salt-sensitive rats. J. Hypertens. 1995;13:883–890. doi: 10.1097/00004872-199508000-00009. [DOI] [PubMed] [Google Scholar]
- TOUYZ R.M., SCHIFFRIN E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- TSUTSUMI K., SAVEEDRA J.M. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 1991;216:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- VEERASINGHAM S.J., YANG H., FRANCIS S.C., SUMNERS C., RAIZADA M.K.Profiling reveals altered Angiotensin II regulation of gene expression in neurons from pre-hypertensive SHR Hypertension 2002. abstract (In press)
- VELTMAR A., CULMAN J., QADRI F., RASCHER W., UNGER T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J. Pharmacol. Exp. Ther. 1992;263:1253–1260. [PubMed] [Google Scholar]
- WANG D., SUMNERS C., POSNER P., GELBAND C.H. A-type K+ current in neurons cultured from neonatal rat hypo-thalamus and brain stem: modulation by angiotensin II. J. Neuro-physiol. 1997;78:1021–1029. doi: 10.1152/jn.1997.78.2.1021. [DOI] [PubMed] [Google Scholar]
- WEYHENMEYER J.A., PHILLIPS M.I. Angiotensin-like immunoreactivity in the brain of the spontaneously hypertensive rat. Hypertension. 1982;4:514–523. doi: 10.1161/01.hyp.4.4.514. [DOI] [PubMed] [Google Scholar]
- WIELBO D., SERNIA C., GYURKO R., PHILLIPS M.I. Antisense inhibition of hypertension in the spontaneously hypertensive rat. Hypertension. 1995;25:314–319. doi: 10.1161/01.hyp.25.3.314. [DOI] [PubMed] [Google Scholar]
- WRIGHT J.W., SULLIVAN M.J., QUIRK W.S., BATT C.M., HARDING J.W. Heightened blood pressure and drinking responsiveness to intracerebroventricularly applied angiotensins in the spontaneously hypertensive rat. Brain Res. 1987;420:289–294. doi: 10.1016/0006-8993(87)91249-2. [DOI] [PubMed] [Google Scholar]
- YANG C.R., PHILLIPS M.I., RENAUD L.P. Angiotensin II receptor activation depolarizes rat supraoptic neurons in vitro. Am. J. Physiol. 1992a;263:R1333–R1338. doi: 10.1152/ajpregu.1992.263.6.R1333. [DOI] [PubMed] [Google Scholar]
- YANG H., FRANCIS S.C., SELLERS K., DEBARROS M., SUN C., SUMNERS C., FERRARIO C.M., KATOVITCH M.J., MURO A.F., RAIZADA M.K. Hypertension-linked decrease in the expression of brain γ-adducin. Circ. Res. 2002;91:633–639. doi: 10.1161/01.res.0000036749.73316.73. [DOI] [PubMed] [Google Scholar]
- YANG H., LU D., RAIZADA M.K. Angiotensin II-induced phosphorylation of the AT1 receptor from rat brain neurons. Hypertension. 1997a;30:351–357. doi: 10.1161/01.hyp.30.3.351. [DOI] [PubMed] [Google Scholar]
- YANG H., LU D., YU K., RAIZADA M.K. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras-dependent mitogen activated protein kinase pathway. J. Neurosci. 1996;16:4047–4058. doi: 10.1523/JNEUROSCI.16-13-04047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG H., RAIZADA M.K. MAP kinase-independent signaling in angiotensin II regulation of neuromodulation in SHR neurons. Hypertension. 1998;32:473–481. doi: 10.1161/01.hyp.32.3.473. [DOI] [PubMed] [Google Scholar]
- YANG H., RAIZADA M.K. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuro-modulation in brain neurons of the spontaneously hypertensive rat. J. Neurosci. 1999;19:2413–2423. doi: 10.1523/JNEUROSCI.19-07-02413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG H., WANG X., RAIZADA M.K. Characterization of signal transduction pathway in neurotropic action of Angiotensin II in brain neurons. Endocrinology. 2001;142:3502–3511. doi: 10.1210/endo.142.8.8348. [DOI] [PubMed] [Google Scholar]
- YANG R.H., JIN H., WYSS J.M., OPARIL S. Depressor effect of blocking angiotensin subtype 1 receptors in anterior hypothalamus. Hypertension. 1992;19:475–481. doi: 10.1161/01.hyp.19.5.475. [DOI] [PubMed] [Google Scholar]
- YANG S.N., LIPPOLDT A., JANSSON A., PHILLIPS M.I., GANTEN D., FUXE K. Localization of angiotensin II AT1 receptor-like immunoreactivity in catecholaminergic neurons of the rat medulla oblongata. Neuroscience. 1997;81:503–515. doi: 10.1016/s0306-4522(97)00057-2. [DOI] [PubMed] [Google Scholar]
- YU K., LU D., ROWLAND N.E., RAIZADA M.K. Angiotensin II regulation of tyrosine hydroxylase gene expression in the neuronal cultures of normotensive and spontaneously hypertensive rats. Endocrinology. 1996;137:3566–3576. doi: 10.1210/endo.137.8.8754788. [DOI] [PubMed] [Google Scholar]
- ZHU D.N., MORIGUCHI A., MIKAMI H., HIGAKI J., OGIHARA T. Central amino acids mediate cardiovascular response to angiotensin II in the rat. Brain Res. Bull. 1998;45:189–197. doi: 10.1016/s0361-9230(97)00338-9. [DOI] [PubMed] [Google Scholar]
- ZHU M., GELBAND C.H., POSNER P., SUMNERS C. Angiotensin II decreases neuronal delayed rectifier potassium current: role of calcium/calmodulin-dependent protein kinase II. J. Neurophysiol. 1999;82:1560–1568. doi: 10.1152/jn.1999.82.3.1560. [DOI] [PubMed] [Google Scholar]
- ZINI S., FOURNIE-ZALUSKI M.C., CHAUVEL E., ROQUES B.P., CORVOL P., LLORENS-CORTES C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc. Natl. Acad. Sci. 1996;93:11968–11973. doi: 10.1073/pnas.93.21.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]