Abstract
Sulphonated aluminium phthalocyanine (SALPC) photodynamic action induces amylase secretion and permanent calcium oscillation in rat pancreatic acinar cells, because of the activation of phospholipase C or signalling proteins upstream. The aim of the present study was to investigate the involvement of muscarinic acetylcholine and cholecystokinin (CCK) receptors.
Muscarinic receptor antagonist atropine (10 μM) blocked amylase secretion induced by bethanechol (100 μM), and CCK1 receptor antagonist (S)-N-[1-(2-fluorophenyl)-3,4,6,7-tetrahydor-4-oxo-pyrrolo-[3,2,1-jk][1,4] benzodiazepine-3yl]-1H-indole-2-carboxamide (FK480) (1 μM) blocked amylase secretion induced by CCK (100 pM).
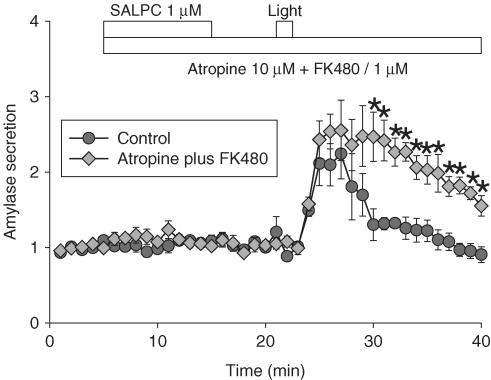
Amylase secretion induced by SALPC photodynamic action was not inhibited when atropine and FK480 were present during photodynamic action. However, addition of FK480 1 μM after initiation of photodynamic action inhibited photodynamic amylase secretion. Bethanechol (10, 100 μM) added after photodynamic action resulted in a full secretory response.
Atropine (10 nM) abolished calcium oscillation induced by bethanechol (5 μM), and FK480 (10 nM) blocked calcium oscillation induced by CCK (10 pM).
Atropine up to 10 μM was without effect on Ca2+ oscillation triggered by photodynamic action, but these oscillations were abolished by FK480 (10 nM). FK480 (10 nM) had no effect on calcium oscillations induced by bethanechol (5 μM). Bethanechol 5 μM, added after FK480 blockade of photodynamic calcium oscillation, still triggered regular calcium oscillation.
It is concluded that SALPC photodynamic action selectively and permanently activates CCK receptor in rat pancreatic acini. Such permanent and selective modulation of signalling proteins has important implications for the treatment of pancreatitis, prion diseases, and neurodegenerative disorders.
Keywords: Amylase secretion, calcium oscillation, CCK receptor, muscarinic receptor, FK480, atropine, SALPC, photodynamic action, pancreatic acinar cell, ligand-independent receptor activation
Introduction
Photodynamic therapy (PDT), or photochemotherapy, is a treatment modality that has been approved in various countries for both cancer and noncancerous lesions (Krammer, 2001; Dougherty, 2002). For optimal therapeutic results, the cellular and molecular basis of PDT has been an intensive area of investigation in recent years (Cui & Matthews, 1998; Oleinick & Evans, 1998; Oleinick et al., 2002).
PDT is largely a type II photodynamic action involving the generation of singlet oxygen; singlet oxygen then triggers different cellular responses (Cui & Matthews, 1998). Previously, it has been found that photodynamic action could trigger smooth muscle contraction (Matthews & Mesler, 1984), stimulate amylase secretion in freshly isolated rat pancreatic acini (Matthews & Cui, 1989, 1990a), inhibit basal amylase secretion in pancreatic tumour cells (Matthews & Cui, 1990b), block degranulation in peritoneal mast cells (Hashikura et al., 2001), and desensitize adrenergic receptors in freshly isolated hepatocytes (Cui et al., 2000). Most significantly, photodynamic action in rat pancreatic acini could trigger calcium oscillations, which were identical to secretagogue-induced or physiological oscillations (Cui & Kanno, 1997; Cui et al., 1997). Detailed investigations indicated that in the freshly isolated rat pancreatic acini, the target of photodynamic action responsible for inducing calcium oscillations was phosphotidylinositol-phospholipase C (PI-PLC) or signalling proteins upstream (Cui & Kanno, 1997). Therefore, the aim of the present study was to investigate in freshly isolated pancreatic acini whether cell surface receptors were directly modulated by photodynamic action leading to calcium oscillations.
Physiological concentrations of endogenous substances are known to induce typical oscillations in cytosolic calcium concentration (Habara & Kanno, 1994; Cui & Guo, 2002). Therefore, calcium oscillations were induced in this study by appropriate concentrations of muscarinic acetylcholine receptor agonist bethanechol, and of cholecystokinin (CCK) receptor agonist CCK octapeptide (CCK8), and by photodynamic action. For comparison, maximal concentrations of bethanechol and CCK were used to induce amylase secretion (Rogers et al., 1988; Murai et al., 2000). A moderate dose of photodynamic action was also used to induce amylase secretion. The effects of muscarinic receptor antagonist atropine and CCK1 receptor antagonist (S)-N-[1-(2-fluorophenyl)-3,4,6,7-tetrahydor-4-oxo-pyrrolo-[3,2,1-jk][1,4] benzodiazepine-3yl]-1H-indole-2-carboxamide (FK480) on secretagogue-induced amylase secretion and calcium oscillations were compared with photodynamically induced amylase secretion and calcium oscillations, to identify the possible involvement of these two major receptors during photodynamic action in the freshly isolated rat pancreatic acini.
Methods
Isolation of rat pancreatic acini
Rat pancreatic acini were isolated according to previous reports (Cui & Kanno, 1997, 2000). Briefly, rat of the Sprague–Dawley strain (150–450 g, from the Institute of Zoology, Academia Sinica or from Vital River Experimental Animals, Beijing) was killed by cervical dislocation. The pancreas was taken out and trimmed off fat, blood vessel, and connective tissue. Collagenase P (0.5 mg ml−1, 5 ml) in standard buffer was infiltrated into the pancreas with a syringe. The tissue was digested at 37°C in a shaking water bath for 3 × 15 min (120 cycles min−1). The digested tissue was then dispersed with a plastic pipette and filtered through a nylon mesh (150 mesh), layered onto buffer containing 4% bovine serum albumin (BSA) and acini clusters gravitated to the bottom. The acini obtained were then washed three times with fresh buffer, and resuspended before use.
The standard buffer used for pancreatic acini isolation had the following composition (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgCl2 1.13, Na2HPO4·2H2O 1.0, D-glucose 5.5, N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) (HEPES) 10, L-glutamine 2.0, bovine serum albumin (BSA) 2 mg ml−1, minimum essential medium (MEM) amino-acid mixture (50 ×) 2%, soybean trypsin inhibitor 0.1 mg ml−1, pH adjusted to 7.4 with NaOH 4 M, and oxygenated for 30 min before use. For pancreatic acini perfusion, the buffer was slightly modified as follows (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgCl2 1.13, Na2HPO4·2H2O 1.0, D-glucose 5.5, HEPES 10, pH adjusted to 7.4 with NaOH 4 M, and oxygenated for 30 min before use.
Assay of amylase secretion
The perfusion chamber was made from a sawn-off syringe (5 ml), at the bottom of which was placed a filter paper (Whatman); on top of the filter paper was placed hydrated Biogel beads (P2, Bio-Rad, 1 cm in height), 1 ml acini suspension was layered onto Biogel beads (Matthews & Cui, 1989). The acini were perfused at a rate of 1 ml min−1 for 15–20 min before measurement of basal amylase secretion. Fractions were collected at 1 fraction min−1; the mean of the first five fractions was taken as 1 and all other fractions normalized to this value. Stimulating chemicals were introduced by directly switching to a buffer containing different stimulating chemicals (Matthews & Cui, 1990a). Amylase was assayed by the reported method, with amylose azure as substrate (Matthews & Cui, 1989, 1990a, 1990b).
Measurement of [Ca2+]i
Pancreatic acini were loaded with Fura-2 AM (with a final concentration of 5 μM) for 30 min, with oxygenation in between. Fura-2 loaded acini were attached to the coverslip of the Sykus-Moore chamber; the coverslip was treated with Cell-Tak before use. [Ca2+]i was measured on the platform of an inverted Olympus fluorescence microscope (IX70) attached to a PTI calcium measurement system (Photon Technology International, New Jersey, U.S.A.), with excitation at 340 nm/380 nm (monochromater M40), and emission at 510 nm (with a band pass filter 510±40 nm); fluorescence ratio F340/F380 was taken as indicative of calcium concentration changes (Cui & Kanno, 1997; Cui & Guo, 2002). The shutter to the photon detector was closed during photodynamic illumination (>580 nm), and all data points during this period took the value of the last data point before light illumination (for Figure 5 and Figure 6). However, we later realized that the red light (>580 nm) did not pass through to the photon detector even when the shutter was open, because of the presence of the band pass emission filter (510±40 nm), so for experiments depicted in Figure 7, normal measurement continued during photodynamic illumination.
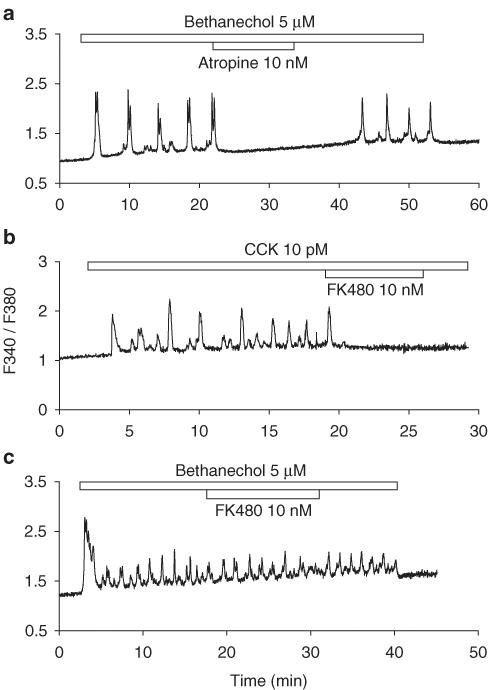
Figure 5.
Blockade of bethanechol- and CCK-induced calcium oscillations by atropine and FK480 respectively. In (a), bethanechol 5 μM and atropine 10 nM were added as indicated by the horizontal bars. In (b), CCK 10 pM and FK480 10 nM were added as indicated by the horizontal bars. In (c), bethanechol 5 μM and FK480 10 nM were added as indicated by the horizontal bars. Note that FK480 had no effect on bethanechol-induced calcium oscillations. Traces (a), (b), and (c) are representative of four, ten, and three identical experiments.
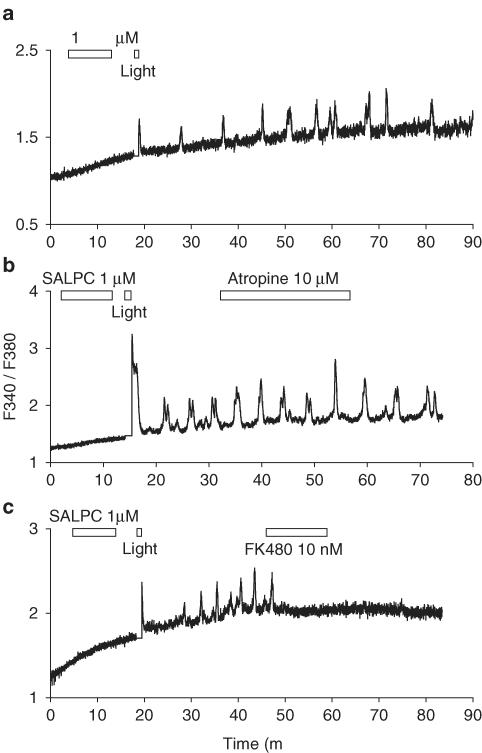
Figure 6.
Photodynamic-action-induced calcium oscillations and the effect of atropine and FK480. In (a), SALPC 1 μM, light (53,000 lx) was delivered as indicated by the horizontal bars. In (b), SALPC 1 μM, light (53,000 lx) and atropine 10 μM were delivered as indicated by the horizontal bars. In (c), SALPC 1 μM, light (53,000 lx) and FK480 10 nM were delivered as indicated by the horizontal bars. Note the complete blockade by FK480 of photodynamically induced calcium oscillations in (c). Traces (a), (b), and (c) are representative of seven, four, and four identical experiments.
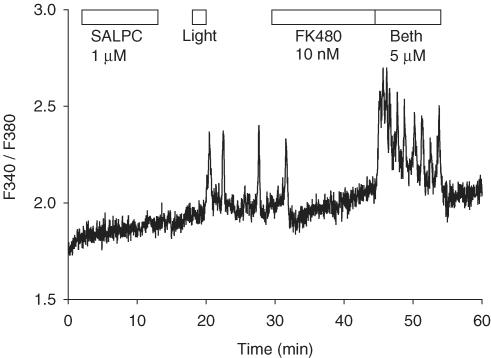
Figure 7.
Addition of bethanechol after SALPC photodynamic action elicited regular calcium oscillations. SALPC 1 μM, light illumination (53,000 lx), FK480 10 nM, and bethanechol 5 μM were delivered as indicated by the horizontal bars. Photodynamic-action-induced calcium oscillations were blocked by the addition of FK480 10 nM. Subsequent addition, after FK480, of bethanechol 5 μM induced regular calcium oscillations. Trace is representative of five identical experiments.
Photodynamic treatment of perfused pancreatic acini
Isolated pancreatic acini were perfused as indicated above, and SALPC 1 μM was applied to the perfusion buffer for a set time of 10 min, before washing off unbound (SALPC) from the perfusion chamber for 5 min. Light from a cold halogen light source (Hoya-Schott, HL100R, Tokyo, Japan), equipped with a condenser (HLL201) and a filter (R60, λ>580 nm), was delivered for 1–1.5 min at an intensity of 53,000 lx, as indicated by the horizontal bars in the figures. This light intensity in terms of irradiance was 7.239 × 10−2 W cm−2 as measured by a radiometer (International Light, model IL1700).
Materials
Cholecystokinin (CCK8), bethanechol, atropine, α-amylase, amylose azure, BSA (fraction V), and soybean trypsin inhibitor were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.). Collagenase P (from Clostridium histolyticum), 4-(2-hydroxyethyl)-1-piperazineethane-sulphonic acid (HEPES) was from Boehringer Mannheim (Mannheim, Germany). Concentrated MEM amino-acid mixture (50 ×) was from GIBCO BRL (Grand Island, NY, U.S.A.). Cell-Tak was from Collaborative Biomedicals (Bedford, MA, U.S.A.). Fura-2 AM was from Molecular Probes (Eugene, OR, U.S.A.). (S)-N-[1-(2-fluorophenyl)-3,4,6,7-tetrahydor-4-oxo-pyrrolo-[3, 2, 1-jk][1,4] benzodiazepine-3yl]-1H-indole-2-carboxamide) FK480 was donated by Fujisawa Pharmaceutical Co. (Osaka, Japan). SALPC was purchased from Porphyrin Products (Salt Lake City, UT, U.S.A.).
Statistical analysis
For statistical analysis, Student's t-test was used, and P<0.05. was taken as statistically significant. All n numbers refer to number of experiments performed in separate animals.
Results
Effects of receptor antagonists on secretagogue-induced amylase secretion
Two major receptor systems dominate in rat pancreatic acini, the muscarinic acetylcholine and CCK1 receptors. The dose–response curve for amylase secretion for both receptors is bell-shaped, maximal stimulating concentration being 100 μM and 100 pM for bethanechol and CCK, respectively (Rogers et al., 1988; Murai et al., 2000). Therefore, these concentrations were used to stimulate amylase secretion.
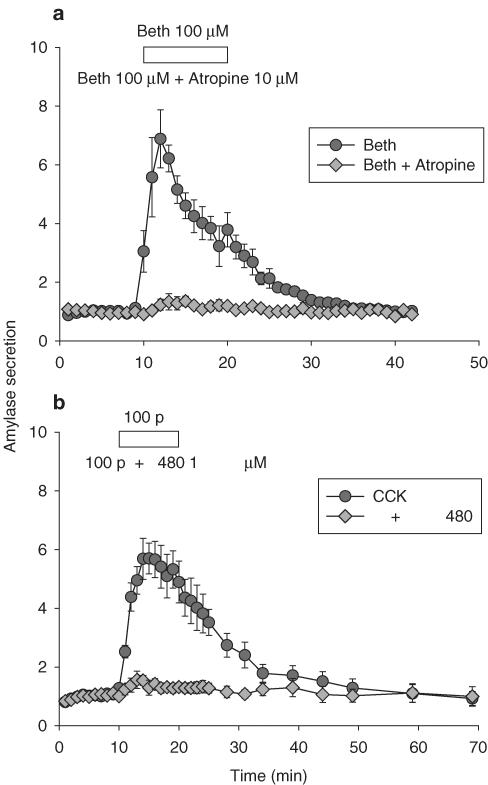
Figure 1a indicates that a 10 min exposure to bethanechol 100 μM induced significant amylase secretion, which reached seven times of basal 4 min after stimulation; secretion rate returned to basal within 15 min (Figure 1a, n=4). Addition of atropine 10 μM at the same time as bethanechol 100 μM completely obliterated bethanechol induced amylase secretion (Figure 1a, n=3).
Figure 1.
Bethanechol- and CCK-stimulated amylase secretion was blocked by atropine and FK480, respectively. Freshly isolated pancreatic acini were perfused. In (a), bethanechol 100 μM (circles, n=4) or bethanechol 100 μM plus atropine 10 μM (diamonds, n=3) was added as indicated by the horizontal bar. In (b), CCK 100 pM (circles, n=4) or CCK plus FK480 1 μM (diamonds, n=4) was added as indicated by the horizontal bar. Data are presented as m±s.e.m. Note the complete blockade of amylase secretion with addition of atropine in (a) and of FK480 in (b). Some standard error bars lie within the symbols.
Similarly, CCK 100 pM in perfused rat pancreatic acini induced significant amylase secretion, with maximal secretion reaching six times of basal. Secretion gradually returned to basal level, reaching basal level 15 min later. When FK 480 1 μM was added at the same time as CCK 100 pM, the amylase secretion observed above was completely obliterated (Figure 1b, n=4).
Effects of receptor antagonists on photodynamically induced amylase secretion
From the above experiments, it is clear that receptor antagonists were effective in blocking maximal receptor-mediated amylase secretion. Therefore, the effect of antagonists on photodynamically induced amylase secretion was examined next. In these experiments, SALPC was incubated with perfused pancreatic acini for 10 min, then unbound SALPC was washed away. Light illumination (53,000 lx, 90 s) 5 min later induced marked amylase secretion (Figure 2, n=3). SALPC addition alone was without effect on amylase secretion, but only when cellularly bound SALPC was activated by light was amylase secretion induced.
Figure 2.
Simultaneous presence of atropine and FK480 did not block photodynamic amylase secretion. SALPC 1 μM, atropine 10 μM plus FK480 1 μM, and light illumination (53,000 lx) were delivered as indicated by the horizontal bars. Photodynamic action induced significant amylase secretion (circles, n=3). The presence of atropine and FK480 from the time of SALPC addition until the end of experiment did not block photodynamic-action-induced amylase secretion (diamonds, n=4), but rather enhanced it (*indicates P<0.05). Data are presented as m±s.e.m. Some standard error bars lie within the symbols.
To investigate the possible involvement of acetylcholine and CCK receptors in photodynamically induced amylase secretion, atropine 10 μM, and FK480 1 μM were added to the perfusion medium at the same time as SALPC until the end of the experiment. When both atropine and FK480 were present, light illumination still induced amylase secretion (Figure 2, n=4). This indicates that the simultaneous presence of atropine and FK480 did not inhibit photodynamically induced amylase secretion. On the contrary, the presence of antagonists seemed to have enhanced photodynamic secretion (P<0.05, from 30 to 40 min). There are two possible explanations for this lack of inhibition (see below also). One would be that FK480 was inactivated by photodynamic action. Alternatively, the presence of FK480 on the CCK receptor enhanced the photodynamic activation of CCK receptor (by a photochemical reaction of FK480 with the receptor), and the subsequent presence of FK480 being unable to replace the already bound FK480 on the CCK receptor, although FK480 was continuously perfused until the end of the experiment. To circumvent such possible photochemical reactions, in subsequent experiments, FK480 was added after photodynamic action. This could be done both for induced amylase secretion and for induced calcium oscillations, although the latter could be done on the same cells, because both secretagogue and photodynamic actions could induce regular calcium oscillations to which many modulators could be subsequently introduced.
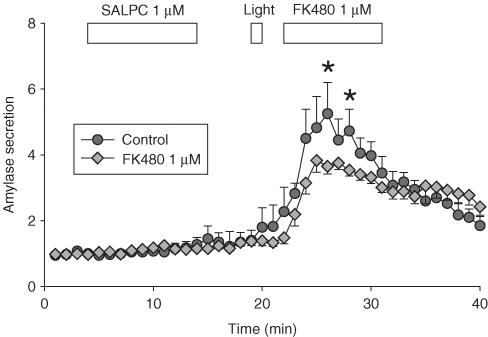
In separate experiments, light was delivered to the perfused pancreatic acini after previously incubating with SALPC as before, followed either by perfusion with buffer alone, or by perfusion with FK480 1 μM 2 min after light (Figure 3). SALPC photodynamic action induced amylase secretion as before (Figure 3, n=4). When FK480 1 μM was added 2 min after light illumination, a marked reduction in amylase secretion was observed (Figure 3, P<0.05, n=13). These data indicated that CCK1 receptor activation was indeed responsible for photodynamic amylase secretion, at least partially.
Figure 3.
Addition of FK480 after initiation of SALPC photodynamic action inhibited photodynamic amylase secretion. SALPC 1 μM, light illumination (53,000 lx), and FK480 1 μM were delivered as indicated by the horizontal bars. Photodynamic action induced significant amylase secretion (circles, n=4), which was inhibited by the addition of FK480 1 μM (diamonds, n=13). Data are presented as m±s.e.m. Some standard error bars lie within the symbols.
SALPC photodynamic action may activate the CCK1 receptor to induce amylase secretion, but whether the overall cellular responsiveness to secretagogue stimulation was affected by photodynamic action was not known. Therefore, in additional experiments, bethanechol was added to the perfused pancreatic acini after SALPC photodynamic secretion had completed. As shown in Figure 4, SALPC photodynamic action induced amylase secretion as expected. Subsequent addition of bethanechol induced significant additional amylase secretion. This bethanechol stimulation after SALPC photodynamic action was also concentration dependent. Bethanechol 10 μM (n=5) stimulated amylase secretion three-fold, whereas at 100 μM (n=6), the stimulation was close to six-fold. Subsequent experiments indicated that the pancreatic acinar cells after SALPC photodynamic action also retained their responsiveness in terms of induction of calcium oscillations (see below).
Figure 4.
Addition of bethanechol after SALPC photodynamic action elicited full amylase secretion. SALPC 1 μM, light illumination (53,000 lx), and bethanechol (10 or 100 μM) were delivered as indicated by the horizontal bars. Photodynamic action induced significant amylase secretion. Addition of bethanechol at 10 μM (circles, n=5) or at 100 μM (diamonds, n=6) induced a concentration dependent increase in amylase secretion. Data are presented as m±s.e.m. Some standard error bars lie within the symbols.
Effects of receptor antagonists on secretagogue-induced calcium oscillations
In perfused rat pancreatic acini, bethanechol 5 μM induced regular calcium oscillations, and these oscillations were completely blocked by atropine 10 nM (Figure 5a, n=4). The atropine effect was easily reversed, with calcium oscillation reappearing after washout of atropine.
Similarly, CCK 10 pM induced regular calcium oscillations in perfused rat pancreatic acini (Figure 5b, n=10). Subsequent addition of FK480 10 nM completely blocked CCK-induced oscillations. The inhibitory effect of FK480 was rather long lasting, which has also been found before (Kihara & Otsuki, 1995; Cui & He, 2002). After FK480 10 nM exposure, normally much higher CCK concentrations (>40 pM) were needed in order for calcium oscillations to reappear quickly (Cui & He, 2002), which indicates tight binding of FK480 to CCK1 receptors.
To exclude the possibility that the long-lasting inhibitory effect of FK480 was because of interference with signalling steps downstream of CCK receptor, the effect of FK480 on bethanechol-induced calcium oscillations was examined. It was obvious from Figure 5c (n=3) that FK480 (10 nM) had no effect on bethanechol 5 μM induced calcium oscillations, confirming that FK480 inhibition was at the site of CCK1 receptor.
Effects of receptor antagonists on photodynamically induced calcium oscillations
As shown in (Figure 6a, n=7), a 10 min incubation of SALPC with perfused rat pancreatic acini and subsequent light illumination (53,000 lx, 1 min) 5 min later induced regular calcium oscillations, which were quite similar to that induced by bethanechol or CCK. There was one notable difference, however; after a brief period of photodynamic action (1 min in this case), calcium oscillations persisted until the end of the experiment (Figure 6a). Therefore, in these experiments, it was possible to add receptor antagonists after photodynamic action, to dissect the involvement of acetylcholine and CCK1 receptors.
Addition of atropine 10 μM did not change calcium oscillations induced by photodynamic action (Figure 6b, n=4). It is interesting to note that atropine at 10 nM was able to block calcium oscillations induced by bethanechol 5 μM (Figure 5), whereas atropine at 1 μM (data not shown) and 10 μM (Figure 6b) was unable to inhibit calcium oscillations induced by photodynamic action. Since atropine 10 μM was able to completely block amylase secretion induced by bethanechol 100 μM (Figure 1a), which was the maximal concentration for inducing amylase secretion, atropine 10 μM should be able to completely block calcium oscillations which would induce only submaximal amylase secretion. Since atropine at 10 nM could effectively block bethanechol-induced calcium oscillations (Figure 5a), whereas at 10 μM unable to block photodynamically induced oscillation (Figure 6b), it may be concluded that acetylcholine receptors were not involved in photodynamically induced calcium oscillations.
In contrast, FK480 10 nM completely blocked calcium oscillations induced by photodynamic action (Figure 6c, n=4), indicating that CCK receptors may be specifically activated by SALPC photodynamic action. Since calcium oscillation disappeared completely after addition of FK480, there was still the possibility that FK480 may be blocking calcium oscillations at steps other than the CCK1 receptor. However, this was effectively ruled out by the fact that addition of the CCK1 receptor antagonist FK480 10 nM did not inhibit calcium oscillations induced by bethanechol 5 μM (Figure 5c). Were the site of FK480 action other than CCK1 receptors, but some other steps in the calcium oscillating machinery instead, FK480 would also have inhibited bethanechol-induced calcium oscillations.
To elucidate whether the pancreatic acinar cells were still functional after photodynamic calcium oscillations were completely blocked by FK480, bethanechol, at a concentration (5 μM) that normally would induce calcium oscillations in previously untreated pancreatic acinar cells, was added. As shown in Figure 7 (n=5), regular calcium oscillations appeared immediately after addition of bethanechol. Similar experiments could not be performed with further addition of CCK after FK480, because FK480 has been shown to have a long-lasting inhibitory effect, which is not easily reversed after wash-off of FK480 (Cui & He, 2002). All the same, this set of experiments indicated that although SALPC photodynamic action preferentially activated CCK1 receptors, the photodynamically treated acinar cells remained fully responsive to muscarinic stimulation, probably also to stimulation by other secretagogues.
Discussion
That photodynamic action may directly stimulate cell signal transduction pathways was first suggested in 1989 (Matthews & Cui, 1989). At that time, it was found that photodynamically induced amylase secretion was not because of plasma membrane permeabilization as indicated by 86Rb efflux and LDH leakage experiments (Matthews & Cui, 1990a, 1990b). Subsequently, it was found that photodynamic action triggered permanent calcium oscillations (Cui & Kanno, 1997; Cui et al., 1997), and it was concluded that PI-PLC or signalling proteins upstream was/were the likely target(s) for photodynamic activation. In the present paper, photodynamically induced calcium oscillations in freshly isolated rat pancreatic acini were reproduced, and it was found that photodynamically induced calcium oscillation was readily blocked by CCK1 receptor antagonist FK480, but the muscarinic acetylcholine receptor antagonist atropine was without effect.
The specificity of atropine on bethanechol stimulation and the specificity of FK480 on CCK stimulation were confirmed by complete blockade of amylase secretion and calcium oscillation induced by bethanechol and CCK, respectively. For amylase secretion, a bethanechol concentration of 100 μM, and a CCK concentration of 100 pM were used, because these were the concentrations that stimulate amylase secretion maximally in the freshly isolated rat pancreatic acini (Rogers et al., 1988; Murai et al., 2000). Atropine 10 μM and FK480 1 μM completely blocked the induced amylase secretion, respectively (Figure 1). For induction of calcium oscillations, much lower concentrations for both bethanechol and CCK were sufficient. Bethanechol 5 μM and CCK 10 pM both induced regular calcium oscillations, which were effectively blocked by atropine 10 nM and FK480 10 nM, respectively (Figure 5a, b). The lack of effect of FK480 10 nM on bethanechol 5 μM induced calcium oscillations (Figure 5c) further confirmed the specificity of FK480 on CCK1 receptors. However, note that FK480 inhibition on [Ca2+]i oscillation was not easily reversed (Figure 5b and Figure 6c), consistent with previous reports (Kihara & Otsuki, 1995; Cui & He, 2002). The complete blockade of photodynamic calcium oscillation by FK480 (Figure 6c), and the lack of effect of atropine (Figure 6b) clearly indicated that the CCK1 receptor was selectively activated by SALPC photodynamic action. The fact that bethanechol 5 μM induced regular calcium oscillations after photodynamic action and subsequent FK480 blockade (Figure 7) indicated that the pancreatic acinar cells remained fully functional after photodynamic action.
In comparison with calcium oscillations, amylase secretion was not sustained, but rather phasic (Figure 1). Therefore, when designing inhibitory experiments with amylase secretion, it was always best to add inhibitors prior to or at the time of stimulation, to ensure that the inhibition could be readily observed. However, in the case of photodynamic action, this did not work. Figure 2 indicated that the simultaneous presence of atropine and FK480 during photodynamic action actually resulted in an enhancement of photodynamic secretion. This may be because of the altered structure of FK480, and probably also some linkage formation with CCK1 receptor, making subsequent addition of FK480 ineffective. The detailed photochemical mechanisms involved warrant further investigation in the future.
When FK480 (1 μM) was added after photodynamic action, a clear inhibition of photodynamic amylase secretion was observed; this inhibition was statistically significant (P<0.05, Figure 3). It was obvious that indeed CCK receptor activation was responsible for photodynamic amylase secretion. Since FK480 at 1 μM completely blocked maximal amylase secretion induced by CCK 100 pM, it was likely that amylase secretion as a result of photodynamic CCK receptor activation was completely blocked by FK480 1 μM. Therefore, the remaining component of amylase secretion must be because of the photodynamic activation of separate plasma membrane mechanisms, the localization of SALPC after only a brief period of incubation being restricted to the plasma membrane (Hubmer et al., 1996; Cui et al., 1997). Similar to the observation with calcium oscillations, addition of bethanechol after photodynamic action resulted in a full secretory response (Figure 4), confirming again that after SALPC photodynamic action, the pancreatic acinar cells remained fully functional.
The existence of a receptor-independent component of amylase secretion induced by photodynamic action at an intensity whereby the corresponding induced calcium oscillation was completely blocked by receptor antagonist (FK480) indicates that photodynamic action may directly modulate the plasma membrane component of the exocytotic machinery, the SNARE assembly.
Direct modulation of the SNARE assembly would override any effect that [Ca2+]i would have on the exocytotic process. Interestingly, SALPC photodynamic action stimulates amylase secretion in normal pancreatic acinar cells (this work; and Matthews & Cui, 1990a), but inhibits amylase secretion in the pancreatic tumour cell line AR4-2J (Matthews & Cui, 1990b) and in rat peritoneal mast cells. Most noteworthy, mast cell exocytosis could be inhibited by SALPC photodynamic action when [Ca2+]i remained high (Hashikura et al., 2001).
One or another isoform of Rab3, a modulator of the SNARE assembly, enhances secretion in pancreatic acinar cells (Ohnishi et al., 1997; Chen et al., 2002), but inhibits secretion in AR4-2J cells (Piiper et al., 2001) and in mast cells (Roa et al., 1997; Pombo et al., 2001; Blank et al., 2002). Dissociation of Rab3 from secretory granules and translocation to the plasma membrane after stimulation of the cells (Tuvim et al., 1999; Valentijn et al., 2000) identify it as a likely target for plasma membrane-localized photodynamically generated singlet oxygen; Rab3 photodynamic activation would lead to inhibition of secretion in AR4-2J and mast cells, but to stimulation in normal pancreatic acinar cells.
Free radicals have also been shown to induce calcium oscillations in rat pancreatic acinar cells (Klonowski-Stumpe et al., 1997). Hydrogen peroxide has been reported to induce calcium oscillations in mouse pancreatic acinar cells (Pariente et al., 2001), in human endothelial cells (Hu et al., 1998), and in rat mesangial cells (Meyer et al., 1996). However, these effects were rather transient. In addition, the sites of action have not been identified; therefore, the specificity of action has not been demonstrated. The present work is the first report of photodynamically induced selective and permanent activation of a cell surface receptor. Specificity may signify a physiological role for singlet oxygen in calcium oscillations or other physiological functions. A short lifetime in cellular milieu (1 μS) and a restricted effective reactive distance (<20 nm) may indeed be some of the qualifying characteristics for singlet oxygen to be an endogenous mediator (Deadwyler et al., 1997; Cui & Matthews, 1998).
At this time, it is not known how singlet oxygen would specifically activate the CCK1 receptor. Certain amino-acid residues are known to be especially susceptible to singlet oxygen modification: His, Cys, Tyr, Met, Trp. His-His and His-Lys crosslinks have also been reported to be formed during photodynamic action (Shen et al., 1996). For the latter case, SALPC photodynamic desensitization of adrenergic receptors may be a case in point. It was suggested that His–His, His–Lys crosslinks formed between transmembrane domains in the same receptor or between different receptors would confer spatial restraints on the receptor; therefore receptor activation was inhibited (Cui et al., 2000). This may be similar to the inhibitory effect of disulphide bond formation on muscarinic receptor activation (Zeng et al., 1999).
Met121 (third transmembrane domain), Met195 (second extracellular loop) (Gigoux et al., 1998; Escrieut et al., 2002), Trp 39(N-terminal domain) and Tyr338 (third extracellular loop), residues important for CCK1 receptor activation (Pellegrini & Mierke, 1999; Giragossian & Mierke, 2001), all present ready targets for singlet oxygen attack and receptor activation. The seventh transmembrane domain being mainly involved in nonpeptidyl antagonist binding (Mantamadiotis & Baldwin, 1994; Ding & Miller, 2001) will explain why photodynamic activation of CCK1 receptor still leaves it susceptible to FK480 inhibition.
Other than a structural basis for differential activation of CCK1 and muscarinic receptors, it is noteworthy that CCK1 and acetylcholine receptors have distinct signalling pathways for calcium mobilization in regard to the involvement of cyclic ADP ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (Cancela et al., 2000, 2002; Cancela, 2001; Ashby et al., 2002). NAADP and cADPR were found to be involved in CCK-induced calcium oscillations, but not essential for ACh-induced oscillations (Cancela et al., 2000, 2002). Impressively, localized apical UV photon uncaging of caged Ca2+ in the presence of subthreshold CCK leads to calcium oscillations (Ashby et al., 2002), but removal of CCK results in immediate cessation of the oscillation (Ashby et al., 2002), which is different from the permanent photodynamic oscillation (this work; and Cui & Kanno, 1997). Additionally, it has been found that a full coordination of IP3, cADPR, and NAADP is required for the globalization of local calcium spikes (Cancela et al., 2002). These findings implicate that the spatial aspect of photodynamic oscillation and the involvement of cADPR and NAADP should be further investigated in the future.
In summary, in the freshly isolated rat pancreatic acini, SALPC photodynamic action induced persistent calcium oscillations, because of selective activation of CCK1 receptor. SALPC photodynamic action may do so by modification of certain amino-acid residues susceptible to singlet oxygen modification in the extracellular loops or in the transmembrane domains. Photodynamic action can, permanently and selectively, activate cell surface receptors; this ligand-independent receptor activation has important pharmacological implications, for the treatment of pancreatitis, for example. Selective protein modulation may also have implications for the treatment of prion diseases and neurodegenerative disorders.
Acknowledgments
This work was supported by grants from The Natural Science Foundation of China (NSFC, No. 39825112, 30070286). We thank Fujisawa Pharmaceutical Co. Ltd (Osaka, Japan) for providing FK480.
Abbreviations
- BSA
bovine serum albumin
- cADPR
cyclic ADP ribose
- CCK
cholecystokinin
- NAADP
nicotinic acid adenine dinucleotide phosphate
- PDT
photodynamic therapy
- PI-PLC
phosphotidylinositol-phospholipase C
- SALPC
sulphonated aluminium phthalocyanine
References
- ASHBY M.C., CRASKE M., PARK M.K., GERASIMENKO O.V., BURGOYNE R.D., PETERSEN O.H., TEPIKIN A.V. Localized Ca2+ uncaging reveals polarized distribution of Ca2+-sensitive Ca2+ release sites: mechanism of unidirectional Ca2+ waves. J. Cell. Biol. 2002;158:283–292. doi: 10.1083/jcb.200112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANK U., CYPRIEN B., MARTIN-VERDEAUX S., PAUMET F., POMBO I., RIVERA J., ROA M., VARIN-BLANK N. SNAREs and associated regulators in the control of exocytosis in the RBL-2H3 mast cell line. Mol. Immunol. 2002;38:1341–1345. doi: 10.1016/s0161-5890(02)00085-8. [DOI] [PubMed] [Google Scholar]
- CANCELA J.M. Specific Ca2+ signaling evoked by cholecystokinin and acetylcholine: the roles of NAADP, cADPR, and IP3. Annu. Rev. Physiol. 2001;63:99–117. doi: 10.1146/annurev.physiol.63.1.99. [DOI] [PubMed] [Google Scholar]
- CANCELA J.M., GERASIMENKO O.V., GERASIMENKO J.V., TEPIKIN A.V., PETERSEN O.H. Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J. 2000;19:2549–2557. doi: 10.1093/emboj/19.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANCELA J.M., VAN COPPENOLLE F., GALIONE A., TEPIKIN A.V., PETERSEN O.H. Transformation of local Ca2+ spikes to global Ca2+ transients: the combinatorial roles of multiple Ca2+ releasing messengers. EMBO J. 2002;21:909–919. doi: 10.1093/emboj/21.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X., EDWARDS J.A., LOGSDON C.D., ERNST S.A., WILLIAMS J.A. Dominant negative Rab3D inhibits amylase release from mouse pancreatic acini. J. Biol. Chem. 2002;277:18002–18009. doi: 10.1074/jbc.M201248200. [DOI] [PubMed] [Google Scholar]
- CUI Z.J., GUO L.L. Assessing physiological concentrations of endogenous substances in situ by inducing calcium oscillations in vitro. Case of liver. Acta Pharmacol. Sin. 2002;23:27–32. [PubMed] [Google Scholar]
- CUI Z.J., HABARA Y., Satoh Y. Photodynamic modulation of adrenergic receptors in the isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 2000;277:705–710. doi: 10.1006/bbrc.2000.3742. [DOI] [PubMed] [Google Scholar]
- CUI Z.J., HABARA Y., WANG D.Y., KANNO T. A novel aspect of photodynamic action: induction of recurrent spikes in cytosolic calcium concentration. Photochem. Photobiol. 1997;65:382–386. doi: 10.1111/j.1751-1097.1997.tb08574.x. [DOI] [PubMed] [Google Scholar]
- CUI Z.J., HE X.H. The pre-synaptic blocker toosendanin does not inhibit secretion in exocrine cells. World J. Gastroenterol. 2002;8:918–922. doi: 10.3748/wjg.v8.i5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI Z.J., KANNO T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J. Physiol. 1997;504:47–55. doi: 10.1111/j.1469-7793.1997.047bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI Z.J., KANNO T. Cholecystokinin analog JMV-180-induced intracellular calcium oscillations are mediated by inositol 1,4,5-trisphosphate in rat pancreatic acini. Acta. Pharmacol. Sin. 2000;21:377–380. [PubMed] [Google Scholar]
- CUI Z.J., MATTHEWS E.K. Photodynamic modulation of cellular function. Acta. Pharmacol. Sin. 1998;19:297–303. [PubMed] [Google Scholar]
- DEADWYLER G., SIMA P.D., FU Y., KANOFSKY J.R. Singlet oxygen-mediated inactivation of acetylcholinesterase: a comparison of purified enzyme in solution and enzyme bound to K562 leukemia cells. Photochem. Photobiol. 1997;65:884–894. doi: 10.1111/j.1751-1097.1997.tb01939.x. [DOI] [PubMed] [Google Scholar]
- DING X.Q., MILLER L.J. Characterization of the type A cholecystokinin receptor hormone-binding domain: use of contrasting and complementary methodologies. Peptides. 2001;22:1223–1228. doi: 10.1016/s0196-9781(01)00445-4. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T.J. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- ESCRIEUT C., GIGOUX V., ARCHER E., VERRIER S., MAIGRET B., BEHRENDT R., MORODER L., BIGNON E, SILVENTE-POIROT S., PRADAYROL L., FOURMY D. The biologically crucial C terminus of cholecystokinin and the non-peptide agonist SR-146, 131 share a common binding site in the human CCK1 receptor. Evidence for a crucial role of Met-121 in the activation process. J. Biol. Chem. 2002;277:7546–7555. doi: 10.1074/jbc.M108563200. [DOI] [PubMed] [Google Scholar]
- GIGOUX V., ESCRIEUT C., SILVENTE-POIROT S., MAIGRET B., GOUILLEUX L., FEHRENTZ J.A., GULLY D., MORODER L., VAYSSE N., FOURMY D. Met-195 of the cholecystokinin-A receptor interacts with the sulfated tyrosine of cholecystokinin and is crucial for receptor transition to high affinity state. J. Biol. Chem. 1998;273:14380–14386. doi: 10.1074/jbc.273.23.14380. [DOI] [PubMed] [Google Scholar]
- GIRAGOSSIAN C., MIERKE D.F. Intermolecular interactions between cholecystokinin-8 and the third extracellular loop of the cholecystokinin A receptor. Biochemistry. 2001;40:3804–3809. doi: 10.1021/bi002659n. [DOI] [PubMed] [Google Scholar]
- HABARA Y., KANNO T. Stimulus–secretion coupling and Ca2+ dynamics in pancreatic acinar cells. Gen. Pharmacol. 1994;25:843–850. doi: 10.1016/0306-3623(94)90085-x. [DOI] [PubMed] [Google Scholar]
- HASHIKURA S., SATOH Y., CUI Z.J., HABARA Y. Photodynamic action inhibits compound 48/80-induced exocytosis in rat peritoneal mast cells. Jpn. J. Vet. Res. 2001;49:239–247. [PubMed] [Google Scholar]
- HU Q.H., CORDA S., ZWEIER J.L., Capogrossi M.C., ZIEGELSTEIN R.C. Hydrogen peroxide induces intracellular calcium oscillations in human aortic endothelial cells. Circulation. 1998;97:269–275. doi: 10.1161/01.cir.97.3.268. [DOI] [PubMed] [Google Scholar]
- HUBMER A., HERMANN A., UBERRIEGLER K., KRAMMER B. Role of calcium in photodynamically induced cell damage of human fibroblasts. Photochem. Photobiol. 1996;64:211–215. doi: 10.1111/j.1751-1097.1996.tb02444.x. [DOI] [PubMed] [Google Scholar]
- KIHARA Y., OTSUKI M. Different inhibitory effects of the newly developed CCK receptor antagonists FK480 and KSG-504 on pancreatic exocrine and endocrine secretion in the isolated perfused rat pancreas. Pancreas. 1995;10:109–117. doi: 10.1097/00006676-199503000-00001. [DOI] [PubMed] [Google Scholar]
- KLONOWSKI-STUMPE H., SCHREIBER R., GROLIK M., SCHULZ H.U., HAUSSINGER D., NIEDERAU C. Effect of oxidative stress on cellular functions and cytosolic free calcium of rat pancreatic acinar cells. Am. J. Physiol. 1997;272:G1489–G1498. doi: 10.1152/ajpgi.1997.272.6.G1489. [DOI] [PubMed] [Google Scholar]
- KRAMMER B. Vascular effects of photodynamic therapy. Anticancer Res. 2001;21:4271–4277. [PubMed] [Google Scholar]
- MANTAMADIOTIS T., BALDWIN G.S. The seventh trans-membrane domain of gastrin/CCK receptors contributes to non-peptide antagonist binding. Biochem. Biophys. Res. Commun. 1994;201:1382–1389. doi: 10.1006/bbrc.1994.1856. [DOI] [PubMed] [Google Scholar]
- MATTHEWS E.K., CUI Z.J. Photodynamic action of rose bengal on isolated rat pancreatic acini: stimulation of amylase release. FEBS Lett. 1989;256:29–32. doi: 10.1016/0014-5793(89)81711-9. [DOI] [PubMed] [Google Scholar]
- MATTHEWS E.K., CUI Z.J. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on isolated rat pancreatic acini. Biochem. Pharmacol. 1990a;39:1445–1457. doi: 10.1016/0006-2952(90)90426-l. [DOI] [PubMed] [Google Scholar]
- MATTHEWS E.K., CUI Z.J. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on AR4-2J cells, a carcinoma cell line of rat exocrine pancreas. Br. J. Cancer. 1990b;61:695–701. doi: 10.1038/bjc.1990.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS E.K., MESLER D.E. Photodynamic effects of erythrosine on smooth muscle cells of guinea-pig taenia coli. Br. J. Pharmacol. 1984;83:555–566. doi: 10.1111/j.1476-5381.1984.tb16520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER T.N., GLOY J., HUG M.J., GREGER R., SCHOLLMEYER P., PAVENSTADT H. Hydrogen peroxide increases the intracellular calcium activity in rat mesangial cells in primary culture. Kidney Int. 1996;49:388–395. doi: 10.1038/ki.1996.57. [DOI] [PubMed] [Google Scholar]
- MURAI A., SATOH S., OKUMURA J., FURUSE M. Factors regulating amylase secretion from chicken pancreatic acini in vitro. Life Sci. 2000;66:585–591. doi: 10.1016/s0024-3205(99)00631-1. [DOI] [PubMed] [Google Scholar]
- OHNISHI H., SAMUELSON L.C., YULE D.I., ERNST S.A., WILLIAMS J.A. Over-expression of Rab3D enhances regulated amylase secretion from pancreatic acini of transgenic mice. J. Clin. Invest. 1997;100:3044–3052. doi: 10.1172/JCI119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLEINICK N.L., EVANS H.H. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat. Res. 1998;150:S146–S156. [PubMed] [Google Scholar]
- OLEINICK N.L., MORRIS M.L., BELICHENKO I. The role of apoptosis in response to photodynamic therapy: what, where, why and how. Photochem. Photobiol. Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- PARIENTE J.A., CAMELLO C., CAMELLO P.J., SALIDO G.M. Release of calcium from mitochondrial and non-mitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J. Membr. Biol. 2001;179:27–35. doi: 10.1007/s002320010034. [DOI] [PubMed] [Google Scholar]
- PELLEGRINI M., MIERKE D.F. Molecular complex of cholecystokinin-8 and N-terminus of the cholecystokinin A receptor by NMR spectroscopy. Biochemistry. 1999;38:14775–14783. doi: 10.1021/bi991272l. [DOI] [PubMed] [Google Scholar]
- PIIPER A., LESER J., LUTZ M.P., BEIL M., ZEUZEM S. Subcellular distribution and function of Rab3A-D in pancreatic acinar AR42J cells. Biochem. Biophys. Res. Commun. 2001;287:746–751. doi: 10.1006/bbrc.2001.5651. [DOI] [PubMed] [Google Scholar]
- POMBO I., MARTIN-VERDEAUX S., IANNASCOLI B., Le Mao J., DERIANO L., RIVERA J., BLANK U. IgE receptor type I-dependent regulation of a Rab3D-associated kinase: a possible link in the calcium-dependent assembly of SNARE complexes. J. Biol. Chem. 2001;276:42893–42900. doi: 10.1074/jbc.M103527200. [DOI] [PubMed] [Google Scholar]
- ROA M., PAUMET F., LE MAO J., DAVID B., BLANK U. Involvement of the ras-like GTPase rab3d in RBL-2H3 mast cell exocytosis following stimulation via high affinity IgE receptors (Fc epsilonRI) J. Immunol. 1997;159:2815–2823. [PubMed] [Google Scholar]
- ROGERS J., HUGHES R.G., MATTHEWS E.K. Cyclic GMP inhibits protein kinase C-mediated secretion in rat pancreatic acini. J. Biol. Chem. 1988;263:3713–3719. [PubMed] [Google Scholar]
- SHEN H.R., SPIKES J.D., KOPECKOVA P., KOPECEK J. Photodynamic crosslinking of proteins. II. Photocrosslinking of a model protein–ribonuclease A. J. Photochem. Photobiol. B. Biol. 1996;35:213–219. doi: 10.1016/s1011-1344(96)07300-9. [DOI] [PubMed] [Google Scholar]
- TUVIM M.J., ADACHI R., CHOCANO J.F., MOORE R.H., LAMPERT R.M., ZERA E., ROMERO E., KNOLL B.J., DICKEY B.F. Rab3D, a small GTPase, is localized on mast cell secretory granules and translocates to the plasma membrane upon exocytosis. Am. J. Respir. Cell Mol. Biol. 1999;20:79–89. doi: 10.1165/ajrcmb.20.1.3279. [DOI] [PubMed] [Google Scholar]
- VALENTIJN J.A., VALENTIJN K., PASTORE L.M., JAMIESON J.D. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZENG F.Y., HOPP A., SOLDNER A., WESS J. Use of a disulfide cross-linking strategy to study muscarinic receptor structure and mechanisms of activation. J. Biol. Chem. 1999;274:16629–16640. doi: 10.1074/jbc.274.23.16629. [DOI] [PubMed] [Google Scholar]