Abstract
Activation of the hypothalamic–pituitary–adrenal axis by fasting seems to involve cholecystokinin (CCK) receptors.
This work aims to characterize the role of endogenous CCK in the activity of the paraventricular nucleus (PVN) of the hypothalamus during food withdrawal. We investigated, by c-Fos immunohistochemistry, the effect of CCK1 and CCK2 receptor antagonists (SR-27,897 and L-365,260, respectively) on c-Fos levels expression induced by food deprivation.
Under our conditions, the number of cells expressing c-Fos was reduced both by SR-27,897 and L-365,260 in food-deprived rats.
To investigate the importance of glucose availability, we studied the effect of CCK receptor antagonists on c-Fos synthesis induced by the glucose antimetabolite 2-deoxyglucose. In these animals, only SR-27,897 decreased c-Fos expression in the PVN.
Our results indicate that the effect of CCK antagonists is mainly perceptible when glucose availability decreases, and suggest that CCK-ergic inputs could drive the activity of the PVN under fasting/low glucose conditions.
Keywords: Cholecystokinin, fasting, c-Fos, hypothalamus, feeding, paraventricular nucleus, hypothalamic–pituitary–adrenal axis, 2-deoxyglucose
Introduction
Food intake, in addition to the circadian cycle light/darkness and stress, is an important factor in regulating the activity of the hypothalamic–pituitary–adrenal (HPA) axis. Acute fasting in rats during the dark period, which corresponds to the peak of food intake in rodents, leads to a significant increase of both adrenocorticotropin (ACTH) hormone and corticosterone plasma level (Akana et al., 1994). The activation of the HPA axis seems to be aimed at increasing glucose availability (Baxter & Forshman, 1972). In fact, a significant decrease of glucose plasma concentration is an early metabolic response that occurs during starvation (Dallman et al., 1999). Such a fall in glucose level can be detected by hypothalamic neurons responsive to glucose (Oomura et al., 1969; Spanswick et al., 1997,2000), and which are involved in modulating the activity of the HPA axis (Schwartz et al., 1996). The importance of glucose in regulating HPA activity has been stressed in a recent work showing that the increase in the mRNA of the corticotropin-releasing hormone (CRH) that occurs in the hypothalamic paraventricular nucleus (PVN) in adrenalectomized rats is prevented by a high-glucose diet (Laugero et al., 2001).
The hypothalamus is a relay area that integrates both peripheral metabolic signals and neuroendocrine responses to experimental manipulations of feeding behavior. Most neurotransmitters that regulate feeding also modulate the activity of the HPA axis (Kalra et al., 1999). In this context, we were interested in better characterizing the eventual role of endogenous cholecystokinin (CCK) in the regulation of the HPA axis. CCK is a gastrointestinal hormone released postprandially, which acts as a peripheral signal of satiety, and also a neurotransmitter synthesized in the central nervous system (CNS), where it seems to be also involved in food-intake regulation (Crawley & Corwin, 1994). CCK binds two different receptors: the CCK1 receptor (CCK1R), which is located in peripheral organs and in some regions of the CNS, such as the nucleus tractus solitarius (NTS) and the hypothalamus, and the CCK2R, mainly located in the CNS (Noble et al., 1999).
In a previous work focusing on the effect of CCKR antagonists on the activity of the HPA axis, we have shown that CCK1R antagonists prevent the increase of both ACTH and corticosterone that occurs during acute fasting in rats, but that they do not modify the basal release of these hormones (Ruiz-Gayo et al., 2000). This finding suggests that endogenous CCK could be involved in the activation of the HPA axis under conditions of metabolic stress. Since exogenous CCK has been shown to stimulate CRH release in rat hypothalamus (Kamilaris et al., 1992), our hypothesis is that, under fasting, endogenous CCK might stimulate hypothalamic sites to drive the activity of the HPA axis. The aim of the present study was to characterize the effect of both CCK1R and CCK2R antagonists on the activation of different brain areas induced by food deprivation. To address this issue, rats fasted during the dark phase were treated with CCK1R or CCK2R antagonists. Then, we examined the effect of both food deprivation and treatment with CCKR antagonists on c-Fos expression in the PVN of the hypothalamus as well as in NTS. These nuclei, which are responsive to CCK, are involved in regulating food intake as well as the activity of the HPA axis (Raybould et al., 1988; Schick et al., 1990). The PVN contains neuronal cell bodies that express both CCK1R and CCK2R mRNAs (Honda et al., 1993; Hinks et al., 1995) and all subdivisions of the PVN possess neurons that are immunopositive for both subtypes of CCKRs (Mercer, 2000; Mercer et al., 2000). To further investigate the role of glucose on the activity of the PVN, we characterized the effect of CCKR antagonists on fed rats treated with the glucose antimetabolite, 2-deoxyglucose (2-DOG).
Methods
Animals
Adult male Wistar rats (200–230 g) were housed, four per cage, under a light/dark cycle (12 h/12 h) in a temperature-controlled room (22°C), with standard chow (Panlab, Spain) and water available ad libitum. Animals were handled daily, for at least 1 week, to avoid stress by manipulation on the day of the experiment. Fasted rats were housed in the same room as the fed animals. All experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC) for the care and use of laboratory animals.
Chemicals
1-[[2-(4-(2-chlorophenyl)thiazol-2-yl)aminocarbonyl]-indolyl]-acetic acid (SR-27,897) was kindly provided by Sanofi Synthélabo (France) (Poncelet et al., 1993). (3R)-(+)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl)-3-methylphenylurea (L-365,260) was a gift of MSD (U.S.A.) (Chang et al., 1989). Other chemicals and solvents were from Sigma (U.S.A.).
Treatment
The variables of the study were food availability and pharmacological treatment. For fasting, food was withdrawn 120 min before the lights were off. At this time, CCKR antagonists were administered. The CCK1R antagonist SR-27,897 was administered, s.c., at doses of 0.03, 0.3 and 1 mg kg−1. The CCK2R antagonist L-365,260 was given s.c. at 0.1, 1 and 10 mg kg−1. The vehicle was 4% carboxymethylcellulose.
In experiments with 2-DOG, this drug was administered in saline to the fed animals, i.p., at the dose of 600 mg kg−1, 60 min before the lights were off and 60 min after the administration of vehicle, SR-27,897 (0.3 mg kg−1) or L-365,260 (1 mg kg−1).
Rats were euthanized 210 min after CCKR antagonist administration. The gastric content in food-deprived rats was about 50% of the content found in ad libitum animals.
Tissue preparation and immunohistochemical procedures
Rats were anesthetized with urethane 210 min after CCKR antagonist administration and immediately perfused transcardially with 300 ml saline, followed by 300 ml 4% paraformaldehyde. After perfusion, brains were removed and postfixed overnight in 4% paraformaldehyde. Coronal sections (40 m) containing hypothalamic PVN (bregma between −1.60 and −1.88), or NTS (bregma between −13.68 and −14.30), were obtained on a Vibratome (Leica 1000 M).
Free-floating sections were bathed in 60% methanol containing 0.3% H2O2 for 30 min to block the endogenous peroxidase activity. Sections were rinsed 3 × 5 and 1 × 10 min in 0.1 M phosphate-buffered saline (PBS) pH 7.4, and then 1 × 10 min in PBS containing 0.1% Triton X-100 (PBS-Triton). Sections were preincubated 1 × 30 min in PBS-Triton containing 5% normal goat serum (PBS-Triton-NS). Anti-c-Fos rabbit antiserum (Calbiochem, U.S.A.) was added at a final dilution of 1 : 5000, and incubated overnight at 4°C. The next day, sections were washed with PBS (3 × 5 and 1 × 10 min) and incubated with goat anti-rabbit secondary antiserum (Vector, U.S.A.) diluted in PBS 1 : 200 for 2 h. Sections were rinsed in PBS (3 × 5 and 1 × 10 min) and incubated with the avidin–biotin–peroxidase complex (ABC kit, Vector, U.S.A.). After washing with 0.05 M Tris-HCl (pH 7.4), sections were developed with 3,3′-diaminobenzidine (Vector, U.S.A.), then mounted and coverslipped with DPX (Aldrich, U.S.A.). Counting was performed through a × 20 air objective by using a Leika DMLS microscope. For each animal, the number of c-Fos-stained cells, either in PVN or NTS, was an average value from 4–6 sections, taken between the coordinates indicated above. For counting cells in the parvocellular area of the PVN, a region of approx. 300 μm from the midline was considered (Paxinos & Watson, 1986). Cell counts were made randomly by two individuals. The specificity of c-Fos antiserum was checked by using the corresponding blocking peptide (Calbiochem, U.S.A.) (data not shown).
Plasma sample analysis
Plasma concentrations of glucose and insulin were determined in troncal blood from fasted and fed rats. In fasted animals, food was withdrawn 120 min before the lights were off. Rats were killed by decapitation 90 min after the lights were off. Glucose was measured by a spectrophotometric method (Glucose Trinder Method, Roche) (Barham & Trinder, 1972). Insulin was determined by using a specific EIA kit for rat insulin (Mercodia, Denmark), based on the direct sandwich technique in which two monoclonal antibodies are directed against separated antigenic determinants on the insulin molecule. Plasma samples were within the detection range of the assay 0.7–5.5 μg insulin ml−1 (1.8% intra-assay variation, 3.8% interassay variation).
Statistical analysis
Individual group comparisons were made using a two-way ANOVA. The factors of variation were treatment and food availability. Individual dose effects within a given group were analyzed by using a one-way ANOVA, followed by the Newman–Keuls' test. Statistical significance was set at P<0.05.
Results
Effect of food deprivation on glucose and insulin plasma concentration
Plasma samples were obtained from troncal blood from both fed and fasted animals. As summarized in Table 1 , the plasma concentration of both glucose and insulin were significantly lower in fasted than in fed rats, confirming that our experimental conditions lead to characteristic metabolic signals of acute fasting.
Table 1.
Plasma concentration of glucose and insulin in fed and fasted rats
| Fed | Fasted | |
|---|---|---|
| Glucose (mg/dl) | 144.9±3.8 | 130.9±3.8* |
| Insulin (μg/l) | 2.0±0.2 | 0.7±0.2* |
Values are mean±s.e.m. of eight individual determinations.
P<0.05 compared to the fed group (Newman–Keuls' test). Rats were fasted during 210 min from 2 h before the lights were off.
Effect of the CCK1R antagonist SR-27,897 and the CCK2R antagonist L-365,260 on c-Fos expression in the hypothalamus
The effect of both SR-27,897 (0.03, 0.3 and 1 mg kg−1) and L-365,260 (0.1, 1 and 10 mg kg−1) on c-Fos expression was examined, in both food-deprived and fed rats, 90 min after the lights were off and 210 min after drug administration (Figure 1).
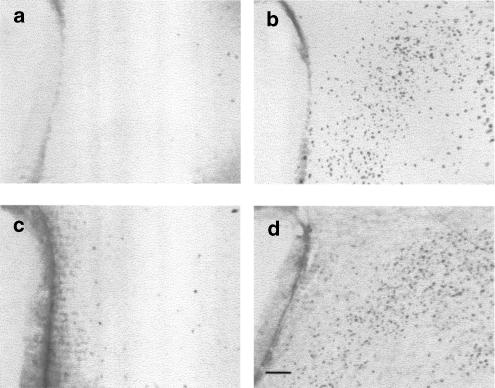
Figure 1.
Effect of CCKR antagonist on c-Fos expression in the hypothalamic PVN in both fed and fasted rats. Images (a–f) are representative of c-Fos immunostaining in a PVN containing section from a fasted rat (a) or a fasted rat treated either with 0.3 mg kg−1 SR-27,897 (b) or 1 mg kg−1 L-365,260. (c) Images (d–f) are representative of fed rats treated with vehicle (d), 0.3 mg kg−1 SR-27,897 (e), or 1 mg kg−1 L-365,260 (f). Scale bar corresponds to 100 μm.
Table 2 summarizes the effect of SR-27,897 in the PVN. Two-way ANOVA revealed significant treatment (F(3,30)=8.151, P<0.01) and fasting effects (F(1,30)=17.766, P<0.01) as well as a significant interaction between treatment and fasting (F(3,30)=7.182, P<0.01). In food-deprived animals, SR-27,897 significantly decreased the expression of c-Fos (one-way ANOVA, F(3,20)=13.378, P<0.01) from the dose of 0.3 mg kg−1. In the parvocellular area of PVN, all treatment, food deprivation and their interaction were significant (F(3,32)=16.083, P<0.01; F(1,32)=40.543, P<0.01, F(3,32)=8.980, P<0.01, respectively). In this area, treatment with SR-27,897 induced significant change in the number of c-Fos-positive cells from the dose of 0.03 mg kg−1 in food-deprived animals (one-way ANOVA, F(3,20)=12.238, P<0.01), but lacked any effect in fed rats.
Table 2.
Effect of treatment with CCKR antagonists on c-Fos expression in total PVN and in the parvocellular (PVNp) zone of the PVN of the hypothalamus
| Treatment | Fed | Fasted | ||
|---|---|---|---|---|
| PVN | PVNp | PVN | PVNp | |
| Vehicle | 43.3±13.5 (5) | 15.3±3.5 (5) | 188.3±29.7♦(5) | 104.1±20.8♦(5) |
| SR-27,897 (0.03 mg kg−1) | 24.3±7.6 (3) | 9.6±1.0 (3) | 125.2±23.0 (3) | 65.2±6.2*(3) |
| SR-27,897 (0.3 mg kg−1) | 64.6±8.9 (4) | 24.3±3.6 (4) | 65.5±14.4** (5) | 28.2±3.7**(5) |
| SR-27,897 (1 mg kg−1) | 87.0±13.1 (3) | 28.0±4.0 (3) | 40.0±9.5**(5) | 16.0±5.2**(5) |
| L-365,260 (0.1 mg kg−1) | 51.5±8.5 (4) | 14±7.8 (4) | 166.2±24.2 (5) | 73.5±11.3 (5) |
| L-365,260 (1 mg kg−1) | 86.6±4.5 (4) | 26.3±7.0 (4) | 117.5±18.2# (5) | 48.7±9.9## (5) |
| L-365,260 (10 mg kg−1) | 80.4±6.3 (3) | 33.6±9.1 (3) | 111.3±14.8# (3) | 26.3±8.2## (3) |
Values are mean±s.e.m. from 3–5 animals. For each animal, 4–6 sections were counted and an average value obtained.
Fasting increased c-Fos expression in both magno- and parvocellular PVN (♦P<0.01 compared to the vehicle-fed group; Newman–Keuls' test).
The effect of SR-27,897 was significant, in fasted rats, from 1 mg kg−1 in PVNm and from 0.03 mg kg−1 in PVNp (*P<0.05, **P<0.01 compared to the vehicle-fasted group; Newman–Keuls' test).
The effect of L-365,260 was significant also in fasted rats from 1 mg kg−1 only in PVNp (#P<0.05, ##P<0.01 compared to the vehicle-fasted group; Newman–Keuls' test). The number within parentheses corresponds to the number of animals used.
Table 2 also shows the effect of the CCK2R antagonist L-365,260. Two-way ANOVA revealed, in PVN, significant treatment (F(3,31)=10.811, P<0.01) and fasting effects (F(1,31)=21.834, P<0.01) with significant interaction (F(3,31)=8.659, P<0.01). In fasted animals, L-365,260 significantly decreased c-Fos expression (F(3,19)=11.747, P<0.01), at the dose of 1 mg kg−1, but was without effect in fed rats. In the parvocellular area of this nucleus, all treatment, fasting and their interaction were significant (F(3,32)=16.083, P<0.01; F(1,32)=40.543, P<0.01, F(3,32)=8.980, P<0.01, respectively). In this area, treatment with L-365,260 also induced significant change in the number of c-Fos-positive cells from the dose of 1 mg kg−1 (one-way ANOVA, F(3,20)=12.238, P<0.01), but lacked any effect in fed rats.
Effect of SR-27,897 and L-365,260 on c-Fos expression in the NTS
Figure 2 illustrates the effect of SR-27,897 (0.3 mg kg−1) and L-365,260 (1 mg kg−1) on c-Fos expression in the NTS. In the case of SR-27,897, two-way ANOVA revealed a significant effect of fasting (F(1,14)=170.591, P<0.01), but both treatment effect and the interaction between fasting and pharmacological treatment lacked statistical significance (F(1,14)=2.659, P=0.119; F(1,14)=1.604, P=0.176). Similar results were obtained with L-365,260 (F(1,14)=121.857, P<0.01 for fasting; F(1,14)=3.109, P=0.096 for treatment; F(1,15)=1.904, P=0.157 for the interaction). Table 3 resumes the effect of SR-27,897 and L-365,260 on c-Fos expression in the NTS.
Figure 2.
c-Fos expression in the NTS of fasted rats treated with vehicle (a), SR-27,897 (b) or L-365,260 (c). (d–f) Representative of fed rats treated with vehicle, SR-27,897 or L-365,269, respectively. Scale bar corresponds to 100 μm.
Table 3.
Effect of CCKR antagonists on the expression of c-Fos in the NTS
| Treatment | Fed | Fasted |
|---|---|---|
| Vehicle | 6.5±2.1 | 92.5±13.3** |
| SR-27,897 (0.3 mg kg−1) | 4.5±1.1 | 59.0±10.2** |
| L-365,260 (1 mg kg−1) | 4.0±0.9 | 44.5±11.9* |
Values are mean±s.e.m. from four animals. For each animal, 4–6 sections were counted and an average value obtained (**P<0.01; *P<0.05, compared to the vehicle-fed group; Newman–Keuls' test).
Effect of SR-27,897 and L-365,260 on c-Fos expression in the hypothalamus and NTS of rats treated with 2-DOG
The effect of i.p. 2-DOG on hypothalamic c-Fos immunostaining in fed rats is illustrated in Figure 3. 2-DOG was administered 60 min before the lights were off and 60 min after administration of vehicle, SR-27,897 (0.3 mg kg−1) or L-365,260 (1 mg kg−1). Treatment with 2-DOG (Figure 3b) led to a significant increase of c-Fos expression in PVN, which was not detectable in animals previously treated with the CCK1R antagonist SR-27,897 (Figure 3c) (F(3,8)=186.4, P<0.01). In contrast, the CCK2R antagonist L-365,260 was without effect (Figure 3d). In the parvocellular area, the CCK1 receptor antagonist also prevented the effect of 2-DOG (F(3,8)=113.3, P<0.01; P<0.01 between 2-DOG and 2-DOG+SR-27,897 groups, Newman–Keuls test). Under the same conditions, the CCK2R antagonist L-365,260 (1 mg kg−1) was without effect (Table 4 ).
Figure 3.
c-Fos expression on the hypothalamic PVN of fed rats treated with (a) vehicle, (b) 2-DOG, (c) 0.3 mg kg−1 of SR-27,897+2-DOG and (d) 1 mg kg−1 of L-365,260+2-DOG. Scale bar corresponds to 100 μm.
Table 4.
Effect of CCKR antagonists on the expression of c-Fos in fed rats treated with 2-deoxyglucose (2-DOG)
| Treatment | PVN | PVNp | NTS |
|---|---|---|---|
| Vehicle+vehicle | 43.3±11.1 (5) | 15.3±2.9 (5) | 6.5±1.5 (5) |
| Vehicle+2-DOG | 2210.0±269.7** (5) | 236.3±19.3** (5) | 119.7±8.4** (5) |
| SR-27,897+vehicle | 64.7±12.7 (4) | 21.0±3.5 (4) | 4.5±0.9 (4) |
| SR-27,897+2-DOG | 113.0±39.9 (5) | 23.3±3.9 (5) | 31.3±4.7*(5) |
| L-365,260+Vehicle | 86.7±3.7 (3) | 26.3±5.7 (3) | 4.0±0.9 (3) |
| L-365,260+2-DOG | 2732.0±338.7 (5) | 224.0±15.4 (5) | 130.7±6.1 (5) |
Values are mean±s.e.m. from 3–5 animals. For each animal, 4–6 sections containing the area were counted and an average value obtained. The effect of SR-27,897 (0.3 mg kg−1) was significant in total PVN and also in parvocellular PVN (**P<0.01, compared to vehicle+vehicle, SR-27,897+vehicle and SR-27,897+2-DOG groups; Newman–Keuls' test). In the NTS, SR-27,897 significantly decreased c-FOS expression (**P<0.01, compared to vehicle+vehicle, SR-27,897+vehicle and SR-27,897+2-DOG groups; *P>0.05 compared to vehicle+vehicle, SR-27,897+vehicle groups; Newman–Keuls' test). The number within parentheses corresponds to the number of animals used.
In NTS (Table 3), 2-DOG led to significant increase of c-Fos expression that was prevented by previous treatment with SR-27,897 (F(3,10)=159.6), but not with L-365,260.
Discussion
The anatomical data reported in this paper show that food deprivation, during the dark phase of the circadian cycle, increases c-Fos expression in the rat hypothalamus, through a mechanism involving CCK. Previous reports have demonstrated that food deprivation stimulates c-Fos expression in the hypothalamus (Timofeeva & Richard, 1997). This effect is probably linked to a decrease of glucose plasma level, since insulin-induced hypoglycemia also stimulates c-Fos expression in this area (Niimi et al., 1995).
Our experimental conditions, that is, food withdrawal only during the initial nocturnal period of feeding, were chosen to avoid deep metabolic changes occurring during starvation but leading to characteristic metabolic signals of acute fasting (Dallman et al., 1999), such as a slight decrease of glucose and insulin plasma concentration. The main result of our work is that either CCK1R or CCK2R blockade decreased, in a dose-dependent manner, c-Fos expression in the PVN of the hypothalamus of fasted rats to values similar to those of fed animals. This finding firstly suggests that, during fasting, endogenous CCK drives, at least partially, the activity of the PVN. The effect of CCKR antagonists seems to be relatively specific of this metabolic situation, since, in a similar experimental design, we have previously reported that CCK1R antagonists reduce the increase of both corticosterone and ACTH that occurs in fasted rats (Ruiz-Gayo et al., 2000), but are without effect in other stressful situations (Hernando et al., 1996).
In this work, the effect of CCKR antagonists was only detected in fasted rats since these drugs, even at the highest doses used, did not modify the expression of c-Fos in ad libitum fed rats. This result points to an effect of CCKR antagonists linked to a situation of metabolic stress and suggests a physiological role for endogenous CCK in regulating neuroendocrine adaptative responses to acute fasting.
Concerning the CCKR subtype involved in this response, we have shown that both SR-27,897 (a CCK1R antagonist) and L-365,260 (a CCK2R antagonist) decreased c-Fos immunoreactivity in the PVN of fasted rats to the values found in fed animals. The effect was significant at the doses of 0.03 and 1 mg kg−1, which are considered selective for CCK1Rs and CCK2Rs, respectively (Dourish et al., 1989; Poncelet et al., 1993; Ruiz-Gayo et al., 2000). Thus, it seems that both CCK1Rs and CCK2Rs are involved in the effect of endogenous CCK. Nevertheless, the fact that SR-27,897 is effective at a very low dose (0.03 mg kg−1) suggests that the partial contribution of CCK1Rs would be more important than the contribution of CCK2Rs. In fact, the effect of L-365,260 could eventually be related to the anxiolytic/antidepressive effect of this drug (Crawley & Corwin, 1994; Hernando et al., 1994), as food deprivation is a stressful situation that could, theoretically, evoke mood alterations involving the hypothalamus (Hauger & Dautzenberg, 2000). The importance of CCK1Rs was stressed in experiments carried out with 2-DOG, which clearly showed that, under low glucose availability, the effect of CCK in the PVN is mediated by CCK1R subtype.
An important question raised from our data is whether the effect of CCKR antagonists is due to the binding of these drugs to central receptors or is an indirect effect linked to the blockade of vagal receptors (Corp et al., 1993). Both SR-27,897 and L-365,260 are known to enter the brain and elicit strong neurobehavioral effects (Dourish et al., 1989; Singh et al., 1991; Poncelet et al., 1993; Vasar et al., 1994). Although we cannot discard a vagus-mediated effect of CCKR antagonists, our results point to a central effect since we have not detected a significant effect of CCKR antagonists on c-Fos expression in the NTS of fasted rats. This nucleus, which receives vagal inputs, is very sensitive to peripheral CCK (Crawley et al., 1981; Barber et al., 1990; Reidelberger, 1992; Yuhan et al., 2000) and also contains also CCK1Rs and CCK2Rs (Branchereau et al., 1992). In fact, central actions elicited by peripheral CCK often involve the NTS (Broberger et al., 1999) and it can be assumed that an eventual antagonism of the central effects of peripheral CCK by CCKR antagonists should be detected at the level of NTS. On the other side, a peripheral effect of CCK in fasted rats is rather unexpected since peripheral CCK is a postprandial hormone with low plasma levels during fasting (Linden, 1989; Linden & Södersten, 1990). The increase in c-Fos expression in the NTS observed in fasted rats could be related to the presence of glucose-responsive neurons in this nucleus (Adachi et al., 1995). Thus, our data suggest that endogenous CCK could be released in brain during fasting and stimulate central CCKRs involved in the activation of hypothalamic nuclei. Such a hypothesis has anatomical support since there is increasing evidence of CCK neurons and somata that are immunopositive for CCKRs in the PVN itself (see Introduction) as well as in both the supraoptic and ventromedial hypothalamic nuclei, which are connected to the PVN (Thompson & Swanson, 2003), and are involved in feeding/satiety mechanisms. Interestingly, both the hippocampus and amygdala, which are involved in regulating the activity of the HPA axis (see review in Jacobson & Sapolsky, 1991), contain mRNA encoding CCK (Vanderhaeghen et al., 1980; Schiffmann & Vanderhaeghen, 1991) and are connected to the PVN (Sawchenko & Swanson, 1983; Swanson & Sawchenko, 1983; Herman et al., 2002). This allows to speculate about a putative CCKergic input from those areas, recruited under fasting/low glucose conditions and aimed at increasing HPA activity. In order to assess the importance of glucose availability in the effect of endogenous CCK in hypothalamic neuronal activity, we have simulated fasting by an i.p. dose of 2-DOG. This nonmetabolizable glucose analogue impairs glucose utilization by neurons and can theoretically simulate a metabolic situation of low glucose intake. Under our conditions (administration 2 h before the onset of the dark phase), 2-DOG produced a significant increase of c-Fos expression in the PVN of the hypothalamus as well as in the NTS. Such an effect of 2-DOG in the hypothalamus and in the brain stem has been previously described by several authors (Briski & Brandt, 2000; Briski & Marshall, 2000). Under our experimental conditions, the effect of 2-DOG was dramatically abolished by SR-27,897 but not by L-365,260. This result, taken together with the preceding data, demonstrates that, when glucose availability decreases, endogenous CCK can activate the PVN, which is involved in the activation of the HPA axis and in feeding control. The strong dependence on glucose of the effect observed suggests that hypothalamic glucose-responsive neurons are involved in the response observed. An attractive hypothesis is that, under acute fasting, paraventricular neurons endowed with CCKRs become more sensitive to the effect of endogenous CCK, released by CCKergic neurons (Schiffmann & Vanderhaeghen (1991) and references cited therein). The increase of c-Fos immunoreactive cells in the PVN, which express both types of CCK receptors (Day et al., 1994; Raybould et al., 1988; Schick et al., 1990; O'Shea & Gundlach, 1993; Mercer & Beart, 1997), allows us to speculate that inhibitory inputs to this nucleus are reduced under fasting. In fact, PVN receives inhibitory glucose responsive afferents (Spanswick et al., 1997,2000). When glucose level falls, such inputs may be blocked, and neurons expressing CCKRs would be more sensitive to the effect of endogenous CCK.
In summary, our results show that (i) CCK pathways are involved in the activation of discrete hypothalamic nuclei that occurs under acute fasting, and that (ii) the effect observed is mediated by CCK receptors in the brain, suggesting that endogenous CCK is physiologically involved in driving hypothalamic activity during fasting.
Acknowledgments
This work was supported by Grants from CICYT – Ministerio de Ciencia y Tecnología (PM 99-0010, PM 99-0011) and FSP-CEU (2/01 USP). MVC is a fellow of FUSP-CEU. We would like to acknowledge Dr Philip M. Beart and Dr Linda D. Mercer (Howard Florey Institute, University of Melbourne, Australia) for invaluable help and critical reading of the manuscript. SR-27,897 and L-365,260 were a generous gift of Sanofi Synthélabo (France) and MSD (U.S.A.), respectively.
Abbreviations
- ACTH
adrenocorticotropin hormone
- CCK
cholecystokinin
- CCKR
cholecystokinin receptor
- CRH
corticotropin-releasing hormone
- HPA
hypothalamic–pituitary–adrenal
- NTS
nucleus tractus solitarius
- PVN
paraventricular nucleus
References
- ADACHI A., KOBASHI M., FUNAHASHI M. Glucose-responsive neurons in the brainstem. Obes. Res. 1995;3:735S–740S. doi: 10.1002/j.1550-8528.1995.tb00493.x. [DOI] [PubMed] [Google Scholar]
- AKANA S.F., STRACK A.M., HANSON E.S., DALLMAN M.F. Regulation of activity in the hypothalamo–pituitary–adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- BARBER W.D., YUAN C.S., BURKS T.F. Local effects of CCK-8 on gastric vagally-evoked responses in nucleus tractus solitarius. FASEB J. 1990;4:A979. [Google Scholar]
- BARHAM D., TRINDER P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- BAXTER J.D., FORSHAM P.H. Tissue effects of glucocorticoids. Am. J. Med. 1972;52:573–579. doi: 10.1016/0002-9343(72)90154-4. [DOI] [PubMed] [Google Scholar]
- BRANCHEREAU P., BOHME G.A., CHAMPAGNAT J., MORIN-SURUN M.P., DURIEUX C., BLANCHARD J.C., DENAVIT-SAUBIE M. Cholecystokinin-A and cholecystokinin-B receptors in neurons of the brainstem solitary complex of the rat: pharmacological identification. J. Pharmacol. Exp. Ther. 1992;260:1433–1440. [PubMed] [Google Scholar]
- BRISKI K.P., BRANDT J.A. Oxytocin and vasopressin neurones in principal and accessory hypothalamic magnocellular structures express Fos-immunoreactivity in response to acute glucose deprivation. J. Neuroendocrinol. 2000;12:409–414. doi: 10.1046/j.1365-2826.2000.00469.x. [DOI] [PubMed] [Google Scholar]
- BRISKI K.P., MARSHALL E.S. Caudal brainstem Fos expression is restricted to periventricular catecholamine neuron-containing loci following intraventricular administration of 2-deoxy-D-glucose. Exp. Brain Res. 2000;133:547–551. doi: 10.1007/s002210000448. [DOI] [PubMed] [Google Scholar]
- BROBERGER C., HOLMBER G.K., KUHAR M.J., HÖKFELT T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: a putative mediator of cholecystokinin-induced satiety. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13506–13511. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R.S., CHEN T.B., BOCK M.G., FREIDINGER R.M., CHEN R., ROSEGAY A., LOTTI V.J. Characterization of the binding of [3H]-L-365,260: a new potent and selective brain cholecystokinin (CCK-B) and gastrin receptor antagonist radioligand. Mol. Pharmacol. 1989;35:803–808. [PubMed] [Google Scholar]
- CORP E.S., MCQUADE J., MORAN T.H., SMITH G.P. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res. 1993;623:161–166. doi: 10.1016/0006-8993(93)90024-h. [DOI] [PubMed] [Google Scholar]
- CRAWLEY J.N., CORWIN R.L. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- CRAWLEY J.N., HAYS S.E., PAUL S.M. Vagotomy abolishes the inhibitory effects of cholecystokinin on rat exploratory behaviors. Eur. J. Pharmacol. 1981;73:379–380. [Google Scholar]
- DALLMAN M.F., AKANA S.F., BHATNAGAR S., BELL M.E., CHOI S.J., CHU A., HORSLEY C., LEVIN N., MEIJER O., SORIANO L., STRACK A.M., VIAU V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- DAY H.E., MCKNIGHT A.T., POAT J.A., HUGHES J. Evidence that cholecystokinin induces immediate early gene expression in the brainstem, hypothalamus and amygdala of the rat by a CCKA receptor mechanism. Neuropharmacology. 1994;33:719–727. doi: 10.1016/0028-3908(94)90111-2. [DOI] [PubMed] [Google Scholar]
- DOURISH C.T., RYCROFT W., IVERSEN S.D. Postponement of satiety by blockade of cholecystokinin (CCK-B) receptors. Science. 1989;245:1509–1511. doi: 10.1126/science.2781294. [DOI] [PubMed] [Google Scholar]
- HAUGER R.L, DAUTZENBERG F.M.Neuroendocrine correlates of stress, behaviour, and biological processes. Regulation of the stress response by corticotropin-releasing factor receptors Neuroendocrinology in Physiology and Medicine 2000Totowa: Humana Press; 261–286.ed. Conn, P.M. & Freeman, M.E. pp [Google Scholar]
- HERMAN J.P., TASKER J.G., ZIEGLER D.R., CULLINAN W.E. Local circuit regulation of paraventricular nucleus stress intgration: glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- HERNANDO F., FUENTES J.A., ROQUES B., RUIZ-GAYO M. The CCK-B receptor antagonist, L-365,260, elicits antidepressant-type effects in the forced-swimming test in mice. Eur. J. Pharmacol. 1994;261:257–263. doi: 10.1016/0014-2999(94)90115-5. [DOI] [PubMed] [Google Scholar]
- HERNANDO F., FUENTES J.A., RUIZ-GAYO M. Impairment of stress adaptive behaviours in rats by the CCKA receptor antagonist, devazepide. Br. J. Pharmacol. 1996;118:400–406. doi: 10.1111/j.1476-5381.1996.tb15416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKS G.L., POAT J.A., HUGUES J. Changes in hypothalamic cholecystokinin A and cholecystokinin B receptor subtypes and associated neuropeptide expression in response to salt-stress in the rat and mouse. Neuroscience. 1995;68:765–781. doi: 10.1016/0306-4522(95)00148-c. [DOI] [PubMed] [Google Scholar]
- HONDA T., WADA E., BATTEY J.F., WANK S.A. Differential gene expression of CCK-A and CCK-B receptors in the rat brain. Mol. Cell. Neurosci. 1993;4:143–154. doi: 10.1006/mcne.1993.1018. [DOI] [PubMed] [Google Scholar]
- JACOBSON L., SAPOLSKY R. The role of the hipp'ocampus in feedback regulation of the hypothalamic–pituitary–adrenocortical axis. Endocrine Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- KALRA S.P., DUBE M.G., PU S., XU B., HORVATH T.L., KALRA P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocrine Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- KAMILARIS T.C., JOHNSON E.O., CALOGERO A.E., KALOGERAS K.T., BERNARDINI R., CHROUSOS G.P., GOLD P.W. Cholecystokinin-octapeptide stimulates hypothalamic–pituitary–adrenal function in rats: role of corticotropin-releasing hormone. Endocrinology. 1992;130:1764–1774. doi: 10.1210/endo.130.4.1312423. [DOI] [PubMed] [Google Scholar]
- LAUGERO K.D., BELL M.E., BHATNAGAR S., SORIANO L., DALLMAN M.F. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenolectomized rats: a glucocorticoid–metabolic brain axis. Endocrinology. 2001;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- LINDEN A. Role of cholecystokinin in feeding and lactation. Acta Physiol. Scand. 1989;585:1–49. [PubMed] [Google Scholar]
- LINDEN A., SÖDERSTEN P. Relationship between the concentration of cholecystokinin-like immunoreactivity in plasma and food intake in male rats. Physiol. Behav. 1990;48:859–863. doi: 10.1016/0031-9384(90)90240-5. [DOI] [PubMed] [Google Scholar]
- MERCER L.D. Immunolocalization of cholecystokinin receptors 2000Monash University, Melbourne, Australia; M.Sc. Thesis [Google Scholar]
- MERCER L.D., BEART P.M. Histochemistry in rat brain and spinal cord with an antibody directed at the cholecystokininA receptor. Neurosci. Lett. 1997;225:97–100. doi: 10.1016/s0304-3940(97)00197-3. [DOI] [PubMed] [Google Scholar]
- MERCER L.D., LE V.Q., NUNAN J., JONES N.M., BEART P.M. Direct visualization of cholecystokinin subtype 2 receptors in rat central nervous system using antipeptide antibodies. Neurosci. Lett. 2000;293:167–170. doi: 10.1016/s0304-3940(00)01504-4. [DOI] [PubMed] [Google Scholar]
- NIIMI M., SATO M., TAMAKI M., WADA Y., TAKAHARA J., KAWANISHI K. Induction of Fos protein in the rat hypothalamus elicited by insulin-induced hypoglycemia. Neurosci. Res. 1995;23:361–364. doi: 10.1016/0168-0102(95)00965-V. [DOI] [PubMed] [Google Scholar]
- NOBLE F., WANK S.A., CRAWLEY J.N., BRADWEJN J., SEROOGY K.B., HAMON M., ROQUES B.P. International Union of Pharmacology XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 1999;54:745–781. [PubMed] [Google Scholar]
- OOMURA Y., ONO T., OOYAMA H., WAYNER M.J. Glucose and osmosensitive neurons of rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- O'SHEA R.D., GUNDLACH A.L. Regulation of cholecystokinin receptors in the hypothalamus of the rat: reciprocal changes in magnocellular nuclei induced by food deprivation and dehydration. J. Neuroendocrinol. 1993;5:697–704. doi: 10.1111/j.1365-2826.1993.tb00542.x. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- PONCELET M., ARNONE M., HEAULME M., GONALONS N., GUEDET C., SANTUCCI V., THURNEYSSEN O., KEANO P., GULLY D., LE FUR G., SOUBRIÉ P. Neurobehavioral effects of SR-27,897, a selective cholecystokinin type A (CCK-A) receptor antagonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:102–107. doi: 10.1007/BF00168544. [DOI] [PubMed] [Google Scholar]
- RAYBOULD H.E., GAYTON R.J., DOCKRAY G.J. Mechanisms of action of peripherally administered cholecystokinin octapeptide on brain stem neurons in the rat. J. Neurosci. 1988;8:3018–3024. doi: 10.1523/JNEUROSCI.08-08-03018.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIDELBERGER R.D. Abdominal vagal mediation of the satiety effects of exogenous and endogenous cholecystokinin in rats. Am. J. Physiol. 1992;263:R1354–R1358. doi: 10.1152/ajpregu.1992.263.6.R1354. [DOI] [PubMed] [Google Scholar]
- RUIZ-GAYO M., GARRIDO M.M., FUENTES J.A. Inhibition of the hypothalamic–pituitary–adrenal axis in food-deprived rats by a CCK-A receptor antagonist. Br. J. Pharmacol. 2000;129:839–842. doi: 10.1038/sj.bjp.0703117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWCHENKO P.E., SWANSON L.W. Organization of the forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J. Comp. Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- SCHICK R.R., HARTY G.J., YAKSH T.L., GO V.L. Sites in the brain at which cholecystokinin octapeptide (CCK-8) acts to suppress feeding in rats: a mapping study. Neuropharmacology. 1990;29:109–118. doi: 10.1016/0028-3908(90)90050-2. [DOI] [PubMed] [Google Scholar]
- SCHIFFMANN S.N., VANDERHAEGHEN J.J. Distribution of cells containing mRNA encoding cholecystokinin in the rat central nervous system. J. Comp. Neurol. 1991;304:219–233. doi: 10.1002/cne.903040206. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M.W., SEELEY R.L., CAMPFIELD L.A., BURN P., BASKIN D.G. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH L., LEWIS A.S., FIELD M.J., HUGUES J., WOODRUFF G.N. Evidence for an involvement of the brain cholecystokinin B receptor in anxiety. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1130–1133. doi: 10.1073/pnas.88.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPANSWICK D., SMITH M.A., GROPPI V.E., LOGAN S.D., ASHFORD M.L.J. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- SPANSWICK D., SMITH M.A., MIRSHAMSI S., ROUTH V.H., ASHFORD M.L.J. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat. Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- SWANSON L.W., SAWCHENKO P.E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- THOMPSON R.H., SWANSON L.W. Structural organization of a hypothalamic visceromotor pattern generator network. Brain Res. Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- TIMOFEEVA E., RICHARD D. Functional activation of CRH neurons and expression of the genes encoding CRH and its receptors in food-deprived lean (Fa/?) and obese (fa/fa) Zucker rats. Neuroendocrinology. 1997;66:327–340. doi: 10.1159/000127256. [DOI] [PubMed] [Google Scholar]
- VASAR E., LANG A., HARRO J., BOURIN M., BRADWEJN J. Evidence for potentiation of the effect of cholecystokinin octapeptide in the elevated plus-maze. Neuropharmacology. 1994;33:729–735. doi: 10.1016/0028-3908(94)90112-0. [DOI] [PubMed] [Google Scholar]
- VANDERHAEGHEN J.J., LOTSTRA F., DE MEY J., GILLES C. Immunohistochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1190–1194. doi: 10.1073/pnas.77.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUHAN C.S., ATTELE A.S., DEY L., XIE J.T. Gastric effects of cholecystokinin and its interaction with leptin on brainstem neuronal activity in neonatal rats. J. Pharmacol. Exp. Therap. 2000;295:177–182. [PubMed] [Google Scholar]