Abstract
Human formyl peptide-receptor-like-1 (FPRL-1) is a promiscuous G protein-coupled receptor (GPCR), and belongs to a chemoattractant receptor family protein. This receptor has been reported to interact with various host-derived peptides and lipids involved in inflammatory responses. We described here, a novel role for FPRL-1 as a high-affinity β-chemokine receptor for an N-terminally truncated form of the CKβ8 (CCL23/MPIF-1) splice variant CKβ8-1 (22–137 aa).
RT-PCR analysis of mRNA derived from human tissues and cells revealed a predominant expression of FPRL-1 in inflammatory cells, particularly in neutrophils.
Intracellular calcium mobilisation assay, used as screening tool, in recombinant Chinese hamster ovary (CHO-K1) and human embryonic kidney (HEK293s) cells coexpressing FPRL-1 and Gα16, demonstrated FPRL-1 is a functional high-affinity receptor for CKβ8-1 (46–137 aa, sCKβ8-1), with pEC50 values of 9.13 and 8.85, respectively.
The FPRL-1 activation in CHO-K1 cells is mediated by Gαi/Gαo proteins, as assessed by pertussis toxin sensitivity and inhibition of forskolin-induced cyclic AMP accumulation.
Binding experiments were performed with a radio-iodinated synthetic peptide, [125-I]-WKYMVm, a known potent FPRL-1 agonist. CHO-K1 cell membranes expressing FPRL-1 bound [125-I]-WKYMVm with a Kd value of 9.34. Many known FPRL-1 agonists were tested and sCKβ8-1 was the most effective nonsynthetic ligand in displacing the radiolabelled agonist, with a pIC50 of 7.97.
The functional significance of sCKβ8-1 interaction with FPRL-1 was further demonstrated by the activation of polymorphonuclear leukocytes (PMNs) calcium mobilisation and chemotaxis. These interactions were shown to be via FPRL-1 by specific blockade of PMNs activation in the presence of an FPRL-1 antibody.
Keywords: G protein-coupled receptor, FPRL-1, β-chemokine, CKβ8-1, calcium mobilisation assay
Introduction
Human formyl peptide-receptor-like-1 (FPRL-1) is a seven-transmembrane G protein-coupled receptor (GPCR), sharing 69% identity at the amino-acid level with formyl peptide receptor (FPR) and 83% identity with FPRL-2. FPR, FPRL-1 and FPRL-2 are located on chromosome 19q13.3 and are phylogenetically clustered with chemokine receptors (Murphy et al., 1992; Haviland et al., 1993; Fredriksson et al., 2003). FPRL-1 is expressed in astrocytes, neutrophils and monocytes (Durstin et al., 1994; Le et al., 2000). Bacterial chemotactic peptides, such as N-formyl-methionyl-leucyl-phenylalanine (fMLF), have been shown to activate FPR at picomolar concentrations and elicit the release of proinflammatory mediators from leukocytes, thereby leading to chemotaxis (Le et al., 2001). FPRL-1 has been reported as a low-affinity fMLF receptor (Le et al., 2001). This class of receptors has been proposed to play a role in host defence mechanisms due to their ability to interact with bacterial formylated peptides (Le et al., 2001; 2002). Whereas, FPRL-2 is expressed in monocytes but not in neutrophils, and is not activated by N-formylpeptides (Le et al., 2000). Thus far, no ligands have been identified for FPRL-2.
The first ligand reported to bind with high affinity to FPRL-1 was the lipid mediator lipoxin A4 (LXA4) eicosanoid and its isomer 15-epi LXA4 (Fiore et al., 1994; Fiore & Serhan, 1995). Recently, a variety of agonists for FPRL-1 have been reported (Le et al., 2001; 2002), including the V3 region of the HIV-1 envelope gp120, glucocorticoid-induced annexin 1 (ANXA1)-derived peptide, and several amyloidogenic proinflammatory polypeptides, such as the acute-phase protein SAA, the 42 amino-acid form of β-amyloid (Aβ42) and human prion peptide (hPrP, 106–126 aa). Moreover, synthetic peptides derived from random peptide libraries, such as MMK-1, W-peptide and its isoform (W-peptide (m)) (Le et al., 1999; Christophe et al., 2001), have been described as highly potent agonists for FPRL-1. The affinities displayed by these ligands, as determined by calcium flux, binding and chemotaxis, were between 1 pM and 5.0 μM (Le et al., 2002). It should be noted that, these ligands bind to the same receptor, despite not sharing any substantial homology or displaying any known structural similarities (Le et al., 2002).
Since human FPRL-1 is expressed in neutrophils and monocytes (Le et al., 2000), and is activated by a diverse class of ligands mostly involved in the release of proinflammatory mediators, we postulated that a chemokine could be a potent endogenous agonist for FPRL-1. We therefore evaluated a collection of inflammatory mediators including more than 77 recombinant chemokines and identified a novel N-terminally truncated form of β-chemokine CKβ8-1 (sCKβ8-1, 46–137 aa), as a high-affinity FPRL-1 agonist.
The chemokines are 8–14 kDa-secreted cytokines, and four subfamilies have been discovered including: CXC(α), CC(β), C(γ) and CX3C (Murphy et al., 2000). Myeloid progenitor inhibitor factor-1 (CCL23/MPIF-1) belongs to the second subfamily. Its cDNA encodes a signal sequence of 21 aa followed by a 99 aa (CKβ8) or 116 aa (CKβ8-1) mature form protein. Both mature forms have been identified as putative ligands for CCR1 receptor (Youn et al., 1998). Multiple chemokines may activate a single chemokine receptor, as CCR1 is activated by RANTES, MIP-1α, MP-1β, CKβ8 and CKβ8-1 (Murphy et al., 2000). However, little is known about the specificity of CKβ8-1 with other receptors.
In the present study, we report the detailed expression profile of FPRL-1, and the findings that this GPCR is activated by sCKβ8-1 at subnanomolar concentrations and is coupled to Gαi/Gαo protein. Furthermore, we demonstrate that sCKβ8-1 induces calcium mobilisation and chemotaxis in PMNs via FPRL-1. These studies emphasise the possible proinflammatory role for FPRL-1 through its interaction with sCKβ8-1. To our knowledge this is the first description of a chemokine as a potent ligand for FPRL-1.
Methods
Cloning of cDNA encoding the human FPRL-1 receptor
The pIRES-neo2 expression vector (BD Biosciences, Palo Alto, CA, U.S.A.) was modified to increase the stability of mRNA cloned into the polylinker (Dr H. Weir, AstraZeneca R&D, Alderley Park, England) and was named pGEN-IRES-neo2. The human FPRL-1 coding sequence (Swissprot accession number P25090) was obtained from Dr. W. Koopmann (AstraZeneca R&D, Lund, Sweden) and subcloned into the pGEN-IRES-neo polylinker, as an EcoRI–XhoI fragment ligated into EcoRI–SalI. A CCACC Kozak consensus sequence was added 5′ to the ATG start codon.
Quantitative reverse transcriptase-PCR analysis
Human tissue cDNAs or RNAs were purchased from Clontech (Palo Alto, CA, U.S.A.) and Invitrogen (Paisley, England). Primary cells were purified from human peripheral blood using standard methods. Total RNA was extracted using TRIzol and cDNA synthesis was using the Superscript system (Gibco BRL Life Technologies). Taqman quantitative PCR was performed on an ABI 7700 (CGRB, Oregon, U.S.A.) apparatus as manufacturer's recommendations, using FPRL-1 probe oligonucleotide 5′-CGGCATGACACGCACAGTCACCACC-3′ and primer sequences 5′-GTGATCTGGGTGGCTGGATT-3′ and 5′-AGGGCCAGGTTCAGGTAACA-3′.
Chemicals
Peptidic and nonpeptidic ligands were obtained from commercial sources and were dissolved either in dimethyl sulphoxide (DMSO), 1 : 1 DMSO/distilled water, or in 1 : 1 ethanol/DMSO. The compounds used for dose–response experiments were: MPIF-1/CKβ8/CCL23 (22–120 aa), sCKβ8 (46–120 aa) (N-terminally truncated form of CKβ8 or sCKβ8), CKβ8-1 (22–137 aa), sCKβ8-1 (46–137 aa), IL-8, RANTES (R&D Systems, Minneapolis, U.S.A.), human Prion protein (hPrP, aa106-126), amyloid β protein (Aβ42, 1–42 aa) acute phase protein (SAA), V3 region of the HIV-1 envelope gp120 (HIV-1Bru gp120, 414–434 aa or F-peptide), MMK-1 (Bachem, Torrance, CA, U.S.A.), W-peptide (WKYMVM) and its isoform (WKYMVm) (Phoenix Pharmaceuticals, Belmont, U.S.A.), Lipoxin A4 (LXA4) (Calbiochem, La Jolla, CA, U.S.A.). Other compounds used were forskolin, pertussis toxin (PTX) (Sigma-Aldrich, Oakville, ON, Canada) and Melanin Concentrating Hormone (MCH) (American Peptide Company, Sunnyvale, CA, U.S.A.). A 17-amino-acid synthetic peptide, named SHAAG peptide which corresponds to the alternatively splice variant region of CKβ8-1 (47LWRRKIGPQMTLSHAAG63), was synthesised using standard procedures.
Cell transfection
HEK293 s cells were transfected with FPRL-1 (or with other GPCRs) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, U.S.A.) following the manufacturer's conditions and by adapting the transfection protocol to a 96-well plate. CHO-K1 cells were transfected by electroporation as described (Groblewski et al., 1997). Following the electro-shock, 8 × 104 cells were seeded per well. HEK293 s and CHO-K1 cells stably expressing Gα16 protein (Molecular Devices, Sunnyvale, CA, U.S.A.) were used to coexpress FPRL-1 or a control GPCR.
Measurement of intracellular Ca2+
HEK293 s cells transiently expressing FPRL-1 or unrelated GPCRs (such as Melanin concentrating hormone receptor, MCH1R (Lembo et al., 1999)) were plated (8 × 104 cells per well) in 96-well, poly-D-lysine-coated black FLIPR plates (Becton Dickinson, ON, Canada), and loaded with 100 μl Dulbecco's modified medium (DMEM)+10% foetal bovine serum containing the calcium-sensitive dye Fluo3-AM (TEF LABS, Austin, TX, U.S.A.) (4 μM final concentration), pluronic acid (20% final concentration) (Molecular Probes, OR, U.S.A.) and were incubated at 37°C for 1 h in a humidified chamber (5% CO2/95% air). Following the incubation step, cells were washed five times in Hanks' balanced salt solution buffer (HBSS) supplemented with 20 mM HEPES+0.1% BSA pH 7.4 (Wisent, Saint Bruno, QC, Canada). CHO-K1 cells were loaded with 100 μl HBSS buffer with 2.5 mM of Probenicid (Sigma-Aldrich, Oakville, ON, Canada) containing Fluo3-AM and pluronic acid as mentioned above. Mobilisation of intracellular Ca2+ was measured online using the Fluorescent Imaging Plate Reader (FLIPRTM) (Molecular Devices) as published (Lembo et al., 1999). Ligands were tested at 1 μM (final concentration, except chemokines were at 66 nM, or different starting concentrations when indicated). [Ca2+]i mobilisation in CHO-K1 and HEK293 s was measured by integrating the kinetic curve on intracellular Ca2+ concentration from 0 to 3 min using the FLIPR system following the addition of mentioned compounds, and plotted as a function of the concentration of the peptide used. To measure [Ca2+]i in HEK293 s cells stably expressing CCR1, cells were plated at 100 × 104 cells per well. Before incubating with test ligands, 0.75 mM carbachol (final concentration) was added for 3 min to prime the cells (without this priming CCR1 calcium responses were not detected). All graphical data in this work were calculated by nonlinear regression method using GraphPrism software, version 3.02.
Pertussis toxin (PTX) treatment
CHO-K1 cells transiently expressing FPRL-1 were treated for 6 h with 100 ng ml−1 of PTX in HAM's F12 modified medium (1 ×) with 2 mM glutamine and 10% foetal bovine serum (Wisent, Saint Bruno, QC, Canada). After the incubation period, cells were loaded with Fluo3-AM dye and pluronic acid as described above, and washed four times in HBSS buffer. To test the viability of CHO-K1 cells after PTX treatment, MCH1R was used as a positive control (Lembo et al., 1999; Hawes et al., 2000).
Cyclic AMP (cAMP) assays
CHO-K1 cells were transfected as above with FPRL-1 (or with a control GPCR) and plated in a P10 culture dish. Two days post-transfection, cells at 70–80% confluency (6× 105 ml−1) were detached using a PBS-based cell dissociation buffer (Invitrogen, Carlsbad, CA, U.S.A.). Cyclic AMP measurement was done using AlphaScreenTM cAMP assay kit (PerkinElmer Life Sciences, Montréal, QC, Canada) following the manufacturer's conditions. To stimulate cAMP production, forskolin was added at 10 μM (final concentration). The concentrations of ligands used were 10 pM to 200 nM for chemokines, 1 pM to 20 nM for W-peptide and 0.5 nM to 10 μM for other compounds. The percentage inhibition was calculated as follows: percentage inhibition=100 ×(F-F: A)/F, where F is the amount of cAMP in the presence of forskolin and F: A is the amount of cAMP in the presence of forskolin plus agonist.
Monoclonal FPRL-1 antibody
A monoclonal FPRL-1 antibody was prepared by genetic immunisation (Genovac AG, Freiburg, Germany). Monoclonal antibodies were screened for anti-FPRL-1 activity by flow cytometry and for receptor blocking activity using the FLIPR assay. For blocking studies, cells transiently or stably expressing FPRL-1 were incubated at 37°C for 1 h with antibody (6C7-3) at a concentration of 5 μg ml−1 (1 : 200 dilution) during the Fluo3-AM dye loading. After the incubation period, cells were washed and [Ca2+]i was measured by FLIPR system.
Isolation of PMNs from human donor blood and calcium flux assays
PMNs were prepared from healthy human donor blood (approval by a local Ethics Committee), collected in 10 U ml−1 of heparin, by centrifugation through Polymorphprep (Robbins Scientific, Solihull, England). Cells were washed with PBS containing 0.2% (w v−1) glucose and red blood cells were removed by two rounds of hypotonic lysis in ice-cold 0.2% (w v−1) NaCl for 1 min. For calcium flux assays, PMNs were resuspended in assay buffer (137 mM NaCl, 11 mM Glucose, 10 mM HEPES, 2.7 mM KCl, 0.4 mM K2H2PO4, 1.8 mM CaCl2, 1.0 mM MgCl2, 0.1% BSA pH 7.4) containing 5 μM Fluo-3AM. Cells were incubated for 90 min at room temperature with gentle mixing. Dye-loaded cells were washed once and resuspended at 4 × 106 cells ml−1. Calcium flux assays were performed using a FLIPR system, in 96-well FLIPR plates containing 2 × 105 cells per well.
Chemotaxis assay in PMNs
Migration assays were performed in 96-well ChemoTx™ microplates (Neuroprobe, Gaithersburg, MD, U.S.A.) in assay buffer (as mentioned above). Test compounds were added to the lower chamber, and 2 × 105 cells per well were placed on to the upper chamber. Following 2 h incubation at 37°C, filters were removed from the chambers, washed, fixed and migrated cells were stained with Alamar BlueTM (Serotec, Kidlington, England). Data are expressed as a percentage of cells migrating.
Radioligand binding assay
A volume of 250 μl of membrane preparation (0.6 mg ml−1, with maximum binding (Bmax) of 1.8 pmol mg−1 membrane protein) of CHO-K1 cells stably expressing FPRL-1 (Perkin-Elmer Life Sciences, Montréal, QC, Canada) were diluted in 7.5 ml of incubation buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 5 mM KCl, 5 mM MgCl2, 2 mM CaCl2, 0.5% BSA). The incubation time was 90 min at room temperature in the presence of 150 μl of diluted membrane preparation, 10 μl DMSO or unlabelled ligand from 10−5 to 10−15 M diluted in DMSO, and 10 μl of labelled ligand ([125I]-WKYMVm at 0.05 nM (PerkinElmer Life Sciences)) diluted in incubation buffer. After incubation, unbound tracer was removed by filtration over GF/C harvest plates (Millipore, Nepean, ON, Canada), presoaked in 50 mM Tris-HCl, pH 7.4, then washed nine times with 500 μl of the same buffer (icecold). Bound label was counted using a TopCount® Microplate Scintillation and Luminescence Counter (PerkinElmer Life Sciences).
Results
Expression profile of FPRL-1
Preliminary studies on the distribution of FPRL-1 mRNA by Northern blot analyses demonstrated abundant levels in lung, placenta and tissues known to have a relatively high degree of phagocytic cell infiltrates, and neutrophils (Fiore et al., 1994; Le et al., 2000). A more detailed expression profile for FPRL-1 was performed, using quantitative-PCR, across different human tissues and blood cells (Figure 1). Of the various tissues tested, the highest relative mRNA levels were observed in bone marrow, synovial tissues and lung. From different blood cells tested, neutrophils and dendritic cells (immature, myeloid) appear to express the highest levels of FPRL-1 mRNA.
Figure 1.
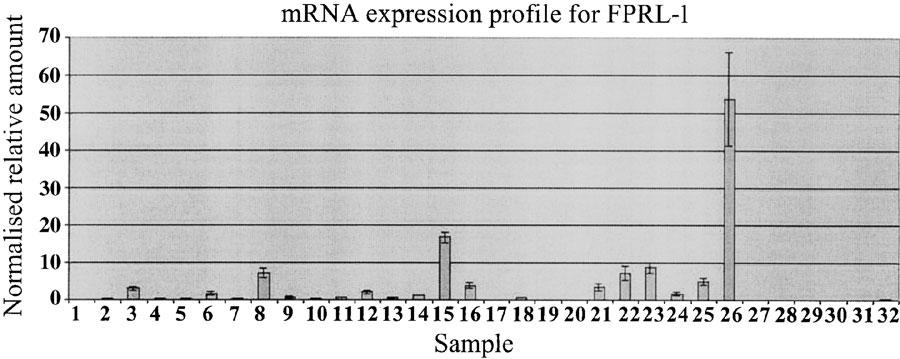
Quantitative reverse transcriptase-PCR analysis of human FPRL-1. RNA samples are derived from various human tissues and cells. FPRL-1 transcripts are detected in bone marrow, synovial tissues, lung, and in various blood cells, such as neutrophils and dendritic cells. Samples are: 1 brain, 2 heart, 3 lung, 4 liver, 5 trachea, 6 placenta, 7 colon, 8 bone marrow, 9 testis, 10 kidney, 11 thymus, 12 spleen, 13 skin, 14 adipose tissue, 15 immature dendritic cells (DC), 16 mature DC, 17 keratinocytes, 18 naïve T cells, 19 activated T cells 1, 20 activated T cells 2, 21 and 22 osteo-arthritis (OA) synovium, 23 rheumatoid-arthritis (RA) synovium, 24 and 25 peripheral blood (PB) monocytes, 26 PB neutrophils, 27 CD4+ T cells, 28 CD8+ T cell, 29 B cells, 30 Type 1 humoral (Th1) cells, 31 Type 2 humoral (Th2) cells, 32 eosinophils. Results are normalised to 18S ribosomal RNA and are presented as relative values. Results are from duplicate PCR reactions.
A truncated form of CKβ8-1 induces mobilisation of intracellular Ca2+ in FPRL-1 expressing cells
We initiated our search for additional FPRL-1 ligands by using a comprehensive collection of peptidic and nonpeptidic ligands obtained from commercial sources. The cellular assay used was based on the measurement of intracellular calcium ion release in cells coexpressing Gα16 protein, a G-protein α subunit belonging to the Gq class, and FPRL-1. The overexpression of Gα16 efficiently switches receptor signalling from Gαi/Gαo or Gαs to the PLCβ pathway (Offermanns & Simon, 1995).
Of all the compounds tested (>1300), including more than 77 chemokines, an N-terminally truncated form of CKβ8-1, sCKβ8-1 (Figure 2), and W-peptide, a known FPRL-1 agonist, were the two most potent compounds to elicit a dose-dependent increase in the mobilisation of intracellular calcium response in CHO-K1 cells coexpressing Gα16 protein and FPRL-1 (Figure 3a and Table 1 ). Compounds depicted in Figure 3a did not elicit responses in nontransfected CHO-K1 cells expressing either Gα16 protein or other unrelated G-protein-coupled receptors (data not shown), suggesting the specificity of sCKβ8-1 for FPRL-1. Similar pEC50 values were obtained in Gα16 and FPRL-1 transfected-HEK293s cells (Table 1). Moreover, in the absence of Gα16, similar calcium mobilisation responses were observed with sCKβ8-1 and W-peptide in CHO-K1 cells transiently expressing FPRL-1 (data not shown). Interestingly, the short form of CKβ8 (46–120 aa), and long form of CKβ8 (22–120 aa), and of CKβ8-1 (22–137 aa) displayed low potency (pEC50 <5.7) at FPRL-1 or were inactive (Figure 3b and Table 1). These results suggested that the structural determinants of CKβ8-1 specificity for FPRL-1 might be the 17-amino-acid peptide at the N-terminus, since the remaining sequence of the molecule is identical to CKβ8. To explore this hypothesis, we synthesised the 17-amino acid stretch peptide (Figure 2), named SHAAG peptide (47LWRRKIGPQMTLSHAAG63) and determined its potency in cells coexpressing Gα16 and FPRL-1. The SHAAG peptide was ∼60–200 times less potent at FPRL-1 as compared to sCKβ8-1, but ∼120 times more potent than long form CKβ8-1 (Figure 3b and Table 1). In CHO-K1 and HEK293s cells, coexpressing Gα16 and FPRL-1, other known FPRL-1 ligands (i.e. Aβ42, SAA, and hPrP) were ∼200 to over 1000-fold less potent at FPRL-1 than sCKβ8-1, and are in agreement with published results (Le et al., 2002), whereas, the LXA4 observed potency for FPRL-1 was low (pEC50 <6). To eliminate the possibility that the low potency displayed by the full-length recombinant CKβ8-1 at FPRL-1 is not due to a misfolding during synthesis and/or degradation during purification process, we measured [Ca2+]i release in cells stably expressing CCR1, and confirmed the biological activity of the samples used (Figure 3c). RANTES, a CCR1 agonist, produced a pEC50 value of 9.57±0.06 (n=4), which is in agreement with published results (Chou et al., 2002). The rank order of potency of CKβ8, CKβ8-1 and of the N-terminally truncated forms at inducing calcium flux via CCR1 was as follows: sCKβ8 (46–120 aa) > CKβ8 (22–120 aa) > sCKβ8-1 (46–137 aa) >CKβ8-1 (22–137 aa) (Figure 3c). Hence, the potency of the long form CKβ8-1 is ∼200–300-fold lower at FPRL-1 than, at the CCR1 receptor.
Figure 2.
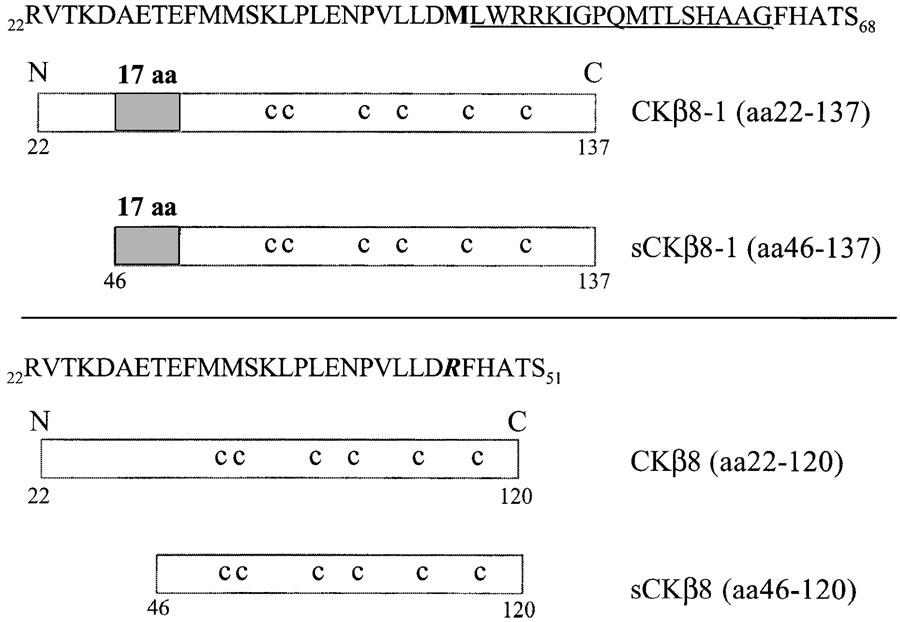
A schematic representation of full-length and truncated forms of CKβ8 and CKβ8-1 used in this study. The signal peptide is from 1 to 21 and is not depicted. The grey box represents the region of 17 amino acid (SHAAG peptide) absent in CKβ8, the corresponding sequence is represented above and underlined. sCKβ8-1 (46–137 aa) starts from methionine (shown in bold) at position 46, sCKβ8 (46–120 aa) starts from arginine (shown in bold and italic) at position 46. c: conserved cysteine residues.
Figure 3.
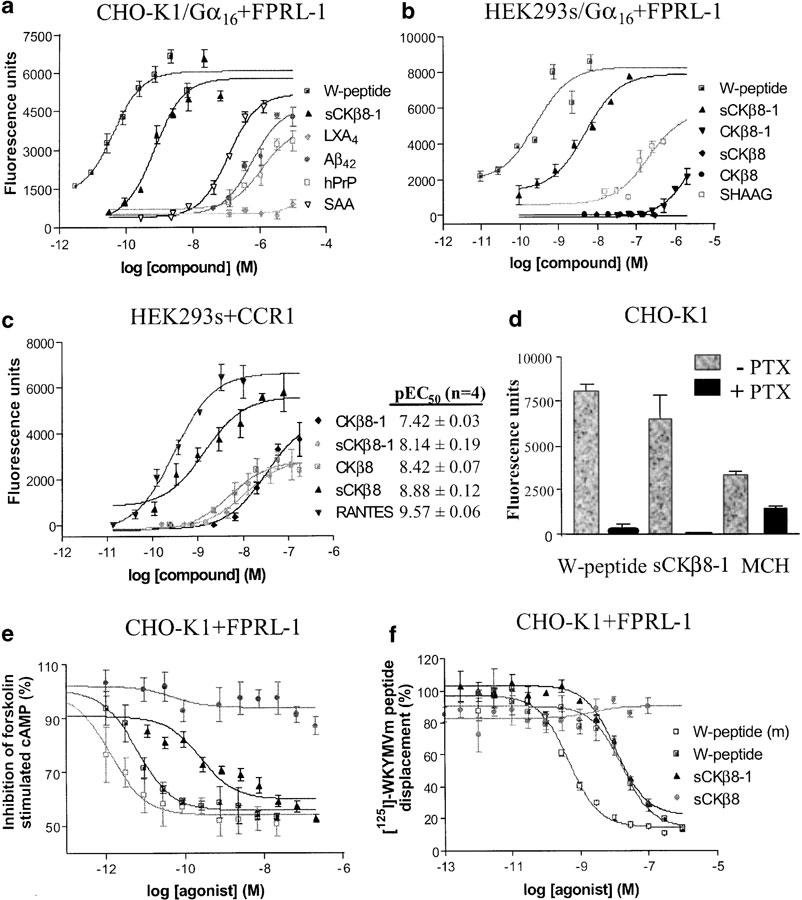
sCKβ8-1 activates and binds to FPRL-1 with high potency, and is coupled to Gαi/Gαo protein. (a) Dose–response curves of the [Ca2+]i changes evoked in the presence of W-peptide, sCKβ8-1, LXA4, Aβ42, hPrP, SAA, in CHO-K1 cells coexpressing Gα16 protein and FPRL-1. (b) [Ca2+]i mobilisation in HEK293s cells coexpressing Gα16 and FPRL-1, in the presence of full-length CKβ8 (22–120 aa), CKβ8-1 (aa22-137), sCKβ8 (46–120 aa), sCKβ8-1 (46–137 aa) and the 17-aa SHAAG peptide. Representative data (from n=3) are shown. (c) CKβ8-1 (22–137 aa) is active on CCR1 stably expressed in HEK293s cells (n=4), pEC50 values are summarised. (d) [Ca2+]i mobilisation was measured after 1 h PTX (100 ng ml−1) treatment, in CHO-K1 cells transiently expressing FPRL-1. PTX blocked (>95%) calcium mobilisation induced by sCKβ8-1 (66 nM) or W-peptide (6.6 nM). In CHO-K1 cells transiently expressing MCH1R, PTX partially inhibited MCH-induced (1 μM) calcium mobilisation response. (e) Dose-dependent inhibition of forskolin-stimulated cAMP accumulation in CHO-K1 cells transiently expressing FPRL-1 with sCKβ8-1. Data are from a representative experiment (from n=3–4) and normalised to the amount of cAMP produced in forskolin-stimulated cells (set to 100%). (f) Dose-dependent displacement of [125I]-WKYMVm by sCKβ8-1. Membrane preparations from CHO-K1 cells stably expressing FPRL-1 were incubated with [125I]-WKYMVm (0.05 nM) in the absence or presence of indicated unlabelled ligands (from 10−5 to 10−15 M). Each point was determined in triplicate, and data are from a representative experiment (n=3). Results are presented as a percentage of displaced [125I]-WKYMVm. pEC50 and pIC50 values are given as mean±s.e.m. Each point is determined in triplicate.
Table 1.
pEC50 and pIC50 values of sCKβ8-1 and known FPRL-1 ligands in various functional assays
| Compound | Intracellular Ca2+ mobilisation pEC50 (n=3) | Adenylyl cyclase pIC50 | [125I]-W-peptide (WKYMVm) displacement, pIC50 (n=3) | |
|---|---|---|---|---|
| CHO-K1 cells | HEK293s cells | CHO-K1 cells | CHO-K1 cells | |
| sCKβ8-1 (aa46-137) | 9.13±0.02 | 8.85±0.07 | 9.02±0.20 (n=4) | 7.97±0.04 |
| SCKβ8 (aa46-120) | <5.0 | Inactive | Inactive (n=2) | Inactive |
| CKβ8-1 (aa22-137) | <5.7 | <5.7 | n.t. | n.t. |
| CKβ8 (aa22-120) | <5.0 | Inactive | n.t. | n.t. |
| SHAAG peptide | 6.74±0.23 | 7.15±0.23 | n.t. | n.t. |
| Amyloid β protein (Aβ42) | 6.09±0.25 | <6.0 | 6.76; 5.90 (n=2) | Inactive |
| Serum amyloid A protein (SAA) | 6.88±0.07 | <6.0 | 6.38; 6.48 (n=2) | <5.52 |
| Lipoxin A4 (LXA4) | <6.0 | <6.0 | <5.7 (n=2) | Inactive |
| Human prion protein (hPrP) | <6.0 | <6.0 | Inactive (n=2) | Inactive |
| W-peptide (WKYMVM) | 10.68±0.25 | 9.56±0.18 | 10.38±0.38 | 7.67±0.06 |
| W-peptide (WKYMVm) | n.t. | n.t. | 11.87; 12.19 (n=2) | 9.34±0.08 (Kd) |
The pEC50 or pIC50 values are given as mean±s.e.m., and were calculated as −log of the EC50 or −log of the IC50 values (50% of the maximal compound effect). n.t.: not tested. The Kd value for WKYMVm was obtained from saturation binding experiments.
FPRL-1 is a Gαi/Gαo-coupled receptor
To determine which Gα protein was involved in the stimulation of PLCβ by the activated human FPRL-1, CHO-K1 cells transiently expressing FPRL-1, in the absence of Gα16 protein, were pretreated with PTX. This pretreatment abolished the calcium responses mediated by W-peptide and sCKβ8-1, suggesting the involvement of Gαi/Gαo protein and not Gαq in this pathway (Figure 3d) (n=3). As a control, CHO-K1 cells transiently expressing MCH1R were treated with PTX and incubated with melanin-concentrating hormone (MCH). In accordance to a proposed dual coupling (Gαi/Gαq) mechanism for this receptor (Hawes et al., 2000), PTX treatment partially (∼50%) inhibited MCH-induced calcium mobilisation response (Figure 3d).
To further demonstrate the involvement of Gαi/Gαo protein in FPRL-1 signalling pathway, the inhibition of forskolin-stimulated cAMP accumulation in CHO-K1 cells was assessed. sCKβ8-1 alone, failed to inhibit basal cAMP levels (data not shown) but did inhibit, in a dose-dependent manner, the forskolin-stimulated cAMP accumulation (n=4) (Figure 3e and Table 1). Previous studies have shown that both forms of the synthetic W-peptide (WKYMVM and WKYMVm) elicited a release of intracellular calcium in cells expressing FPRL-1 with EC50 values of 2 nM and 25 pM, respectively (Christophe et al., 2001). The pIC50 values for W-peptide (10.38±0.38) (n=4) and its isoform (11.87; 12.19) for inhibition of forskolin-stimulated cAMP accumulation are in accordance with published data (Christophe et al., 2001). Nontransfected CHO-K1 cells, or CHO-K1 cells expressing an unrelated GPCR were treated with the same range of agonist concentrations and exhibited no inhibition of cAMP accumulation (data not shown). Aβ42 and SAA, also dose-dependently inhibited forskolin-stimulated cAMP accumulation with pIC50 <6.7, while sCKβ8 and other ligands were found to be weakly active (pIC50 <5.7) or inactive (Table 1). Similar pIC50 values were obtained for sCKβ8-1 and W-peptide in CHO-K1 cells stably expressing FPRL-1 (not shown).
Efficient displacement of [125I]-W-peptide by sCKβ8-1
To characterise the binding properties of sCKβ8-1, membranes prepared from CHO-K1 cells stably expressing FPRL-1 were incubated with the selective FPRL-1 ligand [125I]-WKYMVm (Christophe et al., 2001). The binding was specific and saturable for FPRL-1 using W-peptide (data not shown). Concentrations of unlabelled ligands used to displace [125I]-WKYMVm, were from 0.001 pM to 100 nM for chemokines (sCKβ8-1, sCKβ8), W-peptide and its isoform, and from 1 pM to 10 μM for Aβ42, SAA, LXA4 and hPrP (106-126). The observed Kd value for WKYMVm was 9.34±0.08, and sCKβ8-1 was found to be the most effective, nonsynthetic, agonist at competitively displacing [125I]-WKYMVm (Figure 3f and Table 1), this was followed by SAA with a pIC50 value of <5.52. In agreement with the low potency values observed in the calcium mobilisation assay, other tested compounds did not displace the labelled ligand. Collectively, the data presented clearly demonstrate the ability of sCKβ8-1 to bind and activate FPRL-1 receptor with high efficacy and potency.
sCKβ8-1 induces calcium flux and chemotaxis in polymorphonuclear leukocytes (PMNs)
Neutrophils play a pivotal role in the innate immune response to infection (Ye & Boulay, 1997). Since these cells express FPRL-1, we evaluated the effect of sCKβ8-1 on PMNs calcium mobilisation. In neutrophils, [Ca2+]i increase by PLCβ activation was shown to signal chemotaxis, whereas, high cellular concentrations of cAMP to block that migration (Lang et al., 2003). This is in accordance with FPRL-1 signalling (Gαi/Gαq) pathway (Figures 3d, e). As shown in Figure 4a, sCKβ8-1 elicited a dose-dependent increase in the mobilisation of [Ca2+]i. The rank order of potency for the various FPRL-1 ligands in PMNs was as follows: W-peptide >sCKβ8-1⩾MMK-1. Interleukin-8 (IL-8), known to activate CXCR1 and CXCR2 receptors, induced a dose-dependent calcium response indicating the integrity of the PMNs preparation (Doroshenko et al., 2002).
Figure 4.
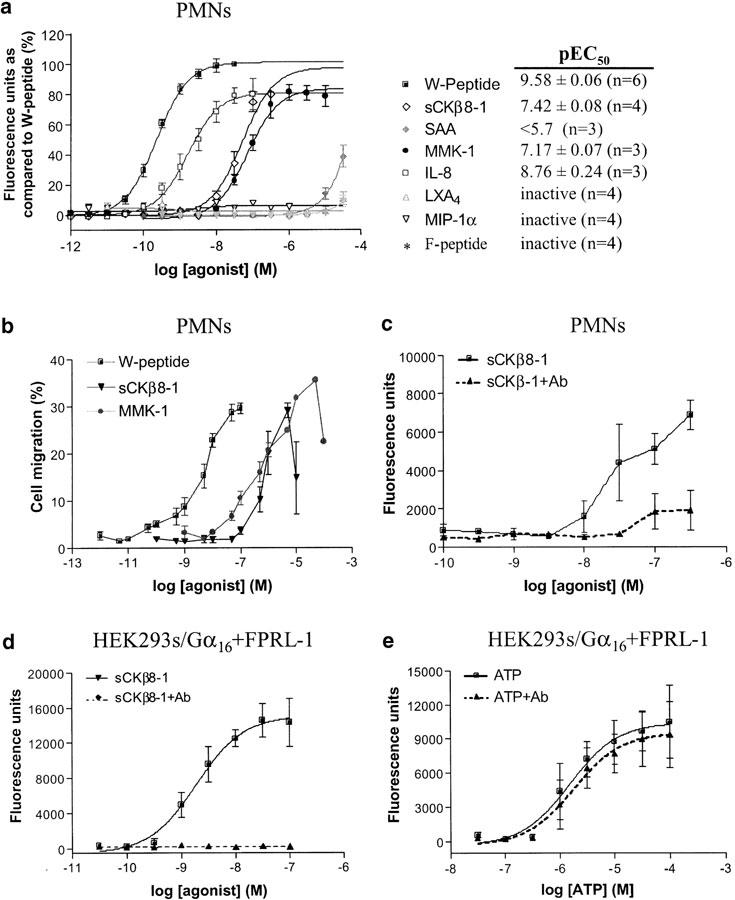
sCKβ8-1 induces calcium flux and chemotaxis in human PMNs through FPRL-1. (a) [Ca2+]i mobilisation in PMNs was measured in response to ligands shown. Data was normalised to the maximum response of W-peptide (WKYMVm) in each donor (n=3–6), and pEC50 values are given as mean±s.e.m. (b) Chemotaxis. Human PMNs were exposed to sCKβ8-1, MMK-1 and W-peptide (WKYMVm) at indicated concentrations. W-peptide and sCKβ8-1 were tested on four donors, and MMK-1 on two donors. Chemotaxis is expressed as the percentage of total cells migrating from the upper chamber through the filter. (c) Human PMNs were preincubated with (dashed lines), or without (solid lines) monoclonal FPRL-1 antibody, and calcium mobilisation in response to sCKβ8-1 was measured. (d) [Ca2+]i mobilisation response in HEK293s cells stably coexpressing FPRL-1 and Gα16, preincubated with FPRL-1 antibody (or not), and activated in the presence of sCKβ8-1 or (e) ATP, at indicated concentrations. In PMNs, the absolute potency of all agonists was found to be variable between donors, therefore the results shown are a representative experiment from one donor (each point was determined in triplicate, except for (c) in duplicate).
The physiological relevance of sCKβ8-1 as a ligand for FPRL-1 was assessed by PMNs chemotaxis experiments. sCKβ8-1, MMK-1 and W-peptide (WKYMVm) induced the migration of PMNs at concentrations ranging from 1 pM to 20 μM. The maximum percentage of cell migration produced by sCKβ8-1 was reached at 1 μM, 12 μM with MMK-1 and 100 nM with W-peptide (Figure 4b).
The cell migration data demonstrates the ability of sCKβ8-1 to activate human PMNs and suggests that this activity is mediated via FPRL-1 receptor endogenously expressed in these cells. To demonstrate the specificity of sCKβ8-1 for FPRL-1, human PMNs were pre-treated in the presence or absence of monoclonal FPRL-1 antibody, and calcium mobilisation in response to sCKβ8-1 was measured. In PMNs, antibody pre-treatment reduced the [Ca2+]i mobilisation by 80–90% when incubated with sCKβ8-1 (Figure 4c, dashed lines). Similar responses were obtained in HEK293s cells stably coexpressing Gα16 and FPRL-1 (Figure 4d). Interestingly, the antibody pretreatment had no effect on ATP-mediated calcium response in these cells (Figure 4e), thus demonstrating the specific blockage of FPRL-1-mediated calcium mobilisation. Collectively, the data confirm the effect produced by sCKβ8-1 in human PMNs is mediated by FPRL-1.
Discussion
In the present study, we have identified a truncated form of the chemokine CKβ8-1 (sCKβ8-1) as a potent agonist for human FPRL-1, capable of inducing the release of intracellular calcium and inhibiting forskolin-stimulated cAMP accumulation in a PTX-sensitive manner. Moreover, sCKβ8-1 mediated a calcium flux and chemotactic activity in human PMNs via FPRL-1 suggesting a physiological role for this β-chemokine.
Lipoxins are members of the eicosanoid family of bioactive lipid mediators generated during cell – cell interactions (Maddox et al., 1997), and Lipoxin A4 was the first reported FPRL-1 agonist (Fiore et al., 1994; Fiore & Serhan, 1995). The binding of [3H]-LXA4 to FPRL-1 has been studied on neutrophils and on membranes expressing FPRL-1 (Kd=6.1 nM) (Fiore et al., 1994; Fiore & Serhan, 1995; Maddox et al., 1997). The activation of FPRL-1 by LXA4 was shown to release arachidonate via phospholipase A2 and phospholipase D pathways (Fiore et al., 1994; Fiore & Serhan, 1995). In various functional assays, we observed that potencies for some putative endogenous ligands were considerably lower than sCKβ8-1. However, the pEC50 values we obtained with these endogenous ligands are in close agreement with published data (Le et al., 2002). In our experimental conditions, LXA4 had no effect on the phospholipase C signalling pathway, nor did it inhibit the forskolin-stimulated cAMP accumulation. Moreover, LXA4 did not competitively displace [125I]-W-peptide on CHO-K1 cells expressing FPRL-1, nor induce calcium mobilisation in PMNs, at concentrations up to 10 μM. The discrepancy between our data and the reported potency values for LXA4 (Fiore et al., 1994; Fiore & Serhan, 1995; Le et al., 2002) may be explained by different experimental conditions: (i) intracellular calcium flux measurement activated by phospholipase C pathway compared to arachidonate release measurement, and (ii) a quite selective radiolabelled FPRL-1 agonist (W-peptide isoform) (Christophe et al., 2001) for displacement studies, as compared to [3 H]-LXA4 displacement (Fiore et al., 1994; Fiore & Serhan, 1995; Chiang et al., 2000). In a recent study using neutrophils, which express FPR and FPRL-1, LXA4 failed to generate any agonist-induced biological responses mediated by W-peptide, such as NADPH-oxidase activation and increase of the CR3 granules, an indicator of neutrophil cells secretory machinery (Christophe et al., 2002). Hence, the authors concluded that LXA4 most likely activated and signalled its effects via a receptor different from FPRL-1. It is also possible to conceive that LXA4 may be recognising other entities expressed on cells, and this interaction may somehow depend or be synergised with the expression of FPRL-1.
The mRNA expression profile for FPRL-1 revealed that the receptor is predominantly distributed in lung, bone marrow, synovial tissues, and in various cell types such as monocytes and neutrophils. The FPRL-1 mRNA expression pattern in inflammatory tissues and cells suggested the possibility this receptor could be activated by a chemokine. CKβ8 and CKβ8-1 mRNAs are abundantly present in pancreas, and at lower extent in skeletal muscle and liver (Youn et al., 1998). Immunocytochemistry studies demonstrated the presence of CKβ8 (and most probably CKβ8-1) in preosteoblasts (Votta et al., 2000). Interestingly, CKβ8 displays chemotactic activity for osteoclast precursors isolated from human osteoclastoma tissues, monocytes and resting lymphocytes, suggesting a possible role for FPRL-1 in proinflammatory and immune reactions (Forssmann et al., 1997). In this regard, we have demonstrated that sCKβ8-1 activates human PMNs and induces chemotaxis. A possible link between high pancreatic CKβ8-1 expression (Youn et al., 1998) and FPRL-1 activation may be inflammatory diseases, such as the acute pancreatitis, mediated through the overexpression and secretion of the pancreatitis-associated protein (PAP). In rat, PAP injection led to neutrophil recruitment and inflammatory reactions in lung (Folch-Puy et al., 2003). Moreover, in pancreas, the β cells and periacinar myofibroblasts were shown to produce and secrete biologically active chemokines (i.e. MCP-1, RANTES, IL-8, MIP-1α) (Piemonti et al., 2002). In this context, since FPRL-1 is expressed in the lung, the possible role of PAP and other proinflammatory stimuli on the expression and secretion of CKβ8-1 from pancreas remains to be investigated.
Youn and colleagues have demonstrated the ability of long-form CKβ8 and CKβ8-1 to induce a rapid release of intracellular calcium via CCR1 in transfected HOS cells, and to trigger chemoattraction in neutrophils (Youn et al., 1998). Surprisingly, they found that MIP-1α, a known CCR1 and CCR5 agonist, induced robust Ca2+ mobilisation in neutrophils but did not provoke chemotaxis (McColl et al., 1993). In the present work however, MIP-1α did not mobilise [Ca2+]i in PMNs, suggesting variable CCR1 and/or CCR5 expression levels. This notion is supported by reports suggesting the variable expression or existence of CCRs (i.e. CCR1) in resting human neutrophils (Cheng et al., 2001). We discovered that the sCKβ8-1-driven calcium flux in PMNs was blocked using a monoclonal FPRL-1 antibody, indicating that in PMNs the sCKβ8-1 response is mediated via FPRL-1.
In this study, we demonstrated the potent activity of a truncated form of CKβ8-1 for FPRL1. Albeit, we have not demonstrated the processing of sCKβ8-1 however, in the literature post-translational modifications including NH2-terminal truncation of chemokines have been shown to affect their biochemical and biological characteristics (Proost et al., 1998). Hemofiltrate CC chemokine 1 (HCC-1), lacking the first eight amino acids was isolated from tumor cell lines (Vakili et al., 2001). The full-length HCC-1 was shown to be a weak agonist for CCR1 and was inactive at CCR5, however, HCC-1[9–74 aa] was characterised as a potent agonist for CCR1, CCR3, CCR5 (Vakili et al., 2001). Similar observations have been made for IL-8 and MIP-1β where the alternatively processed forms elicited different potencies on several chemokine receptors (Hebert et al., 1990). As a final note, the expression of Lkn-1 and CKβ8 in insect cells, two chemokines closely related to CKβ8-1 (Youn et al., 1998), resulted in the synthesis and secretion of N-terminally processed variants lacking the first 24 amino acids (sCKβ8) (Macphee et al., 1998; Lee et al., 2002). These deletions increased the potency ∼100-fold for CCR1 (Macphee et al., 1998; Berkhout et al., 2000; Lee et al., 2002). Interestingly, we have confirmed that the truncated forms of CKβ8 and CKβ8-1 have enhanced potencies at CCR1, and the full-length CKβ8-1 displayed a low potency at activating human FPRL-1. However, sCKβ8-1 was ∼2000 times more potent for this receptor. An integral part of the specificity of sCKβ8-1 at activating FPRL-1 is dependent on the 17-amino-acid region (SHAAG peptide), which is absent in the alternatively spliced variant CKβ8. The data, together with previously published results on Lkn-1, CKβ8 and other CC chemokines (i.e. HCC1, MCP-1, MCP-2, MIP-1β), suggest that the processing of the N-terminus of some members of β-chemokines, including CKβ8-1, may represent a novel mechanism to increase the diversity of inflammatory effects inherent to these ligands.
In conclusion, we have identified the N-terminally truncated form of CKβ8-1, as a highly potent ligand of human FPRL-1. Given their broad chemotactic specificities, β-chemokines may play a central role in development and maintenance of the leukocyte infiltration found in many diseases, such as allergic inflammation, arthritis, nephritis and experimental autoimmune encephalomyelitis (Ye & Boulay, 1997). Our discovery could have interesting implications for the development of anti-inflammatory therapies by developing selective antagonists to FPRL-1.
Acknowledgments
We thank V. Brechler, D. Handfield, D. Rivard and R. Skrovanek (PerkinElmer Life Sciences, Montréal, QC, Canada) for binding experiments, M. Duchesne and M. Valiquette for technical expertise with cell culture (AstraZeneca R&D Montréal, QC, Canada), S. Stinson and T. Phillips (AstraZeneca R&D, Charnwood, England) for antibody screening.
Abbreviations
- cAMP
cyclic AMP
- CHO-K1
Chinese hamster ovary
- FLIPRTM
Fluorescent Imaging Plate Reader
- FPRL-1
formyl peptide-receptor-like-1
- GPCRs
G protein-coupled receptors
- HEK293 s
Human embryonic kidney (s, adapted from suspension)
- MPIF-1
myeloid progenitor inhibitor factor-1
- PMNs
polymorpho-nuclear leukocytes
- PTX
pertussis toxin
- RT
reverse transcription
References
- BERKHOUT T.A., GOHIL J., GONZALEZ P., NICOLS C.L., MOORES K.E., MACPHEE C.H., WHITE J.R., GROOT P.H. Selective binding of the truncated form of the chemokine CKβ8 (25–99) to CC chemokine receptor 1(CCR1) Biochem. Pharmacol. 2000;59:591–596. doi: 10.1016/s0006-2952(99)00354-8. [DOI] [PubMed] [Google Scholar]
- CHENG S.S., LAI J.J., LUKACS N.W., KUNKEL S.L. Granulocyte–macrophage colony stimulating factor up-regulates CCR1 in human neutrophils. J. Immunol. 2001;166:1178–1184. doi: 10.4049/jimmunol.166.2.1178. [DOI] [PubMed] [Google Scholar]
- CHIANG N., FIERRO I.M., GRONERT K., SERHAN C.N. Activation of lipoxin A4 receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOU C.C., FINE J.S., PUGLIESE-SIVO C., GONSIOREK W., DAVIES L., DENO G., PETRO M., SCHWARZ M., ZAVODNY P.J., HIPKIN R.W. Pharmacological characterization of the chemokine receptor, hCCR1 in a stable transfectant and di R1 activation by MIP-1β. Br. J. Pharmacol. 2002;137:663–675. doi: 10.1038/sj.bjp.0704907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOPHE T., KARLSSON A., DUGAVE C., RABIET M.J., BOULAY F., DAHLGREN C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J. Biol. Chem. 2001;276:21585–21593. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- CHRISTOPHE T., KARLSSON A., RABIET M.J., BOULAY F., DAHLGREN C. Phagocyte activation by Trp-Lys-Tyr-Met-Val-Met, acting through FPRL1/LXA4R, is not affected by lipoxin A4. Scand. J. Immunol. 2002;56:470–476. doi: 10.1046/j.1365-3083.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- DOROSHENKO T., CHALY Y., SAVITSKIY V., MASLAKOVA O., PORTYANKO A., GORUDKO I., VOITENOK N.N. Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood. 2002;100:2668–2671. doi: 10.1182/blood.100.7.2668. [DOI] [PubMed] [Google Scholar]
- DURSTIN M., GAO J.L., TIFFANY H.L., MCDERMOTT D., MURPHY P.M. Differential expression of members of the N-formylpeptide receptor gene cluster in human phagocytes. Biochem. Biophys. Res. Commun. 1994;201:174–179. doi: 10.1006/bbrc.1994.1685. [DOI] [PubMed] [Google Scholar]
- FIORE S., SERHAN C.N. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4–lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- FIORE S., MADDOX J.F., PEREZ H.D., SERHAN C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH-PUY E., GARCIA-MOVTERO A., IOVANNA J.L., DAGORN J.C., PRATS N., VACCARO M.I., CLOSA D. The pancreatitis-associated protein induces lung inflammation in the rat through activation of TNFα expression in hepatocytes. J. Pathol. 2003;199:398–408. doi: 10.1002/path.1307. [DOI] [PubMed] [Google Scholar]
- FORSSMANN U., DELGADO M.B., UGUCCIONI M., LOETSCHER P., GAROTTA G., BAGGIOLINI M. CKβ8, a novel CC chemokine that predominantly acts on monocytes. FEBS Lett. 1997;408:211–216. doi: 10.1016/s0014-5793(97)00408-0. [DOI] [PubMed] [Google Scholar]
- FREDRIKSSON R., LAGERSTROM M.C., LUNDIN L.G., SCHIOTH H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- GROBLEWSKI T., MAIGRET B., NOUET S., LARGUIER R., LOMBARD C., BONNAFOUS J.C., MARIE J. Mutation of Asn111 in the third transmembrane domain of the AT1A angiotensin II receptor induces its constitutive activation. J. Biol. Chem. 1997;272:1822–1826. doi: 10.1074/jbc.272.3.1822. [DOI] [PubMed] [Google Scholar]
- HAVILAND D.L., BOREL A.C., FLEISCHER D.T., HAVILAND J.C., WETSEL R.A. Structure, 5′-flanking sequence, and chromosome location of the human N-formyl peptide receptor gene. A single-copy gene comprised of two exons on chromosome 19q.13.3 that yields two distinct transcripts by alternative polyadenylation. Biochemistry. 1993;32:4168–4174. doi: 10.1021/bi00067a003. [DOI] [PubMed] [Google Scholar]
- HAWES B.E., KIL E., GREEN B., O'NEILL K., FRIED S., GRAZIANO M.P. The melanin-concentrating hormone receptor couples to multiple G proteins to activate diverse intracellular signaling pathways. Endocrinology. 2000;141:4524–4532. doi: 10.1210/endo.141.12.7833. [DOI] [PubMed] [Google Scholar]
- HEBERT C.A., LUSCINSKAS F.W., KIELY J.M., LUIS E.A., DARBONNE W.C., BENNETT G.L., LIU C.C., OBIN M.S., GIMBRONE M.A., JR, BAKER J.B. Truncation of NH2-terminal amino acid residues increases agonistic potency of leukotactin-1 on CC chemokine receptors 1 and 3. J. Immunol. 1990;145:3033–3040. [Google Scholar]
- LANG K., HATT H., NIGGEMANN B., ZAENKER K.S., ENTSCHLADEN F. A novel function for chemokines: downregulation of neutrophil migration. Scand. J. Immunol. 2003;57:350–361. doi: 10.1046/j.1365-3083.2003.01247.x. [DOI] [PubMed] [Google Scholar]
- LE Y., GONG W., LI B., DUNLOP N.M., SHEN W., SU S.B., YE R.D., WANG J.M. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation m. J. Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- LE Y., HU J., GONG W., SHEN W., LI B., DUNLOP N.M., HALVERSON D.O., BLAIR D.G., WANG J.M. Expression of functional formyl peptide receptors by human astrocytoma cell lines. J. Neuroimmunol. 2000;111:102–108. doi: 10.1016/s0165-5728(00)00373-8. [DOI] [PubMed] [Google Scholar]
- LE Y., MURPHY P.M., WANG J. Formyl-peptide receptors revisited. Trends Immunol. 2002;10:1–7. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- LE Y., OPPENHEIM J., WANG J.M. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001;12:91–105. doi: 10.1016/s1359-6101(01)00003-x. [DOI] [PubMed] [Google Scholar]
- LEE J.K., LEE E.H., YUN Y.P., KIM K., KWACK K., NA D.S., KWON B.S., LEE C.K. Identification of a truncated form of the CC chemokine CKβ-8 demonstrating greatly enhanced biological activity. J. Biol. Chem. 2002;277:14757–14763. [Google Scholar]
- LEMBO P.M., GRAZZINI E., CAO J., HUBATSCH D.A., PELLETIER M., HOFFERT C., ST-ONGE S., POU C., LABRECQUE J., GROBLEWSKI T., O'DONNELL D., PAYZA K., AHMAD S., WALKER P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat. Cell Biol. 1999;5:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- MACPHEE C.H., APPELBAUM E.R., JOHANSON K., MOORES K.E., IMBURGIA C.S., FORNWALD J., BERKHOUT T., BRAWNER M., GROOT P.H., O'DONNELL K., O'SHANNESSY D., SCOTT G., WHITE J.R. Identification of a truncated form of the CC chemokine CKβ-8 demonstrating greatly enhanced biological activity. J. Immunol. 1998;161:6273–6279. [PubMed] [Google Scholar]
- MADDOX J.F., HACHICHA M., TAKANO T., PETASIS N.A., FOKIN V.V., SERHAN C.N. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J. Biol. Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- MCCOLL S.R., HACHICHA M., LEVASSEUR S., NEOTE K., SCHALL T.J. Uncoupling of early signal transduction events from effector function in human peripheral blood neutrophils in response to recombinant macrophage inflammatory proteins-1α and -1β. J. Immunol. 1993;150:4550–4560. [PubMed] [Google Scholar]
- MURPHY P.M., OZCELIK T., KENNEY R.T., TIFFANY H.L., MCDERMOTT D., FRANCKE U. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- MURPHY P.M., BAGGIOLINI M., CHARO I.F., HEBERT C.A., HORUK R., MATSUSHIMA K., MILLER L.H., OPPENHEIM J.J., POWER C.A. Power. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- OFFERMANNS S., SIMON M.I. Gα15 and Gα16 couple a wide variety of receptors to phospholipase C. J. Biol. Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- PIEMONTI L., LEONE B.E., NANO R., SACCANIM A., MONTI P., MAFFI P., BIANCHI G., SICA A., PERI G., MELZI R., ALDRIGHETTI L., SECCHI A., DI CARLO V., ALLAVENA P., BERTUZZI F. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- PROOST P., STRUYF S., COUVREUR M., LENAERTS J.P., CONINGS R., MENTEN P., VERHAERT P., WUYTS A., VAN DAMME J. Posttranslational modifications affect the activity of the human monocyte chemotactic proteins MCP-1 and MCP-2: identification of MCP-2 (6-76) as a natural chemokine inhibitor. J. Immunol. 1998;160:4034–4041. [PubMed] [Google Scholar]
- VAKILI J., STANDKER L., DETHEUX M., VASSART G., FORSSMANN W.G., PARMENTIER M. Urokinase plasminogen activator and plasmin efficiently convert hemofiltrate CC chemokine 1 into its active [9-74] processed variant. J. Immunol. 2001;167:3406–3413. doi: 10.4049/jimmunol.167.6.3406. [DOI] [PubMed] [Google Scholar]
- VOTTA B.J., WHITE J.R., DODDS R.A., JAMES I.E., CONNOR J.R., LEE-RYKACZEWSKI E., EICHMAN C.F., KUMAR S., LARK M.W., GOWEN M. CKβ-8 [CCL23], a novel CC chemokine, is chemotactic for human osteoclast precursors and is expressed in bone tissues. J. Cell. Physiol. 2000;183:196–207. doi: 10.1002/(SICI)1097-4652(200005)183:2<196::AID-JCP6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- YE R.D., BOULAY F. Structure and function of leukocyte chemoattractant receptors. Adv. Pharmacol. 1997;39:221–289. doi: 10.1016/s1054-3589(08)60073-3. [DOI] [PubMed] [Google Scholar]
- YOUN B.S., ZHANG S.M., BROXMEYER H.E., COOPER S., ANTOL K., FRASER M., JR, KWON B.S. Characterization of CKβ8 and CKβ8-1: two alternatively spliced forms of human β-chemokine, chemoattractants for neutrophils, monocytes, and lymphocytes, and potent agonists at CC chemokine receptor 1. Blood. 1998;91:3118–3126. [PubMed] [Google Scholar]


