Abstract
The ability of the endogenous fatty acid amide, cis-oleamide (ODA), to bind to and activate cannabinoid CB1 and CB2 receptors was investigated.
ODA competitively inhibited binding of the nonselective cannabinoid agonist [3H]CP55,940 and the selective CB1 antagonist [3H]SR141716A to rat whole-brain membranes with Ki values of 1.14 μM (0.52–2.53 μM, Hill slope=0.80, n=6) and 2.63 μM (0.62–11.20 μM, Hill slope=0.92, n=4), respectively. AEA inhibited [3H]CP55,940 binding in rat whole-brain membranes with a Ki of 428 nM (346–510 nM, Hill slope=−1.33, n=3).
ODA competitively inhibited [3H]CP55,940 binding in human CB1 (hCB1) cell membranes with a Ki value of 8.13 μM (4.97–13.32 μM, n=2). In human CB2 transfected (hCB2) HEK-293T cell membranes, 100 μM ODA produced only a partial (42.5±7%) inhibition of [3H]CP55,940 binding.
ODA stimulated [35S]GTPγS binding in a concentration-dependent manner (EC50=1.64 μM (0.29–9.32 μM), R2=0.99, n=4–9), with maximal stimulation of 188±9% of basal at 100 μM. AEA stimulated [35S]GTPγS binding with an EC50 of 10.43 μM (4.45–24.42 μM, R2=1.00, n=3, 195±4% of basal at 300 μM). Trans-oleamide (trans-ODA) failed to significantly stimulate [35S]GTPγS binding at concentrations up to 100 μM.
ODA (10 μM)-stimulated [35S]GTPγS binding was reversed by the selective CB1 antagonist SR141716A (IC50=2.11 nM (0.32–13.77 nM), R2=1.00, n=6).
The anatomical distribution of ODA-stimulated [35S]GTPγS binding in rat brain sections was indistinguishable from that of HU210. Increases of similar magnitude were observed due to both agonists in the striatum, cortex, hippocampus and cerebellum.
ODA (10 μM) significantly inhibited forskolin-stimulated cyclic AMP (cAMP) accumulation in mouse neuroblastoma N1E 115 cells (P=0.02, n=11). ODA-mediated inhibition was completely reversed by 1 μM SR141716A (P<0.001, n=11) and was also reversed by pretreatment with 300 ng ml−1 pertussis toxin (P<0.001, n=6).
These data demonstrate that ODA is a full cannabinoid CB1 receptor agonist. Therefore, in addition to allosteric modulation of other receptors and possible entourage effects due to fatty acid amide hydrolase inhibition, the effects of ODA may be mediated directly via the CB1 receptor.
Keywords: Cis-9,10-octadecanoamide, oleamide (ODA); HU210; 11-hydroxy-dimethylheptyl-Δ8-tetrahydrocannabinol; cannabinoid agonist; endocannabinoid; CB1 receptor; CB2 receptor; rat; human; radioligand binding; [35S]GTPγS binding
Introduction
Cis-9,10-octadecanoamide (oleamide, ODA) is an endogenous sleep-inducing substance, first isolated from the cerebro-spinal fluid of sleep-deprived cats (Cravatt et al., 1995). The primary amide of oleic acid, ODA is an endogenous fatty acid amide of the same family of chemical messengers that includes the endocannabinoid anandamide (AEA). Although its synthetic pathway is unclear, ODA has been shown to be produced in mouse brain microsomes (Sugiura et al., 1996) and mouse neuroblastoma cells where ODA was found to be 78 times more abundant than AEA (Bisogno et al., 1997). ODA is a preferred substrate for the serine hydrolase FAAH, the enzyme responsible for the hydrolysis of all fatty acid amides including AEA (Boger et al., 2000a).
ODA has been shown to have various effects in vitro including inhibition of gap junction-mediated cell–cell communication (Boger et al., 1999), modulation of 5-HT1, 5-HT2A,C and 5-HT7 receptors (Thomas et al., 1997; 1999; Hedlund et al., 1999) and modulation of inhibitory ionotropic receptors such as the GABAA receptor (Coyne et al., 2002).
When administered in vivo, ODA produces similar effects to AEA. Both induce sleep, ODA increasing the slow wave sleep 2 (SWS2) phase (Cravatt et al., 1995; Boger et al., 1998; Yang et al., 1999; Huitron-Resendiz et al., 2001) and AEA increasing SWS2 and rapid eye movement (REM) sleep (Murillo-Rodriguez et al., 1998). ODA induces the classic tetrad of behaviours, used to identify cannabinergic activity, with a similar activity profile to AEA (Mechoulam et al., 1997). ODA produces a dose-dependent hypothermia and a decrease in locomotor activity in both mice and rats (Mechoulam et al., 1997, Huitron-Resendiz et al., 2001). ODA also induces catalepsy in mice (Mechoulam et al., 1997) but not in rats (Fedorova et al., 2001) and produces antinociception in both species (Mechoulam et al., 1997; Fedorova et al., 2001; Murillo-Rodriguez et al., 2001). Studies using the selective CB1 receptor antagonist SR141716A have yielded conflicting results. SR141716A has been shown to reverse the effects of ODA on sleep (Mendelson & Basile, 1999), locomotor activity, ODA-potentiated 5-HT-mediated behaviours (Cheer et al., 1999) and antinociception (Fedorova et al., 2001) yet in a recent report failed to reverse locomotor activity and ptosis (Lichtman et al., 2002). SR141716A failed to reverse ODA-induced hypothermia in both studies that examined the effect (Fedorova et al., 2001; Lichtman et al., 2002). Further, Lichtman et al. (2002) investigated the ability of ODA to induce cannabinoid-like effects in CB1 knockout mice. All three of the effects studied (hypolocomotion, hypothermia and ptosis) were induced by ODA in CB1 knockout mice. However, the ability of ODA to induce sleep, analgesia or catalepsy in CB1 knockout mice was not reported.
In vitro binding studies have also provided conflicting results. Boring et al. (1996) reported that ODA competitively inhibited [3H]CP55,940 binding in rat brain membranes with a relatively low affinity (44 μM). However, ODA apparently failed to activate cannabinoid receptors (as demonstrated by the lack of [35S]GTPγS binding) at concentrations up to 100 μM. Other groups found that ODA negligibly bound (Lichtman et al., 2002) or was unable to bind (Mechoulam et al., 1997; Sheskin et al., 1997) to either the CB1 or CB2 receptor at concentrations up to 10 μM. In order to explain the cannabinergic effects of ODA, with no apparent binding to the CB1 receptor, an ‘entourage' effect was suggested (Lambert & Di Marzo, 1999). ODA may potentiate or prolong the effects of endocannabinoids such as AEA by competitively inhibiting the enzyme FAAH (Mechoulam et al., 1997). A more recent study by Cheer et al. (1999) found that ODA inhibited [3H]CP55,940 binding with an IC50 of 10 μM (with an increase of affinity in the presence of the FAAH inhibitor phenyl-methylsulphonyl fluoride) suggesting that ODA might indeed exert some effects directly via the CB1 receptor.
Owing to the inconsistent nature of both the in vitro and in vivo findings, the involvement of cannabinoid receptors in the actions of ODA is disputed. In the present study, radioligand binding was used to determine the ability of ODA and AEA to bind to the CB1 receptor in rat whole-brain membranes and ODA to human-CB1 (hCB1) transfected human embryonic kidney (HEK)-293T cells. The ability of ODA to bind to the human-CB2 (hCB2) receptor in transfected HEK-293T cells was also tested. The [35S]GTPγS membrane binding assay was used to determine the ability of ODA to activate rat brain CB1 receptors and was compared with two positive controls, HU210 and AEA. Receptor specificity was determined by performing the assay in the presence of selective CB1 receptor antagonists. [35S]GTPγS autoradiography in rat brain sections was used to investigate the distribution of activated CB1 receptors after exposure to ODA compared to the cannabinoid agonist HU210. Cyclic AMP (cAMP) accumulation assays were performed in N1E 115 cells, which endogenously express CB1 receptors, to determine whether ODA inhibits adenylyl cyclase via the CB1 receptor.
The data presented here indicate that ODA is a full endogenous cannabinoid receptor agonist with selectivity for the CB1 receptor.
Some of the data presented have been communicated in preliminary form to the British Pharmacological Society (Ralevic et al., 2000; Leggett et al., 2002).
Methods
Materials
HU210, AEA and ODA were obtained from Tocris Cookson, U.K. SR141716A was supplied via the NIMH synthesis programme (contract NOIMH30003) and LY320135 was a gift from Eli Lilly (U.S.A.). Pertussis toxin was obtained from Sigma (U.K.) and stored at −20°C until required. Trans-ODA was a kind gift from Dr G. Lees (University of Sunderland). ODA and trans-ODA were made as stock solutions in ethanol or DMSO (10 mM) and stored at −20°C until required. HU210, SR141716A and LY320135 were made as stock solutions in ethanol (10 mM) and stored at −20°C until required.
Rat brain membrane preparation
Rats were killed by decapitation and brains (minus brainstem) were removed and rapidly homogenised in ice-cold Tris buffer (50 mM Tris, 100 mM NaCl, 10 mM MgCl2, pH 7.4). with a glass/teflon homogeniser. The homogenate was centrifuged at 20,000 × g at 4°C for 10 min and the pellet resuspended in Tris buffer. Homogenisation and centrifugation were repeated twice and the final pellet was resuspended in storage buffer (50 mM Tris, 1 mM EDTA, pH 7.4) to a protein concentration of 2 mg ml−1 for [35S]GTPγS binding or 5 mg ml−1 for radioligand binding (Bradford, 1976).
hCB1 and hCB2 transient transfection and membrane preparation
HEK-293T cells were grown in Dubelcco's modified Eagle's medium (DMEM) with 10% foetal bovine serum and high glucose. Cells were transiently transfected with pcDNA3 encoding CB1 or CB2 using calcium phosphate precipitation and used for membrane preparation as described previously (Abadji et al., 1999). Briefly, at 24 h post-transfection, cells were harvested, washed twice in phosphate-buffered saline (PBS) and resuspended in PBS with 0.1% v v−1 protease inhibitor cocktail (Sigma Chemical Co., St Louis, MO, U.S.A.). Cell disruption was performed with nitrogen cavitation at 750 psi for 5 min. Following removal of cell debris and nuclei by differential centrifugation, membranes were resuspended in TME buffer (25 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 1 mM EDTA)+7% sucrose. The membrane preparation was diluted to 0.6 μg ml−1 total protein concentration (Bradford, 1976) and stored at −70°C.
Mouse neuroblastoma N1E 115 cell culture
Mouse neuroblastoma N1E 115 cells were grown to confluence in culture flasks and maintained in DMEM with 10% foetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. Cells were then diluted and distributed into 24-well plates to a final volume of 1 ml per well 72 h prior to incubation with [3H]adenine.
Radioligand binding in rat whole-brain membranes
Assay tubes were prepared in triplicate to a final volume of 1 ml containing 0.5 nM [3H]CP55,940 (Perkin-Elmer Life Sciences Inc., U.S.A.) or [3H]SR141716A (Amersham, U.K.) and 250 μg of protein diluted in Tris buffer (50 mM Tris·HCl, 2 mM EDTA, 5 mM MgCl2 also containing 0.2 mg ml−1 bovine serum albumin (BSA), pH7.4). Stock drugs were diluted in the same buffer containing 5 mg ml−1 BSA. Tubes were incubated for 90 min at 30°C. Specific binding was calculated by subtracting nonspecific binding (NSB, determined in the presence of 1 μM HU210). Nonlinear regression analysis was carried out using Graphpad Prism3. Ki values for ODA and AEA were determined from IC50 values using a KD of 550 pM for [3H]CP55940 and 600 pM for [3H]SR141716A, both as described in a recent review (Pertwee, 1997). Results are presented as mean values, with 95% confidence intervals.
Radioligand binding assays with HEK-293T membrane preparations containing hCB1 and hCB2 receptors
Radioligand binding assays were performed as previously described (Abadji et al., 1999). Membrane preparations were incubated with 4 nM [3H]CP55,940 with varying concentrations of ODA. NSB was determined with 1 μM CP55,940. All conditions were prepared in triplicate. A Brandel cell harvester (Brandel Inc., Gaitherburg, MD, U.S.A.) was used to separate bound from unbound ligand and the former quantified by liquid scintillation counting. Results were analysed with Prism GraphPAD Software (GraphPAD Software, San Diego, CA, U.S.A.) as previously described (Chin et al., 1999) using the Cheng–Prusoff equation to determine Ki values with the KD value of 4.7 nM for CP55,940 binding to CB1 HEK 293T membrane preparations. Data are given as the mean from two independent experiments.
[35S]GTPγS membrane binding
Membranes were preincubated with 100 μM GDP (ICN Biomedicals Inc., U.K.) and 1 mM theophylline (Sigma, U.K.) in assay buffer (50 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2 containing 5 mg ml−1 BSA, pH 7.4) for 20 min at 30°C. Assay tubes were prepared in triplicate using the same buffer containing 500 μl preincubated membranes, 0.4 nM [35S]GTPγS (Amersham, U.K.) and the appropriate concentrations of drug. The NSB was determined using 100 μM nonradiolabelled GTPγS and 1 μM HU210 was included as a positive control. Tubes were incubated for 45 min at 30°C. Free and bound [35S]GTPγS were separated by rapid filtration (using filter mats presoaked in Tris buffer (pH 7.4) containing 5% BSA) with ice-cold Tris buffer (pH 7.4). Data are presented as mean values, with 95% confidence intervals for experiments performed in triplicate. EC50 and IC50 values were determined using nonlinear regression analysis of concentration response curves using Graphpad Prism3.
[35S]GTPγS autoradiography
Rats were killed by decapitation and brains were rapidly removed and frozen on dry ice. Coronal brain sections (20 μM) were cut on a cryostat and thaw mounted onto glass microscope slides. Sections were preincubated with 100 mM GDP in buffer A (50 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, pH 7.4) for 30 min at 25°C. Sections were then incubated for 90 min at 25°C with 0.2 nM [35S]GTPγS in the presence or absence of 10 μM ODA or 1 μM HU210 in buffer B (50 mM Tris, 100 mM NaCl, 10 mM MgCl2, pH7.4 containing 0.2 mg ml−1 BSA). The NSB was determined by incubating sections with 0.2 nM [35S]GTPγS in the presence of 100 μM nonlabelled GTPγS. After incubation, sections were washed, dried and exposed to β-max hyperfilm (Amersham, U.K.) for 72 h at −80°C. A [14C] calibration strip was included to enable quantification of the autoradiograms after development. Autoradiographic images were analysed using NIH image (v1.62) for Macintosh computer. Quantification of images was achieved by densitometric analysis using calibrated 14C standards to convert the data to disintegrations per minute. Data were then normalised and presented as % basal binding±s.e.m. The Wilcoxon signed rank statistical tests were carried out using Graphpad Prism3 with P<0.05 considered significant.
Inhibition of forskolin-stimulated cAMP generation in mouse neuroblastoma N1E 115 cells with and without pertussis toxin pretreatment
Mouse neuroblastoma N1E 115 cells that express CB1 but not CB2 receptors (Zhou & Song, 2001) were grown on 24-well plates in DMEM with 10% foetal calf serum at 37°C in an atmosphere of 5% CO2 and air. Cells were maintained at 37°C throughout the experiment. After 48 h, [3H]adenine was added to a final concentration of 74 kBq ml−1 and 300ng ml−1 pertussis toxin was added when appropriate. Cells were incubated for a further 24 h before being washed twice with Hank's HEPES buffer (HHB) pH7.4 and left to equilibrate for 15 min in 1 ml of HHB. Rolipram (10 μM) (phosphodiesterase inhibitor) was added to each well to reduce enzymatic breakdown of cAMP followed immediately by antagonist (if required). Cannabinoids or vehicle (HHB) were added after 5 min and allowed to incubate for a further 5 min before the addition of 10 μl forskolin to a final concentration of 10 μM or 10 μl vehicle (ethanol). Cells were incubated for 15 min before the reaction was terminated by the addition of 50 μl (1 M) HCl. Samples (50 μl) were removed to determine total [3H]adenine incorporation and 100 μl (25–35Bq) [14C]cAMP was added to each well. [3H]cAMP was resolved by single column chromatography according to Alvarez & Daniels (1992) using [14C]cAMP as a recovery marker. Total [3H]cAMP generated was estimated as the percentage conversion from total [3H]adenine nucleotides. Results were expressed as % basal cAMP accumulated. Statistical analysis was performed by analysis of variance followed by Bonferroni/Dunn post hoc tests using superANOVA for Macintosh computer or Graphpad Prism3.
Results
Effect of ODA and AEA on [3H]CP55,940 binding and ODA on [3H]SR141716A binding
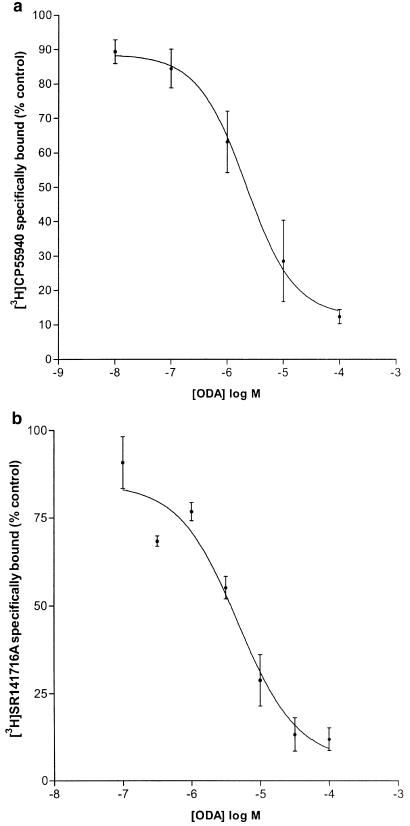
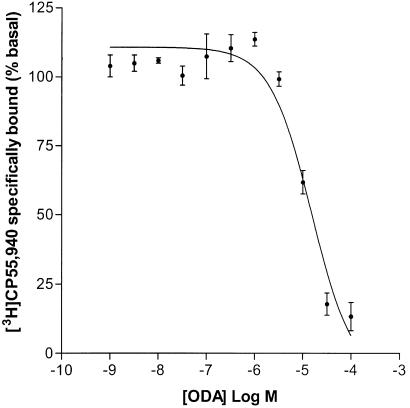
ODA inhibited specific binding of [3H]CP55,940 to rat whole-brain membranes in a concentration-dependent manner (Ki=1.14 μM (0.52–2.53 μM), Hill slope=0.80, n=6; Figure 1a). Similarly, ODA inhibited binding of the selective CB1 receptor antagonist [3H]SR141716A to rat whole-brain membranes (Ki=2.63 μM (0.62–11.20 μM), Hill slope=0.92, n=4; Figure 1b). Analysis showed that the Hill slopes were consistent with single site binding. AEA concentration-dependently displaced 100% of specifically bound [3H]CP55,940 in rat whole-brain membranes (Ki=428±82 nM, Hill slope=−1.33 ±0.15, n=3). The positive cannabinoid agonist control, HU210 (used to determine NSB), inhibited 60% (±5%) of total bound [3H]CP55,940 and 59% (±3%) of total bound [3H]SR141716. In further experiments, ODA inhibited specific binding of [3H]CP55,940 to hCB1 transfected HEK-293T cell membranes in a concentration-dependent manner (Ki=8.14 μM (range 4.97–13.32 μM) n=2; Figure 2). However, in hCB2 transfected HEK-293T cell membranes ODA exhibited only a partial inhibition of [3H]CP55,940 binding (42.5±7%) at the maximal concentration (100 μM) employed.
Figure 1.
(a) Inhibition of specific [3H]CP55,940 binding by cis-ODA (1 nM–100 μM), Ki=1.14 μm (0.52–2.53 μM, Hill slope=0.80, n=6). (b) Inhibition of specific [3H]SR141716A binding by ODA (0.1 μM–100 μM), Ki=2.63 μM (0.62–11.20 μM, Hill slope=0.92, n=4).
Figure 2.
Inhibition of [3H]CP55,940 binding in hCB1 (transiently transfected HEK-293T) cell membranes by cis-ODA, Ki=8.13 μM (range 4.97–13.32 μM, n=2). The figure represents one of two experiments conducted in triplicate.
[35S]GTPγS membrane binding
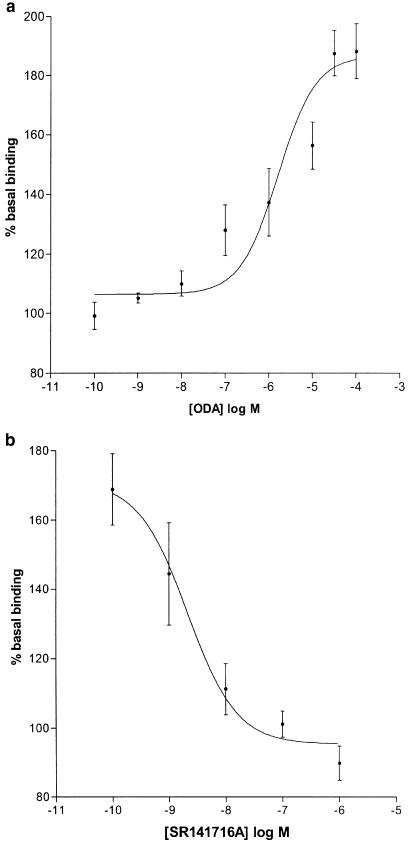
Cis-ODA stimulated [35S]GTPγS binding in rat whole-brain membranes in a concentration-dependent manner (EC50=1.64 μM (0.29–9.32 μM), R2=0.99, n=4–9; Figure 3a). At a maximally effective concentration of 100 μM, ODA enhanced [35S]GTPγS binding to 188±9% of basal. Trans-ODA failed to stimulate [35S]GTPγS binding at concentrations up to 100 μM (n=4).
Figure 3.
(a) ODA-stimulated [35S]GTPγS binding in rat brain membranes (EC50=1.64 μM, n=4–9, R2=0.99). (b) Inhibition of 10 μM ODA-stimulated [35S]GTPγS binding by SR141716A (IC50=2.11 nM, n=6, R2=1.00).
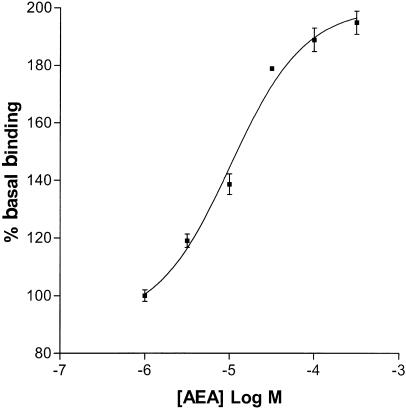
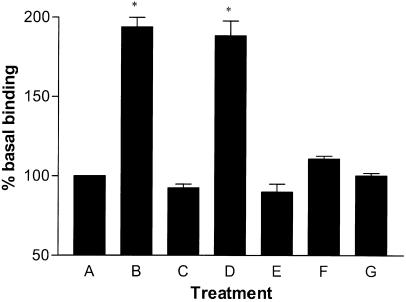
The selective CB1 receptor antagonist SR141716A inhibited [35S]GTPγS binding stimulated by 10 μM ODA (IC50=2.11 nM (0.32–13.77 nM), R2=1.00, n=6; Figure 3b), completely reversing enhanced [35S]GTPγS binding at a concentration of 1 μM. Another selective CB1 receptor antagonist, LY320135 (Felder et al., 1998) also inhibited ODA-enhanced [35S]GTPγS binding (IC50=12.44 nM (1.63–95.16 nM), R2=1.00, n=9). AEA concentration-dependently increased [35S]GTPγS binding (EC50=10.43 μM (4.45–24.42 μM), R2=0.98, n=3; Figure 4). At the maximum concentration used (300 μM), AEA stimulated [35S]GTPγS binding to 195±4% of basal. The positive control, HU210, concentration-dependently increased [35S]GTPγS binding. At a maximally effective concentration, HU210 stimulated binding to 194±6% of basal (EC50=1.46 nM (0.45–4.78 nM), R2=0.98, n=8). SR141716A completely reversed HU210-stimulated [35S]GTPγS binding at a maximally effective concentration of 1 μM (n=9; Figure 5).
Figure 4.
AEA-stimulated [35S]GTPγS binding in rat brain membranes (EC50=10.43 μM (4.45–24.42 μM), n=3, best fit curve: R2=0.98).
Figure 5.
Antagonism of agonist-potentiated [35S]GTPγS binding to rat brain membranes and effects of stereo isomers of ODA. (a) Basal binding, (b) 1 μM HU210, (c) 0.1 μM HU210 plus 1 μM SR141716A, (d) 100 μM cis-ODA, (e) 10 μM cis-ODA plus 1 μM SR141716A, (f) 10 μM cis-ODA plus 10 μM LY320135, (g) 100 μM trans-ODA (significance levels: *P<0.01 compared to basal binding).
[35S]GTPγS autoradiography
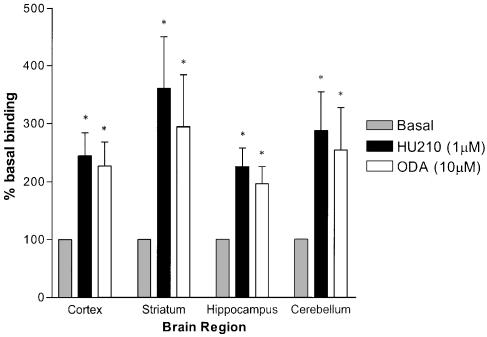
Autoradiography was used to investigate the regional distribution of specific [35S]GTPγS binding in the presence of ODA or the high potency cannabinoid agonist HU210. Four brain regions were examined quantitatively: the striatum, cortex, hippocampus and cerebellum. HU210 (1 μM) significantly increased [35S]GTPγS binding to 360±89% (P<0.05, n=6) of basal in the striatum, 244±39% (P<0.05, n=6) in the cortex, 225±31% (P<0.05, n=6) in the hippocampus and 287±67% (P<0.05, n=5) in the cerebellum. ODA (10 μM) significantly increased [35S]GTPγS binding to 294±89% (P<0.05, n=6) in the striatum, 226±41% (P<0.05, n=6) in the cortex, 196±29% (P<0.05, n=6) in the hippocampus and 254±73% (P<0.05, n=5) in the cerebellum (Figures 6 and 7).
Figure 6.
Regional increases in [35S]GTPγS (autoradiographic) binding after stimulation with HU210 or ODA (bars indicate s.e.m.) compared to [35S]GTPγS binding under basal conditions. Significance levels: *P<0.01 compared to basal.
Figure 7.
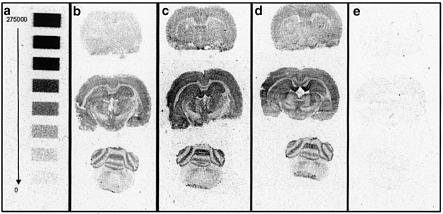
Example autoradiograms showing (a) 14C calibration strip calibrated with disintegrations per minute (DPM) values from 275,000 DPM to 0 DPM (b) basal, (c) 1 μM HU210-treated slices, (d) 10 μM ODA-treated slices and (e) NSB. All examples are from the same experiment and show significantly enhanced [35S]GTPγS binding after treatment with HU210 or ODA.
Effect of ODA on forskolin-stimulated cAMP generation
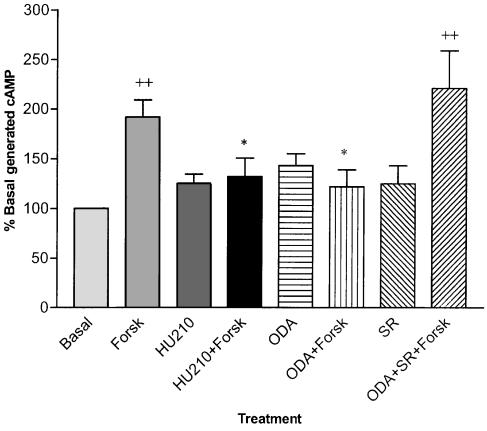
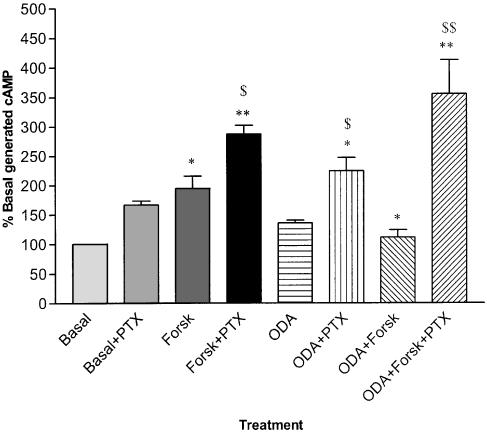
[3H]cAMP accumulation after stimulation with forskolin (10 μM) was 192±17% of basal (P=0.002, n=17). HU210 (1 μM) significantly reduced forskolin-stimulated [3H]cAMP accumulation reducing levels to 132±19% of basal (P=0.05, n=11). ODA (10 μM) significantly inhibited cAMP generation to 122±17% of basal levels (P=0.02, n=11). The selective CB1 receptor antagonist SR141716A completely reversed the inhibitory effect of 10 μM ODA (P=0.0003, n=11) while having no significant effect when administered alone (Figure 8). Pretreatment of cells with pertussis toxin (300 ng ml−1) completely abolished the inhibitory effect of ODA on forskolin-stimulated cAMP generation (P<0.001, n=6). In addition, pretreatment with pertussis toxin significantly elevated cAMP above non-pretreated levels in forskolin-treated (P<0.05, n=6) and ODA-treated cells (P<0.05, n=6, Figure 9).
Figure 8.
Inhibition of forskolin (Forsk)-stimulated cAMP generation in mouse neuroblastoma N1E 115 cells by 1 μM HU210 and 10 μM ODA and attenuation of ODA-mediated inhibition by the selective CB1 receptor antagonist SR141716A (1 μM). ODA, HU210 and SR141716A had no effect alone. ++=P<0.01 compared to basal cAMP generation (n=11), *=P<0.05 compared to forskolin-stimulated cAMP generation (n=11).
Figure 9.
Inhibition of forskolin (Forsk)-stimulated cAMP generation in mouse neuroblastoma N1E 115 cells by 10 μM ODA and blockade of ODA-mediated inhibition by pretreatment with 300 ng ml−1 pertussis toxin (PTX). Pretreatment with PTX significantly increased cAMP accumulation in Forsk only and ODA only treated cells compared to respective non-pretreated cells. *=P<0.05 compared to basal cAMP levels, **=P<0.001 compared to basal cAMP levels, +=P< 0.05 compared to Forsk-stimulated cAMP levels, $=P<0.05 compared to respective non-pretreated condition, $$=<0.001 compared to non-pretreated condition.
Discussion
ODA shares many characteristics with the endocannabinoid AEA. Both ODA and AEA are fatty acid amides produced endogenously in the brain (Di Marzo, 1998) and both are hydrolysed by FAAH (Boger et al., 2000a, 2000b). In vivo administration of ODA or AEA induces sleep that can be reversed by SR141716A. ODA also produces the classical tetrad of behaviours associated with cannabinoid agonists with a similar profile to AEA (Mechoulam et al., 1997). SR141716A has been shown to reverse the effects of ODA on catalepsy, hypolocomotion and analgesia but not hypothermia (Fedorova et al., 2001). Lichtman et al. (2002) were, however, unable to reverse ODA-induced hypolocomotion in their study with the CB1 antagonist. Similarly, SR141716A also fails to completely reverse elements of AEA pharmacology (Breivogel et al., 2001). A recent study has also demonstrated the ability of ODA to induce certain cannabinoid-like effects (hypolocomotion, hypothermia and ptosis) in CB1 receptor knockout mice. The endogenous cannabinoid AEA has also been shown to have residual behavioural effects and stimulates [35S]GTPγS binding in CB1 knockout mice (Di Marzo et al., 2000; Wiley & Martin, 2002). This may be due to AEA activating multiple types of receptor: both CB1 and CB2 cannabinoid receptors, the VR1 vanilloid receptor (Pertwee & Ross, 2002) and possibly a non-CB1/2 cannabinoid receptor subtype (Mang et al., 2001). Despite sharing functional similarities with endocannabinoids, radioligand binding studies indicated that ODA had a low affinity for the cannabinoid CB1 receptor. ODA was found to bind with a Ki of 44 μM in rat brain membranes (Boring et al., 1996), negligibly (Lichtman et al., 2002), not to bind at all (Mechoulam et al., 1997), or more recently, to bind with an IC50 of 10 μM in rat cerebellar membranes (Cheer et al., 1999). Additionally, Boring et al. (1996) reported that ODA, at concentrations up to 1 mM, was unable to enhance GTPγS binding to rat brain membranes, indicating an inability to activate G-protein-coupled receptors (GPCRs). Owing to the reported low affinity of ODA for the CB1 receptor and the apparent inability of ODA to activate the CB1 GPCR, an alternative explanation for its cannabinergic effects was required. One theory presented was that the effects of endocannabinoids are potentiated via the competitive inhibition, by ODA, of the enzyme FAAH; known as the entourage effect (Lambert & Di Marzo, 1999). However, ODA concentrations of ⩾5 μM are required to inhibit FAAH-mediated hydrolysis of AEA (Mechoulam et al., 1997) and a non-hydrolysable analogue of ODA proved to be no more effective at inhibiting AEA hydrolysis (Fedorova et al., 2001). Recently, in a study using FAAH knockout mice, it was concluded that FAAH regulates but does not mediate the effects of fatty acid amides (such as ODA and AEA) in vivo (Lichtman et al., 2002).
Studies have indicated that ODA affects other neurotransmitter systems. ODA has been reported to modulate 5-HT1A and 5-HT2A/2C receptors (Huidobro-Toro & Harris, 1996; Thomas et al., 1997) and has been shown to act via an allosteric site on the 5-HT7 receptor, thereby modulating receptor affinity (Hedlund et al., 1999). Recently, several cannabinoid agonists (tetrahydrocannabinol (THC), WIN55,212-2, AEA, JWH-015 and CP55,940) have been shown to inhibit human 5-HT3A receptors via an allosteric site (Barann et al., 2002) highlighting another similarity in the actions of ODA and endogenous (and classical) cannabinoids. However, a recent report suggests that ODA does not modulate serotonergic transmission in the CA1 pyramidal neurons of the hippocampus (Dougalis et al., 2004) and it has also been shown that 5-HT1A and 5-HT2C antagonists have no effect on ODA-induced hypothermia, locomotor activity, analgesia or anxiety in rats (Fedorova et al., 2001). Cheer et al. (1999) reported a modulation of 5-HT2 receptor affinity but with no effect on associated second messengers. ODA is also a nonselective modulator of inhibitory ionotropic receptors and has been shown to act indirectly via an allosteric site on the GABAA receptor (Coyne et al., 2002). Recently, it has been shown that the GABAA receptor antagonist bicuculline reverses ODA-induced hypothermia and analgesia (Fedorova et al., 2001) and elimination of the β subunit of the GABAA receptor prevents ODA-induced sleep (Laposky et al., 2001).
The radioligand binding results from the present study are similar to those found by Cheer et al. (1999) but contrast sharply with those of Boring et al. (1996), Lichtman et al. (2002) and Mechoulam et al. (1997). Here ODA inhibited [3H]CP55,940 binding in rat whole-brain membranes with a Ki value of 1.14 μM (0.52–2.53 μM). This represents an affinity for ODA much closer to those previously calculated for AEA binding to various rat CB1-containing membranes (52 nM, Devane et al., 1992; 143 nM, Hillard et al., 1995; 44 nM, Petitet et al., 1996; 266 nM, Rinaldi-Carmona et al., 1996) rather than the approximate 800 fold difference between ODA and AEA reported by Boring et al. (1996). Indeed, in the present study under the same experimental conditions, AEA was found to have an affinity of 428 nM (346–510 nM) a less than three-fold difference in affinity. ODA also inhibited binding of the selective CB1 receptor antagonist [3H]SR141716A (Ki=2.63 μM, 0.62–11.20 μM) to rat brain membranes, again demonstrating a relatively high affinity for the CB1 receptor. ODA also successfully inhibited [3H]CP55,940 binding in hCB1 HEK-293T cell membranes, although with marginally less affinity (Ki=8.14 μM). The affinity of AEA binding to transfected hCB1 receptors was reported as 115 nM in HEK-293 cell membranes (Song & Bonner, 1996), although lower binding affinities have been quoted for AEA binding to hCB1 in other model cells (252 nM, Mechoulam and Pride, 1995; 543 nM Felder et al., 1995).
ODA at concentrations up to 100 μM only partially displaced [3H]CP55,940 binding to hCB2 receptors, suggesting little affinity for the hCB2 receptor. It should be noted, however, that selectivity of various agonists for cannabinoid receptors has been reported to vary by more than 2000-fold depending upon the species and assay conditions used (Pertwee, 1997; Iwamura et al., 2001).
The ability of ODA to bind to the CB1 receptor does not necessarily infer agonist activity. Preliminary experiments using [35S]GTPγS autoradiography resulted in significantly increased binding in only the paraventricular nucleus of the hypothalamus, although this was reversed by SR141716A (Beckett et al., 1999) and Boring et al. (1996) failed to observe any ODA-stimulated GTPγS binding in rat brain membranes at concentrations up to 1 mM. However, in the present study ODA enhanced [35S]GTPγS binding in rat whole-brain membranes in a stereoselective manner with an EC50 of 1.64 μM, a 10-fold lower EC50 than AEA under the same conditions. SR141716A concentration-dependently inhibited the increase in ODA-stimulated [35S]GTPγS binding, further indicating the effects of ODA to be mediated via the CB1 receptor.
Although the distribution of ODA-induced increases in Fos protein and c-fos mRNA have been studied (Thomas et al., 1999), the distribution of ODA-stimulated GTPγS binding (and thus the distribution of ODA-activated GPCRs) has not. Having established that ODA stimulates significant increases in [35S]GTPγS binding in rat brain membranes, the distribution of [35S]GTPγS binding was examined in rat brain sections. The distribution of ODA-stimulated [35S]GTPγS binding was indistinguishable from that stimulated by HU210, and the magnitude of [35S]GTPγS binding stimulated by 10 μM ODA was comparable to that produced by 1 μM HU210 (Figure 7). This is consistent with ODA activating the same set of receptors as the cannabinoid HU210.
Since the evidence for ODA binding to and activating the CB1 receptor may be considered controversial, its ability to inhibit cAMP generation via the CB1 receptor would help to confirm its status as an endogenous cannabinoid agonist. Consistent with a previous report (Zhou & Song, 2001), HU210 (1 μM) inhibited forskolin-stimulated [3H]cAMP accumulation by 65% in N1E 115 cells. ODA (10 μM) inhibited 76% of forskolin-stimulated [3H]cAMP accumulation. ODA-mediated attenuation of cAMP accumulation was completely reversed by 1 μM SR141716A and inactivation of Gi/o GPCRs by pretreatment with pertussis toxin (PTX) also completely reversed the inhibitory effect of ODA. These results indicate strongly that the cAMP attenuation is mediated via a Gi/o-linked GPCR likely to be a CB1 receptor. Interestingly, PTX treatment had the effect of unmasking a lesser, stimulatory effect of ODA on cAMP generation. Similar cryptic positive coupling of CB1 receptors to adenylyl cyclase has been reported in striatal neurons and CB1-transfected Chinese hamster ovary cells after Gi/o inactivation with PTX (Glass & Felder, 1997; Felder et al., 1998).
It is difficult to reconcile previous reports of a lack of effect of ODA on CB1 receptors with the present data, although methodological factors may be responsible for some of the differences. The most likely of these is the difficulty in dissolving ODA into saline-based buffers at concentrations higher than 300 μM. Indeed, we were unable to dissolve ODA at any final assay concentration higher than this without an unacceptable increase in the concentration of nonaqueous solvent (DMSO or ethanol). Other factors such as buffer composition, temperature, rat strain and analytical methods may also have contributed to a difference in results. Similar methodological factors may account for the difference in agonist activity, as measured by GTPγS binding, in the present study compared to that reported by Boring et al. (1996). It should be noted that even well-recognised cannabinoid agonists such as THC and AEA have been reported not to enhance [35S]GTPγS binding in membrane preparations (Griffin et al., 1998), a finding that has been negated by the weight of subsequent positive findings.
The apparent ability of ODA to induce certain behaviours in CB1 knockout mice (Lichtman et al., 2002) may be due to interactions with other non-cannabinoid receptors, similar to those produced by a range of cannabinoid receptor agonists, or possibly with as yet uncharacterised cannabinoid receptor subtypes. ODA allosterically modulates GABAA receptors (Coyne et al., 2002) and bicuculline, a GABAA antagonist, reverses ODA-induced hypothermia (Fedorova et al., 2001). In addition, ODA allosterically modulates 5-HT receptors (Thomas et al., 1997) and established cannabinoid agonists have also been shown to act in this manner (Barann et al., 2002). Therefore, in addition to allosteric and possibly, direct modulation of other receptor types and inhibition of FAAH, the effects of ODA may actually be mediated directly via the CB1 receptor. Although the affinity of ODA for the CB1 receptor is somewhat lower than that reported for AEA, it should be noted that the basal concentration of ODA in the brain has not yet been reliably measured. On the basis of the concentration of ODA in neuroblastoma cells being much higher than that of AEA (Bisogno et al., 1997), it is conceivable that endogenous levels of ODA might be sufficient to occupy a significant proportion of CB1 receptors in situ and ODA may, therefore, act as a low-affinity agonist.
In summary, the data presented clearly show that ODA occupies rat and human CB1 (but not CB2) receptors with micromolar affinity and that it has agonist activity comparable to that of HU210 and AEA, revealed by its ability to enhance GTPγS binding to membranes. Furthermore, ODA, at least in mouse neuroblastoma cells, can inhibit or stimulate (after Gi/o inactivation) formation of the intracellular messenger cAMP in a CB1 antagonist-sensitive fashion.
Thus, the evidence strongly supports the contention that ODA is a directly acting endogenous cannabinoid with selectivity for the CB1 receptor.
Acknowledgments
We are grateful for financial support from The University of Nottingham.
Abbreviations
- AEA
Anandamide
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CP55,940
(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]trans-4-(3-hydroxypropyl)-cyclohexanol
- HU210
11-hydroxy-dimethylheptyl-Δ8-tetrahydrocannabinol
- ODA or oleamide
cis-9,10-octadecanoamide
- SR141716A
N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide
- [35S]GTPγS
guanosine 5′-O-[γ-[35S]thio]triphosphate
References
- ABADJI V., LUCAS-LENARD J.M., CHIN C., KENDALL D.A. Involvement of the carboxyl terminus of the third intracellular loop of the CB1 receptor in constitutive activation of Gs. J. Neurochem. 1999;72:2032–2038. doi: 10.1046/j.1471-4159.1999.0722032.x. [DOI] [PubMed] [Google Scholar]
- ALVAREZ R., DANIELS D.V. A separation method for the assay of adenylylcyclase, intracellular cyclic AMP, and cyclic-AMP phosphodiesterase using tritium-labeled substrates. Anal. Biochem. 1992;203:76–82. doi: 10.1016/0003-2697(92)90045-9. [DOI] [PubMed] [Google Scholar]
- BARANN M., MOLDERINGS G., BRUSS M., BONISCH H., URBAN B.W., GOTHERT M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKETT S.R.G., MILLNS P.J., KENDALL D.A. The effect of cannabinoid ligands on [35S]GTPγS binding in the rat brain. Br. J. Pharmacol. 1999;128:192P. [Google Scholar]
- BISOGNO T., SEPE N., DE PETROCELLIS L., MECHOULAM R., DI MARZO V. The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem. Biophys. Res. Commun. 1997;239:473–479. doi: 10.1006/bbrc.1997.7431. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., FECIK R.A., PATTERSON J.E., MIYAUCHI H., PATRICELLI M.P., CRAVATT B.F. Fatty acid amide hydrolase substrate specificity. Bioorg. Med. Chem. Lett. 2000a;10:2613–2616. doi: 10.1016/s0960-894x(00)00528-x. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., HENRIKSEN S.J., CRAVATT B.F. Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signalling molecules. Curr. Pharm. Design. 1998;4:303–314. [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., GUAN X., GILULA N.B. Arachidonic acid amide inhibitors of gap junction cell-cell communication. Bioorg. Med. Chem. Lett. 1999;9:1151–1154. doi: 10.1016/s0960-894x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., HEDRICK M.P., FECIK R.A., MIYAUCHI H., WILKIE G.D., AUSTIN B.J., PATRICELLI M.P., CRAVATT B.F. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc. Natl. Acad. Sci. U.S.A. 2000b;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORING D.L., BERGLUND B.A., HOWLETT A.C. Cerebrodiene, arachidonyl-ethanolamide, and hybrid structures: potential for interaction with brain cannabinoid receptors. Prostaglandins Leukot. Essent. Fatty Acids. 1996;55:207–210. doi: 10.1016/s0952-3278(96)90100-3. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- CHEER J.F., CADOGAN A.K., MARSDEN C.A., FONE K.C.F., KENDALL D.A. Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology. 1999;38:533–541. doi: 10.1016/s0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- CHIN C.N., MURPHY J.W., HUFFMAN J.W., KENDALL D.A. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, the peripheral cannabinoid receptor. J. Pharmacol. Exp. Ther. 1999;29:837–844. [PubMed] [Google Scholar]
- COYNE L., LEES G., NICHOLSON R.A., ZHENG J., NEUFIELD K.D. The sleep hormone oleamide modulates inhibitory ionotropic receptors in mammalian CNS in vitro. Br. J. Pharmacol. 2002;135:1977–1987. doi: 10.1038/sj.bjp.0704651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVATT B.F., PROSPERO-GARCIA O., SIUZDAK G., GILULA N.B., HENRIKSEN S.J., BOGER D.L., LERNER R.A. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V. Endocannabinoids and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim. Biophys. Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C.S., TAO Q., BRIDGEN D.T., RAZDAN R.K., ZIMMER A.M., ZIMMER A., MARTIN B.R. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- DOUGALIS A., LEES G., GANELLIN R.C. The sleep inducing brain lipid cis-oleamide (cOA) does not modulate serotonergic transmission in the CA1 pyramidal neurons of the hippocampus in vitro. Neuropharmacol. 2004;46:63–73. doi: 10.1016/s0028-3908(03)00297-1. [DOI] [PubMed] [Google Scholar]
- FEDOROVA I., HASHIMOTO A., FECIK R.A., HEDRICK M.P., HANUS L.O., BOGER D.L., RICE K.C., BASILE A.S. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J. Pharmacol. Exp. Ther. 2001;299:332–342. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., GLASS M., MACKIE K.P., FAHEY K.J., CULLINAN G.J., HUNDEN D.C., JOHNSON D.W., CHANEY M.O., KOPPEL G.A., BROWNSTEIN M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J. Pharmacol. Exp. Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- GLASS M., FELDER C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN G., ATKINSON P.J., SHOWALTER V.M., MARTIN B.R., ABOOD M.E. Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J. Pharmacol. Exp. Ther. 1998;285:553–560. [PubMed] [Google Scholar]
- HEDLUND P.B., CARSON M.J., SUTCLIFFE J.G., THOMAS E.A. Allosteric regulation by oleamide of the binding properties of 5-hydroxytryptamine7 receptors. Biochem. Pharmacol. 1999;58:1807–1813. doi: 10.1016/s0006-2952(99)00274-9. [DOI] [PubMed] [Google Scholar]
- HILLARD C.J., EDGEMOND W.S., CAMPBELL W.B. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J. Neurochem. 1995;64:677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- HUIDOBRO-TORO J.P., HARRIS R.A. Brain lipids that induce sleep are novel modulators of 5-hydroxytrypamine receptors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8078–8082. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUITRON-RESENDIZ S., GOMBART L., CRAVATT B.F., HENRIKSEN S.J. Effect of oleamide on sleep and its relationship to blood pressure, body temperature, and locomotor activity in rats. Exp. Neurol. 2001;172:235–243. doi: 10.1006/exnr.2001.7792. [DOI] [PubMed] [Google Scholar]
- IWAMURA H., SUZUKI H., UEDA Y., KAYA T., INABA T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- LAMBERT D.M., DI MARZO V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic. Curr. Med. Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- LAPOSKY A.D., HOMANICS G.E., BASILE A., MENDELSON W.B. Deletion of the GABAA receptor β3 subunit eliminates the hypnotic actions of oleamide in mice. NeuroReport. 2001;12:4143–4147. doi: 10.1097/00001756-200112210-00056. [DOI] [PubMed] [Google Scholar]
- LEGGETT J.D., OVERBURY A.L., BECKETT S.R.G., KENDALL D.A. Oleamide stimulates [35S]GTPγS binding in rat brain preparations via a cannabinoid receptor. Br. J. Pharmacol. 2002;137:114P. [Google Scholar]
- LICHTMAN A.H., HAWKINS E.G., GRIFFIN G., CRAVATT B.F. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J. Pharmacol. Exp. Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- MANG C.F., ERBELDING D., KILBINGER H. Differential effects of anandamide on acetylcholine release in the guinea-pig ileum mediated via vanilloid and non-CB1 cannabinoid receptors. Br. J. Pharmacol. 2001;134:161–167. doi: 10.1038/sj.bjp.0704220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E.The unpaved road to the endogenous brain cannabinoid ligands, the anandamides Cannabinoid Receptors. 1995Academic Press, London; 233–258.ed. Pertwee, R.G. pp [Google Scholar]
- MECHOULAM R., FRIDE E., HANUS L., SHESKIN T., BISOGNO T., DI MARZO V., BAYEWITCH M., VOGEL Z. Anandamide may mediate sleep induction. Nature. 1997;389:25–26. doi: 10.1038/37891. [DOI] [PubMed] [Google Scholar]
- MENDELSON W.B., BASILE A.S. The hypnotic actions of oleamide are blocked by a camabinoid receptor antagonist. NeuroReport. 1999;10:3237–3239. doi: 10.1097/00001756-199910190-00021. [DOI] [PubMed] [Google Scholar]
- MURILLO-RODRIGUEZ E., SANCHEZ-ALAVEZ M., NAVARRO L., MARTINEZ-GONZALEZ D., DRUCKER-COLIN R., PROSPERO-GARCIA O. Anandamide modulates sleep and memory in rats. Brain Res. 1998;812:270–274. doi: 10.1016/s0006-8993(98)00969-x. [DOI] [PubMed] [Google Scholar]
- MURILLO-RODRIGUEZ E., GIORDANO M., CABEZA R., HENRIKSEN S.J., DIAZ M.M., NAVARRO L., PROSPERO-GARCIA O. Oleamide modulates memory in rats. Neurosci. Lett. 2001;313:61–64. doi: 10.1016/s0304-3940(01)02256-x. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., ROSS R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- PETITET F., MARIN L., DOBLE A. Biochemical and pharmacological characterization of cannabinoid binding sites using [3H]SR141716A. Neuroreport. 1996;7:789–792. doi: 10.1097/00001756-199602290-00026. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., BENNETT T., GARDINER S.M. Comparison of the effects of anandamide and methanandamide in bioassays. Br. J. Pharmacol. 2000;129:141P. [Google Scholar]
- RINALDI-CARMONA M., PIALOT F., CONGY C., REDON E., BARTH F., BACHY A., BRELIERE J.C., SOUBRIE P., LE FUR G. Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci. 1996;58:1239–1247. doi: 10.1016/0024-3205(96)00085-9. [DOI] [PubMed] [Google Scholar]
- SHESKIN T., HANUS L., SLAGER J., VOGEL Z., MECHOULAM R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- SONG Z.H., BONNER T.I. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Mol. Pharmacol. 1996;49:891–896. [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., KODAKA T., TONEGAWA T., NAKANE S., YAMASHITA A., ISHIMA Y., WAKU K. Enzymatic synthesis of oleamide (cis-9, 10-octadecenoamide), sleep-inducing lipid, by rat brain microsomes. Biochem. Mol. Biol. Int. 1996;40:931–938. doi: 10.1080/15216549600201553. [DOI] [PubMed] [Google Scholar]
- THOMAS E.A., CARSON M.J., NEAL M.J., SUTCLIFFE J.G. Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14115–14119. doi: 10.1073/pnas.94.25.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS E.A., CRAVATT B.F., SUTCLIFFE J.G. The endogenous lipid oleamide activates serotonin 5-HT7 neurons in mouse thalamus and hypothalamus. J. Neurochem. 1999;72:2370–2378. doi: 10.1046/j.1471-4159.1999.0722370.x. [DOI] [PubMed] [Google Scholar]
- WILEY J.L., MARTIN B.R. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem. Phys. Lipids. 2002;121:57–63. doi: 10.1016/s0009-3084(02)00146-9. [DOI] [PubMed] [Google Scholar]
- YANG J.Y., WU C.F., SONG H.R. Studies on the sedative and hypnotic effects of oleamide in mice. Arzneimittelforschung. 1999;49:663–667. doi: 10.1055/s-0031-1300479. [DOI] [PubMed] [Google Scholar]
- ZHOU D., SONG Z.H. CB1 cannabinoid receptor-mediated neurite remodeling in mouse neuroblastoma N1E-115 cells. J. Neurosci. Res. 2001;65:346–353. doi: 10.1002/jnr.1160. [DOI] [PubMed] [Google Scholar]