Abstract
The effects of short-term oral administration of red wine polyphenolic compounds (RWPC, 20 mg kg−1 day−1 for 7 days) on haemodynamics, ex vivo cardiac responsiveness and ischaemia–reperfusion injury were investigated in rats. The involvement of nitric oxide (NO) was evaluated using the NO synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 2 mg kg−1 day−1 for 7 days), at a dose which did not affect blood pressure.
Ex vivo reactivity of hearts from RWPC-treated rats showed lower basal developed pressure, greater heart rate and decreased inotropic responses to either β-adrenoceptor or muscarinic receptor stimulation with isoprenaline or carbachol, respectively.
RWPC treatment did not modify cardiac expression of endothelial NO synthase or Cu/Zn superoxide dismutase. However, it increased nitrite in the coronary effluent.
In ischaemia–reperfusion, RWPC treatment reduced infarct size and oxidative stress, as shown by the myocardial content of the end products of lipid peroxidation, malondialdehyde and 4-hydroxynonenal, without affecting post-ischaemic contractile dysfunction. All the observed effects of RWPC were prevented by L-NAME treatment.
Altogether, these data show that short-term treatment with RWPC decreases blood pressure and cardiac responsiveness, and protects against post-ischaemic infarction via decreased oxidative stress. All the above effects of RWPC are sensitive to NO synthase inhibition that implies an involvement of NO-dependent pathway. This study suggests a basis for the beneficial effects of plant-derived polyphenols against cardiovascular disease.
Keywords: Red wine, polyphenols, NO synthase, heart, ischaemia–reperfusion
Introduction
Various epidemiological studies have indicated that the consumption of foods and beverages rich in polyphenols, including fruit, vegetables, tea or red wine is associated with a lower incidence of coronary heart disease (Renaud & De Lorgeril, 1992; Hertog et al., 1993) and especially with myocardial infarction (Geleijnse et al., 2002). Furthermore, it has been shown recently that moderate wine drinking is associated with reduced risk of complications after myocardial infarction (De Lorgeril et al., 2002). The beneficial effects of polyphenols on the risk of myocardial infarction could be related to their ability to reduce the susceptibility of low-density lipoprotein to oxidation in healthy subjects and patients with coronary heart disease (Stein et al., 1999; Serafini et al., 2000; Abu-Amsha Caccetta et al., 2001). In addition, red wine supplementation of drinking water has been shown to modulate haemostasis and to prevent experimental thrombosis in animals, independently of its alcohol content (Wollny et al., 1999). In a previous work, we have provided evidence for in vivo cardiovascular effects of red wine polyphenolic compounds (RWPC) (Diebolt et al., 2001). Oral administration of RWPC was able to produce a decrease in blood pressure in normotensive rats. This haemodynamic effect was associated with an enhanced endothelium-dependent relaxation and induction of the expression of inducible NO synthase (NOS) and cyclooxygenase 2 within the arterial wall. Moreover, we reported recently that RWPC accelerated the regression of blood pressure and improved structural and functional cardiovascular changes including cardiac fibrosis in hypertensive rats (Bernatova et al., 2002). These effects of RWPC probably involved the NO pathway; in as much as enhanced in vitro endothelium-dependent relaxation was observed as a result of enhanced NO synthesis (Andriambeloson et al., 1997; 1999). Experimental studies on the cardiac effects of RWPC have essentially focused on their acute effects against ischaemia/reperfusion injury. Acute administration of RWPC improved post-ischaemic ventricular function and reduces the extent of myocardial infarction. This acute effect is related mainly to their antioxidant property, but it also implicates KATP channels and NO (Sato et al., 2000; Mosca & Cingolani, 2002).
However, little is known about the consequences of oral administration of RWPC on cardiac reactivity and ischaemia injury. Therefore, the aim of the present study was to determine the effect of short-term oral administration of RWPC on haemodynamic parameters, on cardiac reactivity both under basal conditions as well as in response to β-adrenoceptor and muscarinic agonists and on protection against ischaemia/reperfusion injury. The implication of the nitric oxide (NO) pathway in the effects of treatment with RWPC has been investigated using the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME). We have also obtained insight into the mechanisms involved in the effects of RWPC treatment by looking at the ventricular levels of endothelial NO synthase (eNOS) and Cu/Zn superoxide dismutase (SOD), by measuring nitrite concentration in coronary effluent, and by assaying malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) as an index of oxidative stress after ischaemia–reperfusion.
Methods
Animals
This investigation conforms to authorization number 01918 given by the French Government Department of Agriculture. Four groups of male Wistar rats (12–14 week old) were used and treated daily for 7 days by intra-gastric gavage: the first group (control) received the vehicle containing 5% glucose, the second group received RWPC (20 mg kg−1 day−1), the third group received L-NAME (2 mg kg−1 day−1), a dose at which it had no effect on blood pressure per se, and the fourth group was given a combination of RWPC plus L-NAME. This dose of RWPC has been previously shown to induce cardiovascular effects (Diebolt et al., 2001) and it corresponds to 10 times the concentration that produced maximal relaxation of rat aortic rings in vitro (Andriambeloson et al., 1997; 1999). As only 5–10% of polyphenols can be absorbed in the digestive tract (Duthie et al., 1998), it may be assumed that the concentration of polyphenols present in the plasma is comparable to the concentration at which RWPC produced endothelium-dependent relaxation in rat aorta.
Systolic blood pressure (SBP, mmHg) and heart rate (beats min−1) were measured daily using the pneumatic tail-cuff method (NarcoBio Systems, TX, U.S.A.). Rats were accustomed to the tail-cuff measurements during 4 days and the systolic blood pressure was measured 5 to 10 times in the morning and the mean of these measurements was taken as one measurement.
Cardiac reactivity studies were performed in each group of rats 24 h after administration of the last dose of the treatment.
Isolated perfused heart
Rats were anaesthetized with pentobarbital (60 mg kg−1, i.p.). mixed with 500 units heparin. The heart was rapidly excised and stopped in ice-cold Krebs–Henseleit buffer with the following composition in mM: NaCl 118; NaHCO3 24; KCl 4.7; KH2PO4 1.2; MgSO4 1.2; CaCl2 1.7; glucose 10. Then, the heart was immediately mounted and cannulated in the retrograde mode according to Langendorff method as described previously (Gyorgy et al., 1999). Perfusion pressure was maintained constant at 100 cmH2O with Krebs – Henseleit buffer gassed with 95% O2, 5% CO2 maintained at 37°C, and was monitored with a pressure transducer connected to the perfusion circuit immediately before the heart. A water-filled latex balloon (Ealing S.A.R.L, France) connected to an Isotec® pressure transducer (Hugo-Sachs, Germany) was inserted into the left ventricle for measurement of ventricular pressure. Left ventricular end diastolic pressure was set to 10–15 mmHg by adjusting the volume of the balloon and the heart was allowed to equilibrate for 20 min. Cardiac parameters (i.e. left ventricular developed pressure (LVDP) in mmHg, and heart rate in beats min−1) were recorded continuously. Coronary flow (ml min−1) was determined by collecting and weighing 1-min samples of the effluent from the heart.
After a 20 min period of stabilization, the following experimental protocols were performed. The first protocol consisted of perfusing the heart with increasing concentrations of isoprenaline (10−9 to 10−8 M). When the response to the highest concentration of isoprenaline had reached a steady state, the hearts were perfused with increasing concentrations of carbachol (10−6 to 10−5 M). The second protocol consisted of inducing a global no-flow ischaemia by stopping aortic perfusion for 30 min. Ischaemia was carried out at 37°C and was followed by 60 min of reperfusion. Cardiac parameters were continuously recorded during all experiments. The coronary flow was determined, as described above, at the end of the equilibration period, during the steady-state response to each tested compound and at different times during reperfusion. The steady state was reached between 5 and 15 min after starting perfusion of isoprenaline and carbachol.
Morphological analysis of cardiac ischaemic damage by triphenyltetrazolium chloride (TTC) staining
After the ischaemic insult followed by 60 min of reperfusion, the hearts were stored at −20°C for 24 h. Then, they were sliced transversally (2 mm thickness) and incubated for 20 min at 37°C with 1% TTC in phosphate buffer (0.1 M, pH 7.4). Slices were then fixed with 10% formalin for 20 min. Sections were scanned and the total and infarcted areas were measured using ImageTool (UTHSCSA, U.S.A.).
Western blotting
Heart ventricles were homogenized and approximately 1 μg (for Cu/Zn SOD) or 100 μg (for eNOS) of total proteins from the supernatant fraction were loaded onto 7% (for eNOS) or 15% SDS–PAGE (for Cu/Zn SOD). Total proteins were measured using Lowry methods (BioRad, Marnes-la-Coquette, France). Proteins were transferred to nitrocellulose membranes and were probed with monoclonal mouse anti-eNOS (BD Biosciences, Le Pont de Claix, France) and rabbit anti-Cu/Zn SOD polyclonal antibody (Stressgen Biotechnologies by TEBU, Le Perray en Yvelines, France). The sample loading was verified by staining the membranes with Ponceau red and amido black. Bound antibodies were detected with a secondary peroxidase-conjugated anti-mouse IgG for eNOS and anti-rabbit IgG for Cu/Zn SOD (Promega, Charbonnières, France). The bands were visualized using the enhanced chemiluminescence system (ECL Plus, Amersham, Buckinghamshire, U.K.) and quantified by densitometry.
Determination of nitrite in the coronary effluent
Samples (170 μl) of coronary effluent from hearts in each group of treated rats were collected at the end of the equilibration period during the response to 3 μM carbachol. They were incubated with 17 μl of 2–3-diaminonaphthalene (0.05 mg l−1 in 6 M HCl) at room temperature in the dark for 10 min. Then the reaction was stopped with 30 μl NaOH (10 M). Fluorescence emission was measured at 415 nm, with excitation at 365 nm, using a fluorescence spectrophotometer (F-2000, Hitachi Ltd, Tokyo, Japan). Nitrite concentrations were calculated from standard curves of known concentrations of NaNO2 (30 to 700 nM).
Determination of MDA+ 4-HNE in hearts
Hearts were homogenized in 20 mM Tris–HCl, pH 7.4. Homogenates were centrifuged and the supernatant was used for the assay of total MDA and 4-HNE using a commercial assay kit (Calbiochem, Cat No 437634). This assay is based on the reaction of N-methyl-2-phenylindole with MDA and 4-HNE at 45°C to yield a stable chromophore with its maximal absorbance at 586 nm. Total proteins of the supernatant were measured using the Lowry method (BioRad, Marnes-la-Coquette, France).
Expression of results and statistical analysis
LVDP and heart rate were expressed as changes from the baseline values. The baseline values represented the data recorded before the addition of the drug in the case of isoprenaline. Changes in coronary flow were expressed as a percentage of the baseline values obtained in the absence of any drug; the baseline was taken as 100%. In the case of carbachol, the effects on LVDP and heart rate were expressed as changes from the values obtained after stimulation of the heart with the highest concentration of isoprenaline used (i.e. 10−8 M). Coronary flow changes were expressed as a percentage of the response induced by isoprenaline (10−8 M). For the evaluation of post-ischaemic recovery, data for each cardiac parameter were expressed as percentage of their own respective pre-ischaemic value. Infarct size was expressed as the percentage of total ventricular volume. The level of MDA+4-HNE was expressed in μM per mg of total proteins. All results are expressed as mean±s.e.m. Two-way ANOVA with repeated measures, Student's unpaired t-test or one-way ANOVA followed by Dunnett's post-hoc test were used for statistical analysis as appropriate. P<0.05 was considered significant.
Drugs
The RWPC powder from red wine (Cabernet-Sauvignon grape variety) was provided by Dr Moutounet (Institut National de la Recherche Agronomique, Montpellier, France) and contained 100 mg g−1 of total catechins plus proanthocyanidins expressed as catechin (with only 1.0%. of each monomeric form of catechin and epicatechin), 64 mg g−1 of total anthocyanins (including 36% of malvidin-3-glucoside), 5 mg g−1 of total flavonols and 8.7 mg g−1 of total phenolic acids (including 19.5% of caftaric acid) as previously described (Andriambeloson et al., 1997). The vehicle used for the administration of RWPC and L-NAME was 5% glucose. Pentobarbital was from CEVA SANTE ANIMAL (Libourne, France). All the chemicals were purchased from Sigma Chemical (Grenoble, France).
Results
In vivo haemodynamic parameters
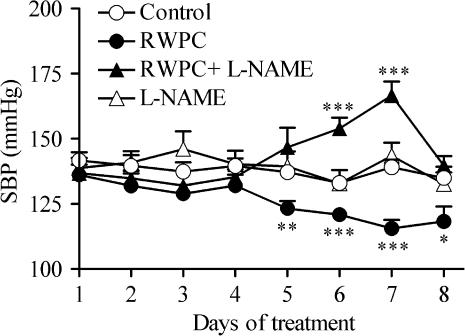
The SBP and heart rates of rats receiving the vehicle, RWPC, L-NAME alone or L-NAME plus RWPC were not significantly different before the beginning of the treatment. Treatment of the rats for 7 days with the vehicle (5% glucose) did not alter the SBP significantly (Figure 1). However, intra-gastric administration of RWPC produced a progressive reduction in SBP during the treatment. When compared to rats receiving the vehicle, the decrease in SBP produced by RWPC was significantly different from day 5 until the end of the treatment. Treatment of rats with L-NAME alone did not affect the SBP, but prevented the decrease in blood pressure produced by RWPC. Furthermore, co-administration of L-NAME with RWPC unmasked a transient hypertension that was significantly different from control rats on days 6 and 7 of the treatment but was not significant at the end of the experiment on day 8.
Figure 1.
Effects of RWPC treatment on systolic blood pressure. Variation of systolic blood pressure in control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1) + L-NAME (2 mg kg−1 day−1), or L-NAME (2 mg kg−1 day−1). Values are the mean±s.e.m. of seven or eight experiments. *P< 0.05; **P< 0.01; ***P< 0.001 show significant differences versus the control group by Student's unpaired t-test.
In contrast, the heart rates of the rats receiving the vehicle, RWPC, L-NAME or RWPC plus L-NAME were not significantly different during the treatment both within the group and between the groups (not shown).
Basal cardiac parameters ex vivo
At 24 h after the end of the treatment, the basal cardiac parameters were recorded in isolated hearts from the four groups of rats (Table 1). When compared to hearts from rats receiving the vehicle, those from RWPC-treated rats displayed a lower LVDP (P<0.05) and a higher heart rate (P<0.05). Alteration of LVDP and heart rate by RWPC were prevented by the concomitant treatment of the rats with RWPC plus L-NAME. It should be noted that L-NAME administered alone during the treatment did not induce any change either in heart rate or in LVDP. The coronary flow rates in hearts from rats receiving the vehicle, RWPC or RWPC plus L-NAME were not significantly different between the groups. However, the coronary flow in hearts from L-NAME treated rats was significantly lower than that from control rats.
Table 1.
Baseline cardiac parameters ex vivo measured after equilibration period in isolated hearts from rats treated with vehicle, RWPC, RWPC plus L-NAME and L-NAME.
| LVDP (mmHg) | Heart rate (beats.min−1) | Coronary flow (ml.min−1) | |
|---|---|---|---|
| Control | 68.5±7.7 | 246.9±9.9 | 12.8±1.2 |
| RWPC | 47.7±5.7* | 285±11.6* | 11±1.2 |
| RWPC + L-NAME | 69.4±9.6 | 245.2±8.8 | 10.3±0.9 |
| L-NAME | 64.5±8.9 | 252.6±17.4 | 9.4±0.6* |
Rats were treated with vehicle (5% glucose, Control), red wine polyphenolic compounds (RWPC; 20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1) + NG-nitro-L-arginine methyl ester (L-NAME; 2 mg kg−1 day−1) or L-NAME (2 mg kg−1 day−1). Values are presented as the mean±s.e.m. of 11–16 experiments.
P<0.05 shows a significant difference versus the control group.
Cardiac reactivity to isoprenaline and carbachol
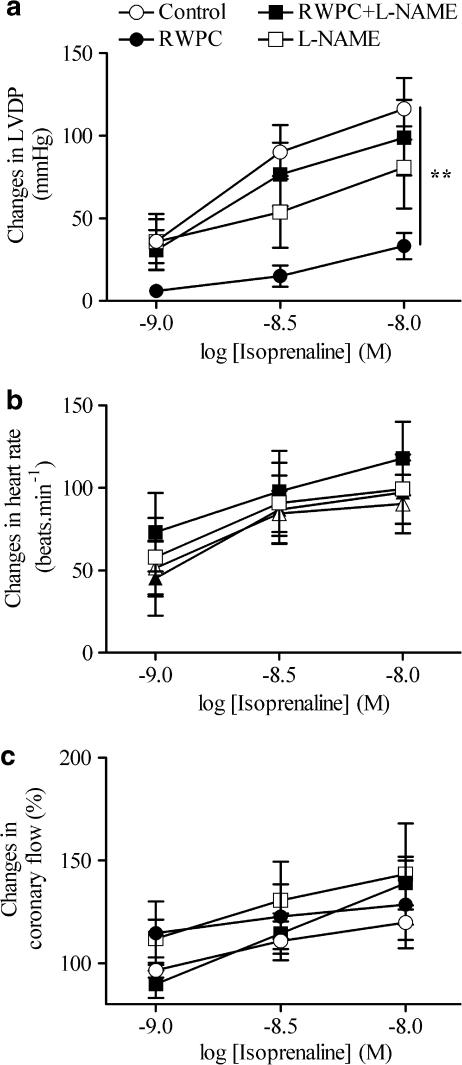
Isoprenaline produced concentration-dependent increase in LVDP, heart rate and coronary flow (Figure 2). The positive inotropic effect of isoprenaline was significantly reduced in hearts from RWPC-treated rats as compared to those from control animals (Figure 2a). Interestingly, treatment with L-NAME alone did not affect the positive inotropic effect of isoprenaline, but it completely prevented the inhibitory effect of the RWPC treatment. However, the positive chronotropic effect and the increase in coronary flow induced by isoprenaline were not significantly different in the hearts from the four groups of rats (Figures 2b and c).
Figure 2.
Cardiac consequences of RWPC treatment on cardiac reactivity in response to isoprenaline. Changes in LVDP (a), heart rate (b) and coronary flow (c) in isolated hearts from control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1)+L-NAME (2 mg kg−1 day−1), or L-NAME (2 mg kg−1 day−1). Columns represent the mean and bars show the s.e.m. of six experiments. **P<0.01 shows a significant difference from the control group by two-way ANOVA with repeated measures.
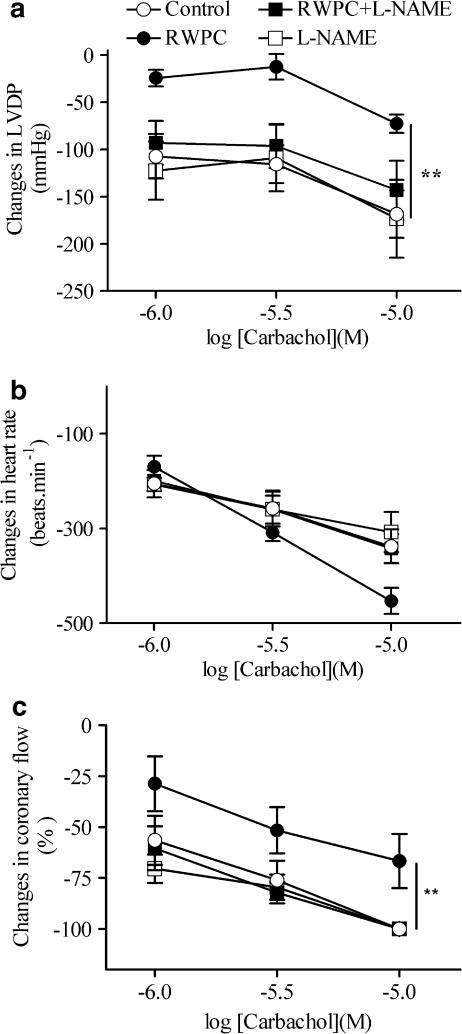
Carbachol decreased LVDP, heart rate and coronary flow in hearts from all groups (Figure 3). RWPC treatment significantly reduced the negative inotropic effect, but not the negative chronotropic effect of carbachol (Figure 3a and b). RWPC significantly attenuated the decrease in coronary flow induced by carbachol (Figure 3c). Treatment with L-NAME alone did not significantly modify the effect of carbachol on cardiac parameters. In contrast, it prevented the inhibitory effect of RWPC treatment on LVDP (Figure 3).
Figure 3.
Cardiac consequences of RWPC treatment on cardiac reactivity in response to carbachol. Changes in LVDP (a), heart rate (b) and coronary flow (c), in isolated hearts from control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1) + L-NAME (2 mg kg−1 day−1) or L-NAME (2 mg kg−1 day−1). Carbachol was perfused into the hearts after obtaining the maximal response to the highest concentration of isoprenaline (10−8 M). Columns represent the mean and bars show the s.e.m. of six experiments. *P<0.05, **P<0.01 show significant differences versus the control group by two-way ANOVA with repeated measures.
Nitrite concentrations in coronary effluent
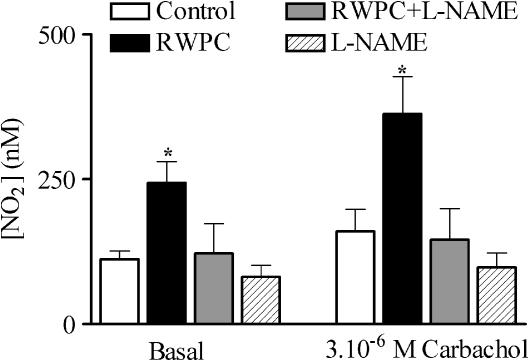
To study the involvement of a change in NO production, nitrite concentration was measured in the coronary effluent from hearts taken from each group of rats. Samples were taken after the equilibration period and during the response to perfusion with 3 μM carbachol. As shown in Figure 4, nitrite concentration was significantly increased in the RWPC-treated group as compared to the control (P<0.05). Addition of L-NAME to the RWPC treatment prevented the increase in nitrite concentration, although treatment with L-NAME alone did not induce any modification in nitrite concentration as compared to control.
Figure 4.
Nitrite concentration in coronary effluent. Histogram showing nitrite concentration in the coronary effluent of hearts from control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1) + L-NAME (2 mg kg−1 day−1), or L-NAME (2 mg kg−1 day−1) under basal conditions and in response to 3μM carbachol. Results are representative of five experiments, *P< 0.05 shows a significant difference from the control group by one-way ANOVA followed by Dunnett's post-hoc test.
Western blot analysis
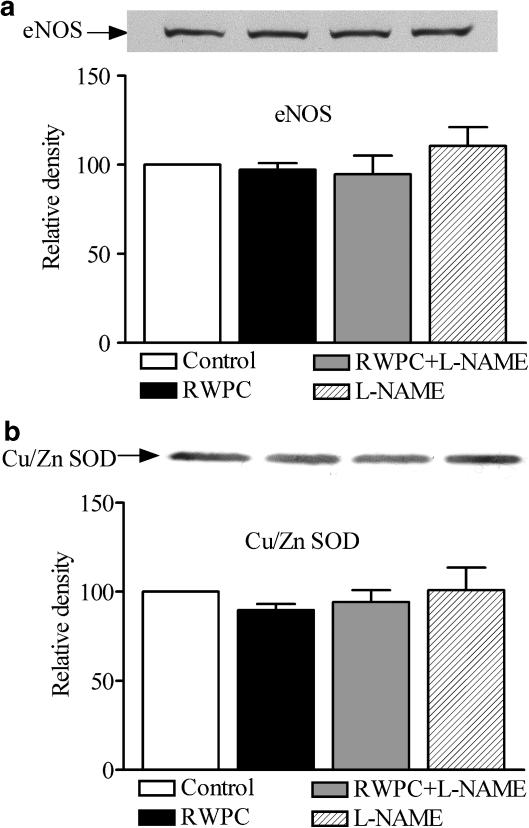
Figure 5a and b showed that eNOS and Cu/Zn SOD protein expression was not significantly different in hearts taken from any of the groups of rats.
Figure 5.
eNOS and Cu/Zn SOD protein level in heart ventricles. Western blot of eNOS (a) and Cu/Zn SOD (b) in heart ventricle from control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1)+L-NAME (2 mg kg−1 day−1), or L-NAME (2 mg kg−1 day−1) treated rats. Density is relative to those measured in lanes of control hearts in each experiment. Results are representative of five experiments.
Ischaemia–reperfusion
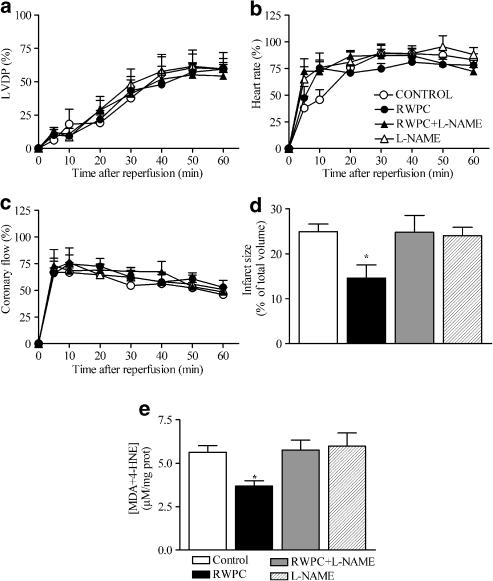
All the hearts from the four groups of rats showed some recovery from the ischaemia. Thus, in all the groups of rats, the recovery of LVDP stabilized after 50 min of reperfusion. However, the level of recovery of LVDP was lower than that measured before the induction of the ischaemia (Figure 6a). In all the groups of rats, the heart rate recovered rather quickly and reached steady-state levels 20 min after reperfusion that were comparable to the heart rates measured before the ischaemia (Figure 6b). Finally, the coronary flow recovered to a steady state 10 min after reperfusion but then remained significantly lower than pre-ischaemic values in the four groups of rats (Figure 6c).
Figure 6.
Effects of RWPC treatment on post-ischaemic recovery. Recovery of LVDP (a), heart rate (b) and coronary flow (c) during post-ischaemic reperfusion in hearts removed from control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1)+L-NAME (2 mg kg−1 day−1), or L-NAME (2 mg kg−1 day−1). Also shown are histograms showing infarct size (d) and the content of MDA+4-HNE (e) in hearts from control rats and those treated with RWPC (20 mg kg−1 day−1), RWPC (20 mg kg−1 day−1)+L-NAME (2 mg kg−1 day−1), or L-NAME (2 mg kg−1 day−1). *P< 0.05 shows a significant difference from the control group by one way ANOVA followed by Dunnett's post-hoc test. Values are mean and the error bars show the s.e.m. of at least 5–10 experiments.
Myocardial infarct size was evaluated morphologically using TTC staining as an index of irreversible myocardial injury. The RWPC treatment significantly reduced the size of myocardial infarction induced by ischaemia by two-fold relative to control hearts (P<0.05). L-NAME, at a dose at which it had no effect by itself, prevented the decrease in infarct size induced by RWPC treatment (Figure 6d).
Total myocardial content of MDA and 4-HNE was decreased in heart ventricles from RWPC-treated rats after ischaemia–reperfusion. L-NAME treatment prevented this effect of RWPC treatment although L-NAME alone did not modify this index of oxidative stress as shown in Figure 6e.
Discussion
The present study provides evidence that the decrease in blood pressure produced by oral administration of RWPC for 1 week was prevented by treatment of rats with L-NAME at a dose at which the NOS inhibitor had no observed effect when administered alone. Furthermore, a transient increase in blood pressure was observed during concomitant treatment with both RWPC and L-NAME. Isolated hearts from rats treated with RWPC displayed a lower basal LVDP and a greater basal heart rate, and showed decreased inotropic responses to both isoprenaline and carbachol. The RWPC treatment also reduced myocardial infarct size and decreased the myocardial content of MDA and 4-HNE, but was not able to improve post-ischaemic functional recovery. These cardiac effects of RWPC treatment were reversed by co-administration of L-NAME with the RWPC. There was no change in eNOS expression in the heart after RWPC treatment, although it increased nitrite concentration in coronary effluent compared to control. Altogether, these data show that the decreased blood pressure, the reduced post-ischaemic infarct size and the cardiac effects induced by chronic treatment of RWPC could be attributable to an increased production of NO.
The present study confirms that short-term administration of RWPC is adequate to produce a sufficient circulating concentration of compounds to induce a decrease in blood pressure and cardiovascular effects as in our previous report (Diebolt et al., 2001). However, the molecular identity of polyphenols responsible for the in vivo effect was still unknown, but they may include oligomeric condensed tannins and anthocyanins (Andriambeloson et al., 1998).
Many authors have advanced the hypothesis that NO accounts for the in vivo effects of RWPC or purple grape juice (Stein et al., 1999). An increase in NO production has been reported to be induced by RWPC in vitro in cultured endothelial cells (Martin et al., 2002), in rat aortic rings with endothelium (Andriambeloson et al., 1997; 1999) and in vivo either in human beings or rats (Wollny et al., 1999; Matsuo et al., 2001). In the present study, evidence is provided that, at a dose at which it had no effect on blood pressure per se, L-NAME treatment completely prevented the hypotensive effect of RWPC, the increase of nitrite production of the heart both under basal and in response to carbachol in RWPC-treated heart. These data suggest the involvement of NO at least for haemodynamic and cardiac effects of RWPC even though the dose of L-NAME used in this work is not sufficient to block entirely NOS activity in accordance with the results reported by Tucker et al. (2000).
With regard to the haemodynamic parameters, L-NAME treatment unmasked a transient hypertensive effect of RWPC. Previously, we have reported that the haemodynamic effects of RWPC were associated with an induction of the expression protein of inducible NOS and cyclooxygenase-2 within the arterial wall which, on balance, allows maintenance of vascular reactivity in response to vasoconstrictor agonists (Diebolt et al., 2001). Thus, it might be that, while blockade of NOS by L-NAME blunted the vascular hyporeactivity component of the effect of RWPC that is due to NO, the treatment allowed the continued release of vasoconstrictor products, such as thromboxane A2, which might explain the transient hypertension observed here.
Short-term administration of RWPC reduced myocardial infarct size even though it did not significantly improve post-ischaemic recovery of cardiac parameters. It has been shown that decreased energy demand during ischaemia allowed improving functional recovery and decrease cellular injury (Vanoverschelde et al., 1994). The decreased contractility observed in hearts from RWPC-treated rats before ischaemia might partially participate in the decreased infarct size by decreasing energy demand and ATP depletion during ischaemia, and thus prevented cellular injury.
Some authors have reported a protective effect from acute administration of RWPC on isolated hearts, as shown by improved post-ischaemic ventricular dysfunction and reduced infarct size (Mosca & Cingolani, 2000, 2002; Sato et al., 2000). It is well documented that NO has protective effects against global ischaemia–reperfusion injury (Ferdinandy & Schulz, 2003). In accordance with the literature, RWPC treatment induced an increase in nitrite concentration in cardiac effluent (which is an index of NO production even though a direct measurement of NO using electronic paramagnetic resonance technique would be more appropriate), which was sensitive to concomitant administration of L-NAME. This basal increase of NO that was also prevented by concomitant treatment with L-NAME might partly explain the protective effect of RWPC in terms of reduced infarct size. Altogether, these results suggest the involvement of NO-related mechanisms in the effects of RWPC. However, eNOS expression in heart ventricles from RWPC-treated rats was not modified, and no expression of inducible NOS could be detected (results not shown). Therefore, an increase of NOS activity, other than through an increase in enzyme protein levels, or a decrease in NO degradation may account for the effects of RWPC. With regard to the former, our previous studies showed that the effects of RWPC treatment on blood pressure, and the amelioration of structural and functional cardiovascular changes in a model of hypertension, were associated with an increase of NOS activity in the left ventricle (Bernatova et al., 2002). In respect of the latter, we did not observe a modification in expression of Cu/Zn SOD in the heart, although we could not exclude an increase in its activity or another pathway that can scavenge oxygen radicals. Cu/Zn SOD is important in cardio-protection against ischaemia and reperfusion injury. Indeed, its deletion in mice makes hearts more susceptible to ischaemia/reperfusion injury (Yoshida et al., 2000) and its overexpression induced prevention of post-ischaemic injury (Wang et al., 1998). However, RWPC treatment induced a decrease in oxidative stress, as evidenced by decreased levels of MDA and 4-HNE which were end products of lipid peroxidation. Thus, an anti-oxidant mechanism may be implicated in the effects of RWPC. Interestingly, L-NAME treatment prevented this decrease in MDA and 4-HNE level observed after RWPC treatment, suggesting that the increase in NO production is necessary to induce the decreased oxidative stress. In accordance with the present study, RWPC can prevent the increased ventricular level of conjugated dienes concentrations, another index of oxidative stress, and this effect is associated to an increased NOS activity in hypertensive rats (O. Pechanova, personal communication). Similar anti-oxidant effects of NO have been shown in literature. Indeed, it has been already shown that NO decreased MDA content in post-ischaemic isolated heart (Oz et al., 2000); this effect could be explained by a protective effect of NO against lipid peroxidation reactions induced by reactive oxygen species (Rubbo et al., 1996).
Although the RWPC treatment may be deduced to be adequate to produce a sufficient circulating concentration of compounds to induce a decrease in infarct size (through either a reduced cell necrosis, apoptosis or both), we did not observe any improvement in functional recovery. As discussed above, NO from eNOS has a protective effect against global ischaemia reperfusion injury. On the one hand, it is possible that increased NO release in hearts from RWPC hearts results in protection against infarction, while, on the other hand, NO depresses myocardial contractility (Sumeray et al., 2000). It should be noted that the experimental conditions used to induce ischaemia are different from those occurring in vivo during pathologic conditions. Indeed, a period of 30 min of global ischaemia with no flow and perfusion might be too severe for the myocardium, and thus a decreased oxidative stress is not sufficient to induce an improvement of functional recovery. Thus, a decreased period of ischaemia might induce less injury and allow the detection of a decrease in the stunning in hearts from RWPC-treated rats. Thus, it cannot be excluded that a longer period of reperfusion might be needed to achieve a complete recovery of cardiac parameters.
The main limitation of the present study is linked to the methodology of the use of isolated heart preparation perfused with physiological buffer rather than whole blood containing different circulating blood cells including neutrophils. The latter play a major role in ischaemia-induced injury. Despite these limitations, the model of Langendorff preparations is largely developed to look at cardiac post-ischaemic dysfunction and data reported here allow to describe the protective effect of RWPC treatment against ischaemia under the experimental conditions used. Another limitations of the present study is that the measurements of eNOS and Cu/Zn SOD at baseline conditions and ischaemia/reperfusion injury may have altered the expression/activity of these proteins, and these partially account for some of the differences seen between experimental groups.
Finally, hearts from RWPC-treated rats developed lower basal LVDP and had a greater heart rate as compared to those from control animals. Also, we found that RWPC treatment resulted in reduced inotropic responses to isoprenaline and carbachol. The observed increase in nitrite concentration in the coronary effluent, and the fact that L-NAME, used at a dose at which it had no effect per se, prevented all the effects of RWPC, suggest the involvement of NO-related mechanisms. In the present study, we have not investigated the transduction pathways implicated in these effects of treatment with RWPC. We could hypothesise that the chronic increase in NO production may explain the reduction of both basal developed pressure and the inotropic response to β-adrenoceptor agonists in hearts from RWPC-treated rats. The mechanisms involved might include decreased intracellular levels of cAMP, reduced calcium influx, or decreased sensitivity of myofilaments to calcium in cardiac myocytes. The reduced negative inotropic effect of the muscarinic agonist carbachol, in hearts from rats treated with RWPC, might result from desensitization of the NO effect on the transduction pathway linked to the activation of muscarinic receptor (Kelly et al., 1996; Balligand, 1999; Kojda & Kottenberg, 1999).
In conclusion, the present study provides evidence that there is a decrease in blood pressure and a subsequent reduction of basal LVDP, increased basal heart rate, as well as decreased inotropic effects of both isoprenaline and carbachol upon short-term oral administration of RWPC, which involves an NO-related mechanism. RWPC treatment also induced protection against myocardial infarction by decreasing oxidative stress after global ischaemia. Instead of an increase in expression of cardiac eNOS, the cardiac effects of RWPC could be attributable to an increase in NO production. Therefore, this study helps us to obtain a better understanding of the beneficial effects of plant-derived polyphenols against cardiovascular disease.
Acknowledgments
Hantamalala Ralay Ranaivo was supported by a ‘Bourse de Docteur Ingénieur' from CNRS. We express gratitude to Dr M.C. Martinez, Dr B. Muller and Dr C.R. Hiley for critical reading of the manuscript. This work was supported by grants from Société Française de Distilleries, ‘Vin et Santé' and the EEC grant Fair program.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- 4-HNE
4-hydroxynonenal
- L-NAME
NG-nitro-L-arginine methyl ester
- LVDP
left ventricular developed pressure
- MDA
malondialdehyde
- NO
nitric oxide
- NOS
nitric oxide synthase
- SOD
superoxide dismutase
- TTC
triphenyltetrazolium chloride
References
- ABU-AMSHA CACCETTA R., BURKE V., MORI T.A., BEILIN L.J., PUDDEY I.B., CROFT K.D. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free Radic. Biol. Med. 2001;30:636–642. doi: 10.1016/s0891-5849(00)00497-4. [DOI] [PubMed] [Google Scholar]
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRIAMBELOSON E., MAGNIER C., HAAN-ARCHIPOFF G., LOBSTEIN A., ANTON R., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998;128:2324–2333. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- ANDRIAMBELOSON E., STOCLET J.C., ANDRIANTSITOHAINA R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J. Cardiovasc. Pharmacol. 1999;33:248–254. doi: 10.1097/00005344-199902000-00011. [DOI] [PubMed] [Google Scholar]
- BALLIGAND J.L. Regulation of cardiac beta-adrenergic response by nitric oxide. Cardiovasc. Res. 1999;43:607–620. doi: 10.1016/s0008-6363(99)00163-7. [DOI] [PubMed] [Google Scholar]
- BERNATOVA I.I., PECHANOVA O., BABAL P., KYSELA S., STVRTINA S., ANDRIANTSITOHAINA R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H942–H948. doi: 10.1152/ajpheart.00724.2001. [DOI] [PubMed] [Google Scholar]
- DE LORGERIL M., SALEN P., MARTIN J.L., BOUCHER F., PAILLARD F., DE LEIRIS J. Wine drinking and risks of cardiovascular complications after recent acute myocardial infarction. Circulation. 2002;106:1465–1469. doi: 10.1161/01.cir.0000029745.63708.e9. [DOI] [PubMed] [Google Scholar]
- DIEBOLT M., BUCHER B., ANDRIANTSITOHAINA R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001;38:159–165. doi: 10.1161/01.hyp.38.2.159. [DOI] [PubMed] [Google Scholar]
- DUTHIE G.G., PEDERSEN M.W., GARDNER P.T., MORRICE P.C., JENKINSON A.M., MCPHAIL D.B., STEELE G.M. The effect of whisky and wine consumption on total phenol content and antioxidant capacity of plasma from healthy volunteers. Eur. J. Clin. Nutr. 1998;52:733–736. doi: 10.1038/sj.ejcn.1600635. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELEIJNSE J.M., LAUNER L.J., VAN DER KUIP D.A., HOFMAN A., WITTEMAN J.C. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am. J. Clin. Nutr. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- GYORGY K., MULLER B., VEGH A., KLESCHYOV A.L., STOCLET J.C. Triggering role of nitric oxide in the delayed protective effect of monophosphoryl lipid A in rat heart. Br. J. Pharmacol. 1999;127:1892–1898. doi: 10.1038/sj.bjp.0702725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTOG M.G., FESKENS E.J., HOLLMAN P.C., KATAN M.B., KROMHOUT D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- KELLY R.A., BALLIGAND J.L., SMITH T.W. Nitric oxide and cardiac function. Circ. Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KOTTENBERG K. Regulation of basal myocardial function by NO. Cardiovasc. Res. 1999;41:514–523. doi: 10.1016/s0008-6363(98)00314-9. [DOI] [PubMed] [Google Scholar]
- MARTIN S., ANDRIAMBELOSON E., TAKEDA K., ANDRIANTSITOHAINA R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol. 2002;135:1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUO S., NAKAMURA Y., TAKAHASHI M., OUCHI Y., HOSODA K., NOZAWA M., KINOSHITA M. Effect of red wine and ethanol on production of nitric oxide in healthy subjects. Am. J. Cardiol. 2001;87:1029–1031. doi: 10.1016/s0002-9149(01)01446-1. [DOI] [PubMed] [Google Scholar]
- MOSCA S.M., CINGOLANI H.E. Post-ischemic myocardial protection with Cabernet-Sauvignon red wine. Medicina (B Aires) 2000;60:609–612. [PubMed] [Google Scholar]
- MOSCA S.M., CINGOLANI H.E. Cardioprotection from ischemia/reperfusion induced by red wine extract is mediated by K(ATP) channels. J. Cardiovasc. Pharmacol. 2002;40:429–437. doi: 10.1097/00005344-200209000-00012. [DOI] [PubMed] [Google Scholar]
- OZ E., ARSAKAY G., DINCER S., ERBAS D. Role of the nitric oxide pathway on ischemia–reperfusion injury in an isolated perfused guinea pig heart. Gen. Pharmacol. 2000;34:3–7. doi: 10.1016/s0306-3623(99)00044-0. [DOI] [PubMed] [Google Scholar]
- RENAUD S., DE LORGERIL M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- RUBBO H., DARLEY-USMAR V., FREEMAN B.A. Nitric oxide regulation of tissue free radical injury. Chem. Res. Toxicol. 1996;9:809–820. doi: 10.1021/tx960037q. [DOI] [PubMed] [Google Scholar]
- SATO M., RAY P.S., MAULIK G., MAULIK N., ENGELMAN R.M., BERTELLI A.A., BERTELLI A., DAS D.K. Myocardial protection with red wine extract. J. Cardiovasc. Pharmacol. 2000;35:263–268. doi: 10.1097/00005344-200002000-00013. [DOI] [PubMed] [Google Scholar]
- SERAFINI M., LARANJINHA J.A., ALMEIDA L.M., MAIANI G. Inhibition of human LDL lipid peroxidation by phenol-rich beverages and their impact on plasma total antioxidant capacity in humans. J. Nutr. Biochem. 2000;11:585–590. doi: 10.1016/s0955-2863(00)00124-8. [DOI] [PubMed] [Google Scholar]
- STEIN J.H., KEEVIL J.G., WIEBE D.A., AESCHLIMANN S., FOLTS J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- SUMERAY M.S., REES D.D., YELLON D.M. Infarct size and nitric oxide synthase in murine myocardium. J. Mol. Cell. Cardiol. 2000;32:35–42. doi: 10.1006/jmcc.1999.1050. [DOI] [PubMed] [Google Scholar]
- TUCKER E.J., LEDINGHAM J.M., ZHENG Y., LAVERTY R. Effects of chronic inhibition of nitric oxide synthase in the genetically hypertensive rat. Clin. Exp. Pharmacol. Physiol. 2000;27:647–649. doi: 10.1046/j.1440-1681.2000.03299.x. [DOI] [PubMed] [Google Scholar]
- VANOVERSCHELDE J.L., JANIER M.F., BERGMANN S.R. The relative importance of myocardial energy metabolism compared with ischemic contracture in the determination of ischemic injury in isolated perfused rabbit hearts. Circ. Res. 1994;74:817–828. doi: 10.1161/01.res.74.5.817. [DOI] [PubMed] [Google Scholar]
- WANG P., CHEN H., QIN H., SANKARAPANDI S., BECHER M.W., WONG P.C., ZWEIER J.L. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLNY T., AIELLO L., DI TOMMASO D., BELLAVIA V., ROTILIO D., DONATI M.B, DE GAETANO G., IACOVIELLO L. Modulation of haemostatic function and prevention of experimental thrombosis by red wine in rats: a role for increased nitric oxide production. Br. J. Pharmacol. 1999;127:747–755. doi: 10.1038/sj.bjp.0702586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA T., MAULIK N., ENGELMAN R.M., HO Y.S., DAS D.K. Targeted disruption of the mouse Sod I gene makes the hearts vulnerable to ischaemic reperfusion injury. Circ. Res. 2000;86:264–269. doi: 10.1161/01.res.86.3.264. [DOI] [PubMed] [Google Scholar]