Abstract
Phytohemagglutinins are widely distributed in common food items. They constitute a heterogeneous group of proteins, which are often resistant to proteolysis in the gastrointestinal tract. Upon binding to the luminal membrane of intestinal cells, they can interfere with digestive, protective or secretory functions of the intestine.
Phytohemagglutinins present in red kidney beans and jackbeans have been shown to induce diarrhea and hypersecretion in human airways, but the underlying mechanisms remain obscure. We examined how agglutinins from wheat germ (WGA), soy bean (SBA), red kidney beans (Pha-E, Pha-L), and jackbeans (Con-A) affect ion transport in mouse airways and large intestine using Ussing chamber techniques.
We found that Pha-E, Pha-L, and Con-A but not WGA and SBA inhibit electrogenic Na+ absorption dose dependently in both colon and trachea. The inhibitory effects of Con-A on Na+ absorption were suppressed by the sugar mannose, by inhibition of phospholipase C (PLC) and protein kinase C (PKC).
Thus, nutritional phytohemagglutinins block salt absorption in a PLC- and PKC-dependent manner, probably by inhibition of the epithelial Na+ channel (ENaC). This effect may be therapeutically useful in patients suffering from cystic fibrosis.
Keywords: Phytohemagglutinin, red kidney beans, jackbeans, concanavalin A, ENaC, epithelial transport, diarrhea, ion transport, airways, intestine
Introduction
Lectins are carbohydrate-binding proteins present in most plants, especially seeds and tubers likes cereals, potatoes, and beans. They constitute a heterogeneous group of proteins, often resistant to proteolysis in the gastrointestinal tract. Some species of beans are particularly rich of these phytohemagglutinins (Pha) such as red kidney beans, which contain Pha-E (erythroagglutinin) and Pha-L (leuagglutinin). These and other lectins are often resistant to cooking and digestive enzymes and sometimes cause food poisoning (Sharon & Lis, 1989). They may not only cause local toxic and inflammatory responses but have also been demonstrated to penetrate the gut wall and may be deposited in distant organs (Wang et al., 1998). Thus, a variety of diseases can be induced by these lectins, such as coeliac disease, peptic ulcer, IgA nephropathy, insulin dependent diabetes, rheumatoid arthritis, and they may even promote upper respiratory infections (Freed, 1999). Lectins bind to carbohydrate residues located in the luminal membrane of intestinal epithelial cells, and thus may induce cellular effects causing interference with digestive, protective, or secretory functions of the intestine. Coeliac disease is a well-known example, induced by gluten, a wheat germ agglutinin (WGA) (Kolberg & Sollid, 1985). Hypersensitivity towards gluten leads to duodenal mucosal damage. These changes in mucosal architecture are accompanied by a change in the electrogenic secretory response in the small intestine (Reims et al., 2002). Stripping of the intestinal mucous coat and bacterial overgrowth has also been observed in the small intestine of rodents fed by lectin from red kidney beans (Phaseolus vulgaris) (Banwell et al., 1988). Moreover, the jackbean lectin concanavalin A (Con-A) causes hypersecretion of mucus in human nose and rat jejunum. It has therefore even been proposed for the treatment of cystic fibrosis patients (Freed & Buckley, 1978; Greer & Pusztai, 1985).
Little is known regarding the cellular effects of lectins on signal transduction and possible changes of ion conductances. For the mitogenic properties of some lectins, intracellular signaling includes activation of phospholipase C (PLC), formation of 1,4,5-inositol trisphosphate (IP3) and activation of protein kinase C (PKC). Activation of this second messenger cascade is likely to affect membrane ion conductances and thus may change epithelial transport properties. The purpose of the present study, therefore, was to determine acute effects of different lectins on electrical properties in mouse colon. As colonic and airway epithelia share many ion transport properties and because a previous report suggested effects of lectins on ion transport in the respiratory epithelium, we also examined the effects of lectins on mouse trachea (Freed & Buckley, 1978). The present results show inhibition of electrogenic Na+ absorption by different lectins, and thus explain why salt absorption is inhibited and how lectins cause diarrheal disease (Banwell et al., 1984; Dobbins et al., 1986).
Methods
Ussing chamber recordings
Distal colon and tracheas were removed from mice (Quackenbush, animal facility of the University of Queensland and BL/6, Charles River, Germany) after killing the animal by cervical dislocation. After removing connective tissues, tracheas were opened by a longitudinal cut. The mucosa of the distal colon was separated mechanically from the submucosal layer. Tissues were put immediately into a ice cold buffer solution of the following composition (mmol l−1): NaCl 145, KCI 3.8, D-glucose 5, MgCI2 1, HEPES 5, Ca-gluconate 1.3. The tissues were mounted into a perfused micro-Ussing chamber with a circular aperture of 0.95 mm2 as described previously (Mall et al., 2003). The apical and basolateral surfaces of the epithelium were perfused continuously at a rate of up to 10 ml min−1 (chamber volume 2 ml). The bath solution contained the following (mmol l−1): NaCl 145, KH2PO4 0.4, K2HPO4 1.6, D-glucose 5, MgCl2 1, HEPES 5 and Ca-gluconate 1.3. The pH was adjusted to 7.4 and all experiments were carried out at 37°C.
Experimental protocols
Experiments were performed under open circuit conditions. Transepithelial resistance (Rte) was determined by applying short (1 s) current pulses (ΔI=0.5 μA) and the corresponding changes in Vte (ΔVte) and basal Vte were recorded continuously (cf. inset in Figure 1a). Values for the transepithelial voltage (Vte) were referred to the serosal side of the epithelium. The equivalent short circuit current (Isc) was calculated according to Ohm's law from Vte and Rte (Isc=Vte/Rte). After mounting the tissues in Ussing chambers, an equilibration time of 30 min was allowed for stabilization of basal Vte and Rte. Amiloride (10 μmol l−1, luminal) was added to inhibit electrogenic Na+ absorption. Continuous bilateral bath perfusion allowed to perform consecutive measurements of amiloride-sensitive Na+ absorption and Cl− secretion activated by carbachol or forskolin and IBMX, in the absence or presence of lectins in the bath solution. For incubation with lectins, luminal or basolateral bath perfusion was reduced and luminal and basolateral compartments were air bubbled.
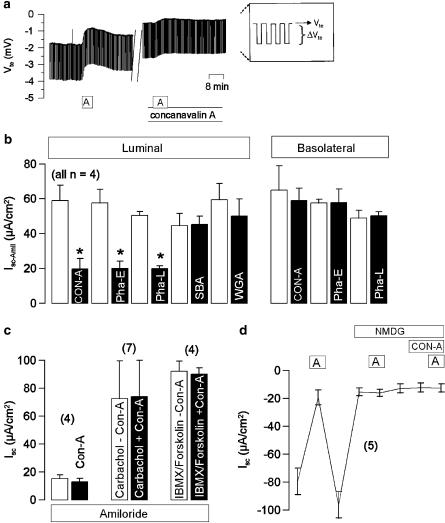
Figure 1.
(a) Original recording of the transepithelial voltage Vte in a mouse colonic epithelium. The voltage deflections (ΔVte) are induced by pulsed current injection (0.5 μA). Effects of amiloride (A, 10 μmol l−1) before and after exposure (20 min) to concanavalin-A (100 μg ml−1). (b) Summary of the effects of the lectins concanavalin-A (Con-A), erythroagglutinin (Pha-E), leuagglutinin (Pha-L), soy bean agglutinin (SBA), and wheat germ agglutinin (WGA) (all 100 μgm l−1) on amiloride-sensitive Na+ absorption (Isc−Amil) in mouse colonic epithelia, when applied to either luminal or basolateral sides. (c) Summary of the short circuit currents Isc induced by Con-A (100 μmol l−1), carbachol (100 μmol l−1), and IBMX (100 μmol l−1) together with forskolin (10 μmol l−1) in mouse colon in the presence of amiloride. (d) Summary of the effects of amiloride (10 μmol l−1) applied under control conditions, after replacing extracellular Na+ by N-methyl D-glucamine (NMDG), and after addition of NMDG in the presence of NMDG. * indicate significant difference when compared to control (number of experiments).
Materials and statistical analysis
All used compounds were of highest available grade of purity. WGA from Triticum vulgaris, soy bean agglutinin (SBA) from Glycine max, phaseolus hemagglutinin, Pha-L and Pha-E, from P. vulgaris (red kidney bean), and Con-A from Canavalia ensiformis (Jack bean) were all obtained from Sigma and BioChemika, respectively (Sigma-Aldrich Australia). 3-Isobutyl-1-methylxanthine (IBMX), forskolin, amiloride, bisindolylmaleimide 1 (BIM), and 1,2-Bis(2-aminophenoxy)ethane-N,N,N¢,N¢-tetraacetic acid tetrakis(acetoxy-methyl ester) (BAPTA-AM) were all from Sigma (Australia and Germany). U73122 was from Calbiochem (Australia, Germany). All other chemicals were obtained from Merck (Darmstadt, Germany). Paired or unpaired student's t-test were used for statistical analysis. P-values <0.05 were accepted to indicate statistical significance (*).
Results
Lectins inhibit amiloride-sensitive Na+ absorption but do not change secretory transport in mouse colon
After mounting mouse colon into the perfused micro-Ussing chamber, the transepithelial voltage (Vte) was measured and the transepithelial resistance (Rte) was calculated from the voltage deflections (ΔVte) induced by pulsed current injection (0.5 μA) (Figure 1a). Under control conditions, Vte of −3.1±0.5 mV and Rte of 41.4±6.5 Ω cm2 were measured and an equivalent short circuit current of −78±15.1 μA cm−2 (n=4) was calculated. The short circuit current was dominated by electrogenic Na+ absorption, since 10 μmol l−1 amiloride reduced Isc reversibly to 17.8±3.6 μA cm−2 (Figure 1a and b). We examined if different lectins, previously described to induce diarrhea, change the ion transport properties of the colonic epithelium. To that end we examined the effects of Con-A, Pha-E and Pha-L from red kidney beans (phaseolus) as well as WGA and SBA. The agglutinins were applied to either luminal or basolateral sides of epithelial tissues at concentrations of 100 μg ml−1. We found that incubation of the luminal side of the mucosa for 20 min with Con-A, Pha-E and Pha-L reduced the transepithelial voltage of the colonic epithelium. An example of an original recording is shown for Con-A in Figure 1a. Calculation of the short circuit currents showed inhibition of amiloride-sensitive Na+ absorption (Isc−Amil) by luminal but not basolateral application of Con-A, Pha-E or Pha-L, while SBA and WGA were without effects (Figure 1b). The results suggest that only specific lectins have the potential to change epithelial ion transport properties and to cause disturbances such as diarrhea. It should be noted that the inhibitory effects on Isc−Amil were not reversible within 3 h after washing out of the lectins.
In order to unmask possible secretory effects of lectins, Con-A was applied in the presence of amiloride. However, no increase in secretory transport could be assessed. Moreover, Ca2+-dependent Cl− secretion by carbachol (100 μmol l−1) and cyclic adenosine monophosphate (cAMP)-dependent Cl− secretion by IBMX (100 μmol l−1) and forskolin (2 μmol l−1) were not different in the presence of the lectin (Figure 1c). Finally, we examined the effect of Con-A when Na+ absorption was eliminated by replacing extracellular Na+ with an equimolar concentration of NMDG+. It is shown that replacement of extracellular Na+ by NMDG largely reduced Isc and abolished the effects of amiloride. Under these conditions Con-A no longer affected Isc (Figure 1d). The experiments therefore suggest that Con-A predominantly, if not exclusively, affects amiloride-sensitive Na+ absorption.
Na+ absorption is inhibited by lectins in mouse trachea
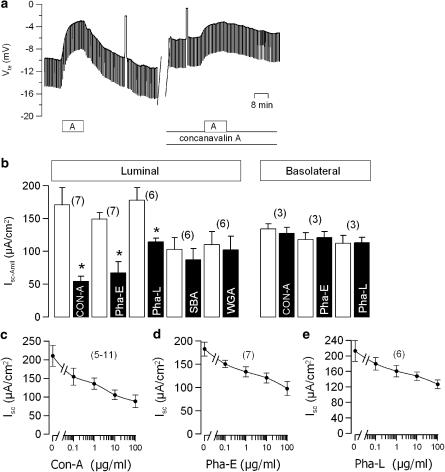
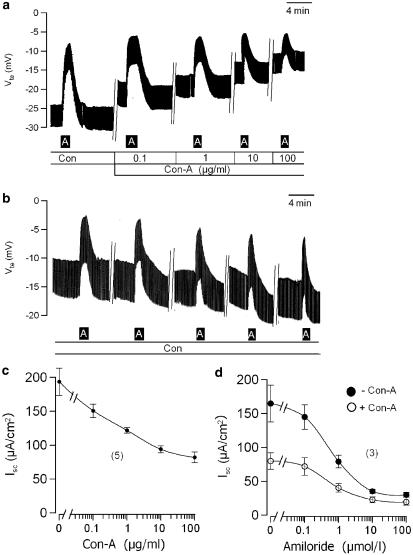
Owing to the similarities in ion transport of colonic and airway epithelia, we examined the effects of lectins on mouse trachea. Under control conditions, Vte and Rte of mouse tracheas were −12.5±2.2 mV and 84.0±6.4 Ω cm2, respectively and an equivalent short circuit current of −215.3±29.1 μA cm−2 (n=7) was calculated. As demonstrated for the colon, application of Con-A (100 μg l−1) reduced Vte and attenuated the effects of amiloride on Vte (Figure 2a). Similar to the effects on mouse colon, amiloride-sensitive Na+ absorption (Isc−Amil) was inhibited by luminal but not basolateral application of Con-A, Pha-E or Pha-L, while SBA and WGA were without any effects (Figure 2b). Again, Con-A did not cause a secretory response of the epithelium. Short circuit currents activated by carbachol (100 μmol l−1) or IBMX (100 μmol l−1) and forskolin (10 μmol l−1) were not augmented in the presence of Con-A, suggesting an exclusive effect on Na+ absorption (data not shown). When we examined the effects of different concentrations of Con-A, Pha-E and Pha-L on the ion transport in mouse trachea, we found a dose-dependent inhibition of Isc (Figure 2c–e). Concentration-dependent inhibition of Isc was due to inhibition of amiloride-sensitive absorption, as the inhibitory effect of amiloride on Vte is gradually reduced (Figure 3a). This was not observed when amiloride was applied repetitively in the absence of the lectin (Figure 3b). Airways of cystic fibrosis patients show enhanced Na+ absorption, which plays a central role for the pathophysiology of the CF lung disease (Kunzelmann & Mall, 2003). Con-A and other lectins inhibit Na+ absorption in both colon and airways, which may be potentially useful for the therapy of CF lung disease (Kunzelmann & Mall, 2003). We therefore asked the question, whether Con-A also inhibits Na+ absorption in the airways of transgenic CF mice, homozygous for the common mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) G551D (Oceandy et al., 2003). In contrast to the most frequent CF mutation ΔF508, G551D-CFTR is expressed in the luminal membrane of epithelial cells, but is nonfunctional. We preferred G551D mice over ΔF508 mice in order to detect any possible direct effects of lectins on transport proteins, including mutant CFTR. In fact, Con-A reduced Vte and enhanced Rte in CF airways similar to that in non-CF mice, and thus inhibited Isc dose dependently (Figure 3c). Thus, natural phytohemagglutinins may be well suitable for attenuating excessive Na+ absorption in the airways of CF patients. Inhibition of Isc is due to blocking of Na+ absorption, indicated by attenuated effects of amiloride. In order to exclude any changes in affinity of the epithelial Na+ channel (ENaC) for amiloride due to incubations with lectins, we examined different concentrations of amiloride in the absence and presence of Con-A. As shown in Figure 3d, incubation with the lectin did not shift the concentration-response to amiloride.
Figure 2.
(a) Original recording of the transepithelial voltage Vte in a tracheal epithelium. Effects of amiloride (A, 10 μmol l−1) before and after exposure (20 min) to concanavalin-A (100 μg ml−1). (b) Summary of the effects of the lectins concanavalin-A (Con-A), erythroagglutinin (Pha-E), leuagglutinin (Pha-L), soy bean agglutinin (SBA), and wheat germ agglutinin (WGA) (all 100 μg ml−1) on amiloride-sensitive Na+ absorption (Isc−Amil) in mouse colonic epithelia, when applied to either luminal or basolateral sides. (c–e) Dose–response of the effects of the lectins Con-A, Pha-E, and Pha-L on ion transport (Isc) in mouse trachea. * indicate significant difference when compared to control (number of experiments).
Figure 3.
(a) Original recording of the transepithelial voltage Vte in mouse trachea. At 20 min incubation of increasing concentrations of Con-A reduced Vte and the inhibitory effect of amiloride on Vte. (b) Incubation for the same time period in control ringer solution did not reduce Vte and did not attenuate amiloride responses. (c) Dose–response curve for the inhibitory effects of Con-A on Isc measured in tracheas of homozygous G551D-CFTR mice. (d) Concentration dependence of the inhibitory effect of amiloride on Isc detected in mouse trachea in the absence or presence of Con-A (number of experiments).
Con-A binds to membrane carbohydrates and inhibits Na+ absorption probably in a PLC- and PKC-dependent manner
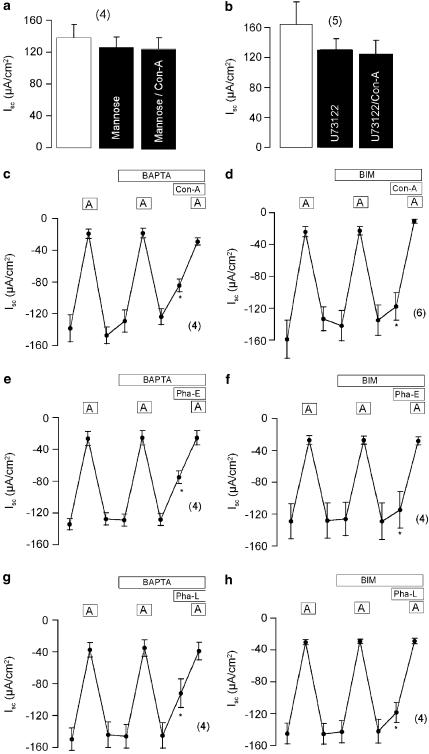
Lectin binding shows a high specificity for carbohydrates (Sharon & Lis, 1989). Thus, binding of Con-A to the cellular proteoglycan mannose is impaired by exogenous application of mannose. We therefore tested whether the inhibitory effects of 100 μg ml−1 Con-A on Isc−Amil are inhibited in the presence of 10 mmol l−1 mannose. Luminal application of mannose itself had no effects on Isc. However, in the presence of mannose, the inhibitory effects of 100 μg ml−1 Con-A on Isc were completely eliminated, suggesting inhibition of epithelial Na+ absorption via specific binding of Con-A to a membrane proteoglycan receptor (Figure 4a). It is known from previous studies that binding to membrane carbohydrates may cause activation of the PLC pathway with consecutive hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2), IP3 formation and increase in intracellular Ca2+. In fact, when preincubated with the PLC inhibitor U73122 (10 μM) for 30 min, Con-A did no longer change Isc (Figure 4b). This result suggests that a PLC-dependent process in involved in the inhibition of Isc and decrease of Na+ absorption. For some phytohemagglutinins, increase in intracellular Ca2+ along with activation of other intracellular signaling molecules, such as mitogen activated protein kinase and PKC has been demonstrated (Sharon & Lis, 1989). We could demonstrate recently that stimulation of the PKC cascade is involved in the attenuation of airway Na+ absorption by influenza virus (Kunzelmann et al., 2000). This prompted us to further examine the intracellular events causing inhibition of Na+ absorption. To that end, amiloride was applied to airway epithelia and Isc was determined. Afterwards, the tissue was incubated from both sides for 30 min with 10 μmol l−1 with the membrane permeable Ca2+ chelator BAPTA-AM, which did not significantly affect amiloride-sensitive Na+ transport. The efficacy of BABTA-AM to chelate Ca2+ was demonstrated by application of carbachol (100 μmol l−1), which is know to increase cytosolic Ca2+. The carbachol induced Isc of 185.6±55.8 μA cm−2 was significantly reduced to 74.5±19 μA cm−2 (n=5) in the presence of BAPTA-AM. However, all three phytohemagglutinins, Con-A, Pha-E, and Pha-L (each 100 μg ml−1) still inhibited Isc to the same extent in the presence of BAPTA-AM. Thus, inhibition of Isc−Amil is not caused by a possible increase in intracellular Ca2+. Similar experiments were performed with an inhibitor of PKC, bisindolylmaleimide (BIM, 0.5 μmol l−1). Similar to BAPTA-AM, BIM did not affect amiloride-sensitive Na+ transport when applied for 30 min to both basolateral and luminal sides of the epithelium. In the presence of BIM, phytohemagglutinins-dependent inhibition of Isc−Amil was still observed, although the effect was reduced when compared to the absence of BIM. Since previous reports have shown a PKC-dependent inhibition of ENaCs, these results suggest that lectins from red kidney beans and jackbeans reduce colonic and airway Na+ absorption by a PLC-dependent mechanism, partially due to PKC mediated inhibition of ENaC.
Figure 4.
Effects of inhibitors of signal transduction on lectin effects in mouse trachea. (a) Summary of the effects on Isc of luminal application of mannose (10 mM) and Con-A (100 μmol l−1) in the presence of mannose. (b) Summary of the effects on Isc of luminal/basolateral application of the PLC inhibitor U73122 (10 μM) and Con-A (100 μmol l−1) in the presence of U73122. (c–h) Effects of Ca2+ chelating by 30 min preincubation with BAPTA-AM (10 μmol l−1) and inhibiting protein kinase C by 30 min preincubation with BIM (1 μmol l−1) on lectin induced changes in transport. (c, e, g) Subsequent application of amiloride (10 μmol l−1) under control conditions, after incubation in BAPTA, and after addition of the lectin in the presence of BAPTA. (d, f, h) Subsequent application of amiloride (10 μmol l−1) under control conditions, after incubation in BIM, and after addition of the lectin in the presence of BIM. * indicate significant difference compared to control (paired t-test) (number of experiments).
Discussion
Lectins are glycoproteins of nonimmune origin that agglutinate cells and/or precipitate complex carbohydrates. The agglutination activity of these highly specific carbohydrate-binding molecules is usually inhibited by a simple monosaccharide, but for some lectins di, tri, and even polysaccharides are required (Sharon & Lis, 1989). In the present study we found inhibition of amiloride-sensitive Na+ transport by the lectin Con-A, which was blocked by the sugar mannose. Since binding of Con-A is specific to mannose linked carbohydrates, we assume that inhibition of Na+ absorption by Con-A occurs through binding of Con-A to these proteoglycan receptors (Sharon & Lis, 1989). Inhibition of electrogenic Na+ absorption by Con-A or the P. vulgaris lectins will shift the net transport by the colonic epithelium from NaCl absorption towards secretion. This is likely to take part in the diarrhea inducing effects of these lectins. Previous reports have shown inhibition of NaCl absorption in the rabbit ileum, although up to 50-fold higher concentrations of the Phaseolus phytohemagglutinin has been used in these experiments (Dobbins et al., 1986). In fact, at higher concentrations additional complications have been described such as translocation of intraluminal enteric bacteria into mesenteric lymph nodes and increased vascular permeability (Greer & Pusztai, 1985; Shoda et al., 1995). These results may explain why several plant lectins may cause severe diarrheal disease in human and animals (Banwell et al., 1984; Freed, 1985, 1999). We were unable to detect a secretory responses by the lectins in both colon and trachea, and the secretory response of cAMP or Ca2+ enhancing secretagogues were not augmented by lectins. Thus, at the low concentrations of lectins used in the present study, their cellular effects appear to be limited to inhibition of Na+ absorption and shift towards electrolyte secretion rather than active secretion or epithelial destruction.
The present results exclude a role of Ca2+ for the inhibition of Na+ absorption by lectins, since the Ca2+ chelator BAPTA-AM did not interfere with the inhibitory effect. Although BAPTA-AM was not able to completely inhibit the carbachol response, it largely reduced carbachol induced secretion. Thus, a role of intracellular Ca2+ appears unlikely. However, phytohemagglutinins inhibit epithelial Na+ absorption obviously via activation of PLC, as indicated by the PLC inhibitor U-73122, which is inhibitory to several PLC subtypes (Chen et al., 1998). Notably, several PKC isoforms are expressed in both colonic and airway epithelia including the Ca2+ independent PKCδ (Cerda et al., 2001; Wang et al., 2003). Activation of PLC and subsequent hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into IP3 and diacylglycerol (DAG) has also been shown for mitogenic stimulation of T lymphocytes (reviewed in Sharon & Lis, 1989). Synthesis of DAG is followed by activation of PKC, which takes part in the mitogenic effects. Inhibition of Na+ absorption may therefore occur via two mechanisms, both of them are likely to act directly at the ENaC: ENaC may be inhibited (i) by a loss of PIP2 through PLC induced hydrolysis and (ii) via activation of PKC. ENaC is formed by a heterotetramer of 2α, 1β, and 1γ subunit (Canessa et al., 1994). The β and γ subunits contain a PIP2 binding motif in their intracellular N-terminus, and it was suggested that binding of β,γ-ENaC to PIP2 is required for full activation of the channel (Ma et al., 2002; Yue et al., 2002). It is therefore entirely possible that lectin induced activation of PLC and hydrolysis of PIP2 reduces binding of ENaC to PIP2 and thus deactivates the channel. Moreover, previous studies also demonstrated inhibition of ENaC by PKC when expressed in Xenopus oocytes (Awayda et al., 1996; Kunzelmann, 2003). In contrast, an effect of PKC on epithelial Cl− secretion could not be demonstrated for the native human colonic epithelium or airways (Kunzelmann, 2003). The present results show inhibition of Na+ absorption by phytohemagglutinins, without stimulation of a Cl− secretion, which is in good agreement with those previous findings.
Previous reports found mucotractive effects of lectins, presumably by a change in ion transport in the respiratory epithelium (Freed & Buckley, 1978; Freed, 1999). In cystic fibrosis, hyperabsorption of NaCl by the respiratory epithelium is a major pathophysiological factor, contributing essentially to the reduced airway surface liquid and reduced mucociliary clearance (Boucher, 2001). As lectins induce a long-lasting inhibition of amiloride-sensitive Na+ absorption in airways and intestine, they may even be considered as a new treatment for the cystic fibrosis lung disease (Freed & Buckley, 1978; Greer & Pusztai, 1985).
Acknowledgments
This work was supported by NHMRC 252823, DFG KU 756/7-1, the Else Kröner-Fresenius Stiftung, Mukoviszidose e.V. and DFG Schr 752/2-1. The excellent technical assistance by Ms Ernestine Tartler and Ms Agnes Paech is gratefully acknowledged. We thank Professor Dr B. Wainwright and Dr B. McMorran (Institute for Molecular Bioscience, University of Queensland, Australia) for supplying the G551D/G551D mice.
Abbreviations
- BAPTA-AM
1,2-Bis(2-aminophenoxy)ethane-N,N,N¢,N¢-tetraacetic acid tetrakis-acetoxymethyl ester
- BIM
bisindolylmaleimide
- cAMP
cyclic adenosine monophosphate
- Con-A
concanavalin A
- DIDS
4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid
- ENaC
epithelial Na+ channel
- IBMX
3-isobutyl-1-methylxanthine
- PHA-E
erythroagglutinin
- PHA-L
leuagglutinin
- SBA
soy bean agglutinin
- WGA
wheat germ agglutinin
References
- AWAYDA M.S., ISMAILOV I.I., BERDIEV B.K., FULLER C.M., BENOS D.J. Protein kinase regulation of a cloned epithelial Na+ channel. J. Gen. Physiol. 1996;108:49–65. doi: 10.1085/jgp.108.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANWELL J.G., ABRAMOWSKY C.R., WEBER F., HOWARD R., BOLDT D.H. Phytohemagglutinin-induced diarrheal disease. Dig. Dis. Sci. 1984;29:921–929. doi: 10.1007/BF01312481. [DOI] [PubMed] [Google Scholar]
- BANWELL J.G., HOWARD R., KABIR I., COSTERTON J.W. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Can. J. Microbiol. 1988;34:1009–1013. doi: 10.1139/m88-177. [DOI] [PubMed] [Google Scholar]
- BOUCHER R.C. Pathogenesis of cystic fibrosis airways disease. Trans. Am. Clin. Climatol. Assoc. 2001;112:99–107. [PMC free article] [PubMed] [Google Scholar]
- CANESSA C.M., SCHILD L., BUELL G., THORENS B., GAUTSCHL I., HORISBERGER J.D., ROSSIER B.C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- CERDA S.R., BISSONNETTE M., SCAGLIONE-SEWELL B., LYONS M.R., KHARE S., MUSTAFI R., BRASITUS T.A. PKC-delta inhibits anchorage-dependent and -independent growth, enhances differentiation, and increases apoptosis in CaCo-2 cells. Gastroenterology. 2001;120:1700–1712. doi: 10.1053/gast.2001.24843. [DOI] [PubMed] [Google Scholar]
- CHEN C.C., WANG J.K., LIN S.B. Antisense oligonucleotides targeting protein kinase C-alpha, -beta I, or -delta but not -eta inhibit lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages: involvement of a nuclear factor kappa B-dependent mechanism. J. Immunol. 1998;161:6206–6214. [PubMed] [Google Scholar]
- DOBBINS J.W., LAURENSON J.P., GORELICK F.S., BANWELL J.G. Phytohemagglutinin from red kidney bean (Phaseolus vulgaris) inhibits sodium and chloride absorption in the rabbit ileum. Gastroenterology. 1986;90:1907–1913. doi: 10.1016/0016-5085(86)90260-x. [DOI] [PubMed] [Google Scholar]
- FREED D.L. Lectins (editorial) Br. Med. J. (Clin. Res. Edn.) 1985;290:584–586. doi: 10.1136/bmj.290.6468.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREED D.L. Do dietary lectins cause disease? (editorial) (see comments) BMJ. 1999;318:1023–1024. doi: 10.1136/bmj.318.7190.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREED D.L., BUCKLEY C.H. Mucotractive effect of lectin. Lancet. 1978;1:585–586. doi: 10.1016/s0140-6736(78)91030-9. [DOI] [PubMed] [Google Scholar]
- GREER F., PUSZTAI A. Toxicity of kidney bean (Phaseolus vulgaris) in rats: changes in intestinal permeability. Digestion. 1985;32:42–46. doi: 10.1159/000199215. [DOI] [PubMed] [Google Scholar]
- KOLBERG J., SOLLID L. Lectin activity of gluten identified as wheat germ agglutinin. Biochem. Biophys. Res. Commun. 1985;130:867–872. doi: 10.1016/0006-291x(85)90496-6. [DOI] [PubMed] [Google Scholar]
- KUNZELMANN K.Control of membrane transport by the cystic fibrosis transmembrane conductance regulator (CFTR) The Cystic Fibrosis Transmembrane Conductance Regulator 2003Landes Bioscience U.S.A.: Kluwer Academic, Plenum Publisher; 55–93.ed. Kirk, K.L. & Dawson, D.C. [Google Scholar]
- KUNZELMANN K., BEESLEY A.H., KING N.J., KARUPIAH G., YOUNG J.A., COOK D.I. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10282–10287. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNZELMANN K., MALL M. Pharmacotherapy of the ion transport defect in cystic fibrosis: potential role of P2Y2 receptor agonists. Am. J. Resp. Med. 2003;2:299–329. doi: 10.1007/BF03256658. [DOI] [PubMed] [Google Scholar]
- MA H.P., SAXENA S., WARNOCK D.G. Anionic phospholipids regulate native and expressed ENaC. J. Biol. Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- MALL M., GONSKA T., THOMAS J., SCHREIBER R., SEYDEWITZ H.H., KUEHR J., BRANDIS M., KUNZELMANN K. Role of basolateral K+ channels in Ca2+ activated Cl− secretion in human normal and cystic fibrosis airway epithelia. Pediatr. Res. 2003;53:608–618. doi: 10.1203/01.PDR.0000057204.51420.DC. [DOI] [PubMed] [Google Scholar]
- OCEANDY D., MCMORRAN B.J., SCHREIBER R., WAINWRIGHT B., KUNZELMANN K. GFP-tagged CFTR transgene is functional in the G551D cystic fibrosis mouse colon. J. Membr. Biol. 2003;192:159–167. doi: 10.1007/s00232-002-1072-y. [DOI] [PubMed] [Google Scholar]
- REIMS A., REDFORS S., ASCHER H., STRANDVIK B. Electrogenic ion transport in duodenal biopsies from children with coeliac disease. Scand. J. Gastroenterol. 2002;37:43–50. doi: 10.1080/003655202753387347. [DOI] [PubMed] [Google Scholar]
- SHARON N., LIS H. Lectins. Cambridge, U.K.: University Press; 1989. [Google Scholar]
- SHODA R., MAHALANABIS D., WAHED M.A., ALBERT M.J. Bacterial translocation in the rat model of lectin induced diarrhoea. Gut. 1995;36:379–381. doi: 10.1136/gut.36.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q., WANG X., EVERS B.M. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J. Biol. Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- WANG Q., YU L.G., CAMPBELL B.J., MILTON J.D., RHODES J.M. Identification of intact peanut lectin in peripheral venous blood. Lancet. 1998;352:1831–1832. doi: 10.1016/S0140-6736(05)79894-9. [DOI] [PubMed] [Google Scholar]
- YUE G., MALIK B., EATON D.C. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J. Biol. Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]