Abstract
Lipoxins (LX) and aspirin-triggered 15-epi-lipoxins (ATL) exert potent anti-inflammatory actions. In the present study, we determined the anti-inflammatory efficacy of endogenous LXA4 and LXB4, the stable ATL analog ATLa2, and a series of novel 3-oxa-ATL analogs (ZK-996, ZK-990, ZK-994, and ZK-142) after intravenous, oral, and topical administration in mice.
LXA4, LXB4, ATLa2, and ZK-994 were orally active, exhibiting potent systemic inhibition of zymosan A-induced peritonitis at very low doses (50 ng kg−1–50 μg kg−1).
Intravenous ZK-994 and ZK-142 (500 μg kg−1) potently attenuated hind limb ischemia/reperfusion-induced lung injury, with 32±12 and 53±5% inhibition (P<0.05), respectively, of neutrophil accumulation in lungs. The same dose of ATLa2 had no significant protective action.
Topical application of ATLa2, ZK-994, and ZK-142 (∼20 μg cm−2) prevented vascular leakage and neutrophil infiltration in LTB4/PGE2-stimulated ear skin inflammation. While ATLa2 and ZK-142 displayed approximately equal anti-inflammatory efficacy in this model, ZK-994 displayed a slower onset of action.
In summary, native LXA4 and LXB4, and analogs ATLa2, ZK-142, and ZK-994 retain broad anti-inflammatory effects after intravenous, oral, and topical administration. The 3-oxa-ATL analogs, which have enhanced metabolic and chemical stability and a superior pharmacokinetic profile, provide new opportunities to explore the actions and therapeutic potential for LX and ATL.
Keywords: Anti-inflammation, treatment, leukocyte, neutrophil infiltration, lipoxins, delivery, ischemia reperfusion injury, peritonitis, skin
Introduction
The acute inflammatory response of the body constitutes recognition of invading pathogens, or chemical and physical tissue alterations, followed by physiological changes that allow recruitment of inflammatory cells that clear the original inflammatory stimulus, and resolution of inflammation allowing tissue homeostasis to recover (Gallin & Snyderman, 1999). Temporal regulation in the order of recruitment and activation of different classes of leukocytes and lymphocytes by proinflammatory mediators allows a coordinated course of events during the inflammatory process (Gallin & Snyderman, 1999). Likewise, the resolution of inflammation is now thought to involve the formation of endogenous anti-inflammatory mediators which signal the termination of recruitment and favor removal of inflammatory cells from the inflammatory locus (Levy et al., 2001; Serhan & Oliw, 2001; Lawrence et al., 2002).
Lipoxins (LX) constitute the first recognized class of endogenous anti-inflammatory lipid-based autacoids which function as endogenous ‘stop signals' that downregulate or counteract the formation and actions of proinflammatory mediators (Serhan, 1991) and promote resolution (Serhan & Oliw, 2001). LX are formed via two sequential lipoxygenase-catalyzed oxygenations of arachidonic acid. In human tissues, LX formation predominantly occurs by transcellular biosynthesis in settings of heterotypic cellular interactions, such as the interaction of polymorphonuclear leukocytes with platelets, endothelia, and epithelia during inflammation (Serhan & Oliw, 2001). Endogenous formation of LX occurs during inflammation, both in man and in animal models (Edenius et al., 1990; Lee et al., 1990; Levy et al., 2001)). The native LX, lipoxin (LX) A4 and its positional isomer LXB4, are potent regulators of leukocyte functions, including migration, degranulation, phagocytosis, and proinflammatory mediator formation (Serhan, 2001; Gewirtz et al., 2002; Gavins et al., 2003). LXA4 exerts its physiological action at least in part via activation of a specific cell-surface binding site, the LXA4-receptor (ALX) (Chiang et al., 2000). Interestingly, aspirin triggers the formation of an epimer of LXA4, 15-epi-lipoxin A4, via alteration of the enzymatic activity of cyclooxygenase-2 (Clària & Serhan, 1995; Fiorucci et al., 2003).
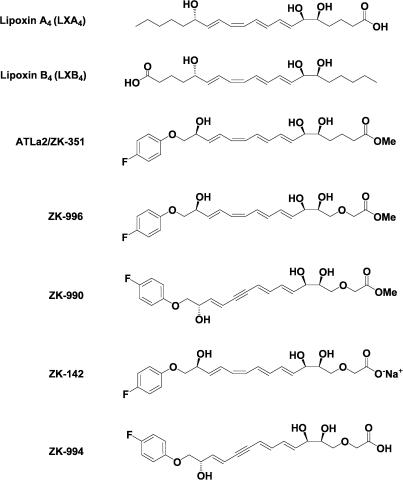
Eicosanoids are known to be rapidly formed, act locally, and are rapidly inactivated by enzymatic degradation. The unique trihydroxy-tetraene structure of LXA4 (Figure 1) is sensitive to metabolism by prostaglandin dehydrogenase (PGDH) at C-15 and ω-oxidation at C-20 (Serhan et al., 1993; 1995). Although aspirin-triggered 15-epi-lipoxin A4 is less susceptible to PGDH deactivation and has a greater biological half-life (Serhan et al., 1995), elimination of ω-oxidation was achieved by replacing carbons 16–20 with a fluorophenoxy group, as in ATLa. However, ATLa was cleared within 15 min after intravenous injection in the mouse (Clish et al., 1999). Eicosanoids were reported to undergo β-oxidation (Pace-Asciak, 1989), but this pathway had not been previously observed in the case of LX. Removal of metabolic inactivation by β-oxidation through the introduction of a 3-oxa group, ZK-142, greatly increased the half-life in rats to 1.3 h (Guilford et al., 2004). LXA4 and ATL analogs are chemically unstable in acid and light, due in part to the central tetraene group. Replacement of the tetraene unit in ZK-142 by a trienyne group gave a more chemically stable analog, ZK-994, which maintained its anti-inflammatory activity in vivo. The pharmacokinetic profiles after oral administration of ATLa, ZK-142, and ZK-994 showed that significant amounts of each compound were present in plasma (Guilford et al., 2004). In the present study, we report on the ability of native LXA4 and B4, the aspirin-triggered LX analog ATLa2, and the new 3-oxa-lipoxin analogs to limit the severity of inflammation in vivo after systemic and topical administration in murine models of inflammation (Serhan et al., 1995; Scalia et al., 1997; Maddox et al., 1998; Filep et al., 1999), as well as after oral administration.
Figure 1.
Structures of LXA4, LXB4, the aspirin-triggered lipoxin (ATL) analog ATLa2 and the novel 3-oxa-ATL analogs ZK-996, ZK-990, ZK-142, and ZK-994.
Experimental procedures
Materials
The following drugs were obtained from the sources indicated: isoflurane and pentobarbital (Abbott Laboratories, North Chicago, IL, U.S.A.). Zymosan A and Evans Blue (Sigma Chemical Company, St Louis, MO, U.S.A.). Synthetic LTB4 and PGE2 (Cayman Chemical Company, Ann Arbor, MI, U.S.A.). LXA4 (5S, 6R, 7E, 9E, 11Z, 13E, 15S)-5,6,15-trihydroxy-7,9,11,13-eicosatetraenoic acid) and LXB4 (5S, 14R, 6E, 8Z, 10E, 12E, 15S)-5,14,15-trihydroxy-6,8,10,12-eicosatetraenoic acid) were purchased from Cascade, Reading, U.K. ATLa2 (ZK-351) (Methyl (5S, 6R, 7E, 9E, 11Z, 13E, 15S)-16-(4-fluorophenoxy)-5,6,15-trihydroxy-7,9,11,13-hexadecatetraenoate), ZK-996 (Methyl (5R, 6R, 7E, 9E, 11Z, 13E, 15S)-16-(4-fluorophenoxy)-3-oxa-5,6,15-trihydroxy-7,9,11,13-hexadecatetraenoate), ZK-990 (Methyl (5R, 6R, 7E, 9E, 13E, 15S)-16-(4-fluorophenoxy)-3-oxa-5,6,15-trihydroxy-7,9,13-hexadecatrien-11-ynoate), ZK-142 (5R, 6R, 7E, 9E, 11Z, 13E, 15S)-16-(4-fluorophenoxy)-3-oxa-5,6,15-trihydroxy-7,9,11,13-hexadecatetraenoic acid, sodium salt), and ZK-994 (5R, 6R, 7E, 9E, 13E, 15S)-16-(4-fluorophenoxy)-3-oxa-5,6,15-trihydroxy-7,9,13-hexadecatrien-11-ynoic acid) were all synthesized at Berlex Biosciences (Richmond, CA, U.S.A.) using the procedure described in Guilford et al. (2004) and were >98% pure by two independent analytical methods. The total organic synthesis of the new 3-oxa-ATLs is described in Guilford et al. (2004).
Animals
All animals used in the present study were male FVB mice (Charles River Laboratory, Wilmington, MA, U.S.A.) that were 6–8 weeks old (weighing 20–25 g). They were maintained in a temperature and light-controlled environment, and had unlimited access to food (Laboratory Rodent Diet, LabDiet) and tap water. All animal experiments were performed in accordance with the guidelines for animal care at the Brigham & Women's Hospital.
Zymosan A-induced peritonitis
Peritonitis was induced by intraperitoneal administration of 1 mg zymosan A in 1 ml of sterile saline. LXA4, ATLa2, ZK-142, ZK-996, ZK-990 or ZK-994 was administered prior to zymosan A, either by intravenous bolus injection or by gavage. For intravenous administration, test compounds were taken from ethanol stocks, dried down almost completely under nitrogen gas, and dissolved in sterile saline (final concentrations of ethanol did not exceed 0.5%). The compounds were administered via the tail vein in a total volume of 120 μl, under isoflurane anesthesia, 5 min prior to zymosan A administration. For intragastric administration (Miyasaka et al., 2001), the compounds were administered by gavage 45 min prior to administration of zymosan A. Sterile disposable animal feeding needles (20G × 1.1/2, Popper and sons, New Hyde Park, NY, U.S.A.) were used to instill test compounds (in 200 μl sterile saline) into the stomach of mice. At 2 h (in experiments where LX were administered i.v.) or 2.5 h (in experiments where LX were administered by gavage) after zymosan A administration, the mice were killed using isoflurane inhalation, and inflammatory cells were harvested from the peritoneum by washing the peritoneum with 5 ml Dulbecco's phosphate-buffered saline. Total cell counts were performed with a hemocytometer. Differential cell counts were performed on Wright–Giemsa-stained peritoneal cells that had been spun onto glass slides (Cytofuge). A total of 300 cells per slide in three different locations were counted. Male FVB mice from a second source (Taconic, Germantown, NY, U.S.A.) were tested as well for the anti-inflammatory actions of intravenously administered ATLa2.
Hind-limb ischemia–reperfusion-induced second-organ lung injury
Mice were anesthetized by intraperitoneal injection of pentobarbital 50 mg kg−1 (Nembutal sodium solution, NDC 0074-3778-04). Hind-limb ischemia was induced by placing a rubber band (no. 30, 2 × 1/32 × 1/8 inches (length × gauge × width: 1800 count pound−1), Pure rubber bands, Plymouth Office Products, Muscatine, IA, U.S.A.) on each hind limb using small clamps (Harvard Apparatus). Mice were exposed to ischemia for 1 h, after which the tourniquets were removed and reperfusion ensued. The reperfusion period was 1 h. At the end of the reperfusion period, the mice were killed with an overdose of pentobarbital (400 μl 10 mg ml−1 i.p.), and the left lung lobes quickly excised and frozen in liquid nitrogen. Test compounds and vehicle (0.5% ethanol in saline) were injected i.v. 5 min before the start of the ischemic period. A second dose was administered 5 min prior to the reperfusion period. The compounds were prepared as described above and administered by tail vein injection in 200 μl sterile saline. Myeloperoxidase activity in the lungs was measured using published methods (Bradley et al., 1982; Takano et al., 1997), and taken as a measurement of polymorphonuclear leukocyte accumulation in the lung. In some experiments, the right lung lobe was fixed in 3% phosphate-buffered formalin, and sections were made for histological evaluation of neutrophil infiltration, edema, and tissue injury.
Mouse ear inflammation
The mouse ear inflammation model (Takano et al., 1997) was used to evaluate the impact of topical administration of ATLa2 and the 3-oxa-ATL analogs ZK-994 and ZK-142, on combined LTB4 plus PGE2-stimulated neutrophil infiltration. Mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg kg−1). The inner side of the left ear (auricle) was treated with acetone (i.e., control), and the inner side of the right ear was treated topically with the indicated amount of LX analog (20 μg in 10 μl of acetone; i.e., 20 μg cm−2). After 5–7 min, LTB4 plus PGE2 (1 μg each in 10 μl acetone) was applied to the inside of both ears. At 20 h, the mice were euthanized with an overdose of isoflurane. Punch biopsy samples (6 mm diameter; Acu-Punch®; Acuderm, Inc., Ft. Lauderdale, FL, U.S.A.) were obtained from both left and right outer ears, and myeloperoxidase activity was quantified as previously detailed (Takano et al., 1997). Percent inhibition of PMN infiltration was calculated after myeloperoxidase activity was corrected for basal levels from mice that received acetone alone. In selected experiments, 6 mm punch biopsies were taken, placed in 10% formalin, and submitted to Children's Hospital (Boston, MA, U.S.A.) for sagittal and longitudinal sectioning and hematoxylin–eosin staining.
For the purpose of quantifying vascular permeability, 120 μl of Evans blue (1% in PBS) was injected intravenously immediately after the topical applications of LX analogs and/or LTB4 plus PGE2 or acetone alone, as detailed above. After 7 or 20 h, punch biopsies (6 mm diameter) were obtained, and Evans blue was extracted in formamide (60°C for 3 h) and quantified by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Inhibition of vascular permeability and PMN infiltration (myeloperoxidase activity) was calculated by the delta difference between the left (LTB4 plus PGE2) and right (LTB4 plus PGE2)+(LX analog, 20 μg cm−2) outer ears. Evans blue and myeloperoxidase values obtained from mouse outer ears that received acetone alone were subtracted from all values as background correction.
Statistical analysis
Statistical significance for differences between groups was determined by Student's t-test, or a conservative one-way ANOVA (Tukey's test) for multiple group comparisons after establishing that data in groups were normally and equally distributed. Differences between or among groups were considered statistically significant at P-values lower than or equal to 0.05.
Results
Oral administration of LXA4, LXB4, ZK-994, and ATLa2 in zymosan A-induced peritonitis
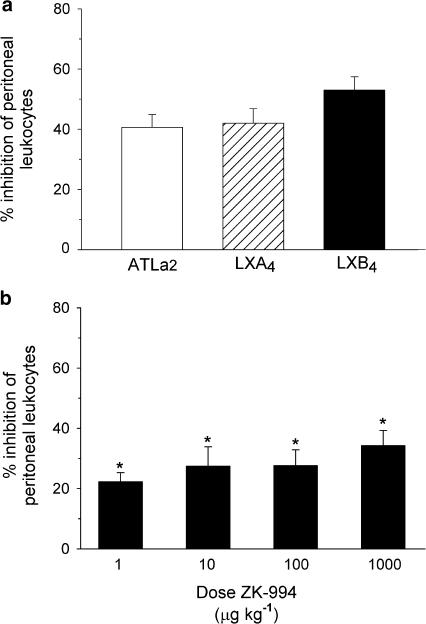
We sought to determine the ability of LX to exert systemic anti-inflammatory actions in vivo by administering native LXA4 and LXB4, and analogs ATLa2 and ZK-994 (Figure 1) by oral gavage in a murine model of acute zymosan A-induced peritonitis. ATLa2 was recently found to display anti-inflammatory efficacy in the acute phase of dextran sodium sulfate-induced colitis in mice when administered via drinking water (Gewirtz et al., 2002). It remained unclear if these effects were the result of topical actions to the gastrointestinal tract or systemic action following absorption. In the experiments, orally administered LXA4, LXB4, and ATLa2 (100 ng; ∼5 μg kg−1) inhibited the acute inflammatory cell recruitment by 40–50% (Figure 2a), without exerting major changes in the differential cellular composition of the inflammatory exudate (Table 1). In the case of ZK-994, a shallow dose–response curve was observed with significant effects at 1 μg kg−1 (22±3%, n=4, P<0.05 versus vehicle) and a maximal effect of 34±5% (n=3, P<0.01 versus vehicle) at a dose of 1000 μg kg−1 (Figure 2b). There were no statistically significant differences among the dose groups after oral administration of ZK-994. The demonstration of similar efficacy after oral dosing of LXA4, LXB4, and ATLa2, which contain the chemically sensitive tetraene group, and ZK-994, which contains the trienyne pharmacophore, suggests rapid uptake of LX and 15-epi-lipoxin analogs.
Figure 2.
(a) LX and aspirin-triggered 15-epi-lipoxin A4 analogs are orally active: inhibition of leukocyte infiltration in zymosan A-induced peritonitis by intragastric administration of 100 ng (∼5 μg kg−1) ATLa2, LXA4, and LXB4. Compounds were administered by gavage 45 min prior to i.p. administration of 1 mg zymosan A. At 2.5 h later, the peritoneum was lavaged with 5 ml sterile phosphate-buffered saline and peritoneal exudate cells were enumerated. Values are expressed as the % inhibition of the leukocyte number in vehicle-treated mice (7.1±0.6 × 106 leukocytes). Values are means±s.e.m., n=4–5. (b) Dose-dependent inhibition of leukocyte infiltration in zymosan A-induced peritonitis by intragastric administration of 3-oxa-ATL analog ZK-994. Values are expressed as the % inhibition of the leukocyte number in vehicle-treated mice (5.1±0.2 × 106 leukocytes). Values are means±s.e.m., n=3–5 (* significantly different from ATLa2, P⩽0.05).
Table 1.
Composition of inflammatory exudate cells: here the percentage of total leukocytes in zymosan A-induced peritonitis is reported after gavage administration of LXA4, LXB4, and ATLa2 (5 μg kg−1 each). Peritoneal exudate cells were collected by lavage 2.5 h after initiation of inflammation by i.p. administration of 1 mg zymosan A
| Treatment | PMN | Monocytes | Lymphocytes |
|---|---|---|---|
| Vehicle | 71.9±4.5 | 25.5±4.7 | 3.1±0.6 |
| ATLa2 100 ng | 65.8±3.2 | 28.5±3.1 | 5.2±0.3* |
| LXA4 100 ng | 67.8±3.9 | 28.7±3.8 | 4.3±1.4 |
| LXB4 100 ng | 61.1±1.5* | 33.4±1.9 | 5.1±1.1 |
Cytospin slides were prepared from the exudates, stained with Wright–Giemsa, and 300 cells per slides were classified as neutrophil, monocyte, or lymphocyte. Values are mean±s.e.m., n=3–5
significantly different from vehicle, P⩽0.05).
Oral versus intravenous administration of ATLa2 in zymosan A-induced peritonitis
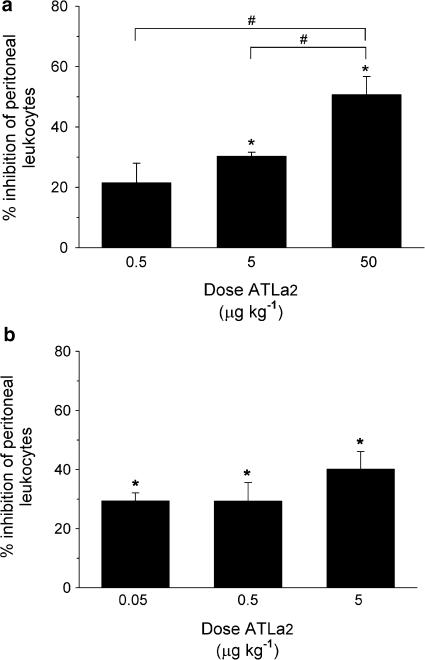
To assess possible differences and importance of route of administration for the systemic anti-inflammatory action of LX, the regulation of peritoneal leukocyte infiltration in zymosan A-induced peritonitis by ATLa2 was assessed and compared after both intravenous and oral administration in doses ranging from 1 ng to 1 μg per mouse (50 ng kg−1–50 μg kg−1). Intravenous ATLa2 induced a dose-dependent reduction in total peritoneal exudate cells 2 h after intraperitoneal administration of zymosan A (Figure 3a). Significant inhibitory actions were demonstrable at doses as low as 100 ng per mouse (∼5 μg kg−1), where total peritoneal exudate cell number was reduced 30±1% (n=7, P<0.05) compared to administration of vehicle alone. At 1 μg (50 μg kg−1), the inflammatory cell recruitment was significantly reduced by 51±5% (n=8, P<0.05). The anti-inflammatory actions of intravenously administered ATLa2 were also observed in the same mouse strain (FVB) from a second source, with 13±4, 63±10, and 44±6% inhibition at 10 ng (500 ng kg−1), 100 ng (5 μg kg−1), and 1 μg (50 μg kg−1), respectively (n=3–6). Direct comparisons after intragastric administration of 100 ng ATLa2 (5 μg kg−1) showed that the total inflammatory exudate cell numbers were similarly reduced by 40±6% (P<0.05) (Figure 3b). Lower doses of 1 ng (50 ng kg−1) and 10 ng (500 ng kg−1) ATLa2 significantly reduced the inflammatory response by 29±3 and 29±6% (P<0.05), respectively, demonstrating very potent oral activity for this analog. In contrast to intravenous administration of ATLa2, there were no significant differences within this dose range among the treatment groups after oral administration. Total leukocyte exudate cell numbers in zymosan A-induced peritonitis with intragastrically administered vehicle control (5.2±0.1 × 106 cells; mean±s.e.m., n=15) were similar to those obtained after intravenously administered vehicle alone (5.2±0.2 × 106 cells; mean±s.e.m., n=33). These results confirmed lack of any effect of the dosing regimen on the inflammatory response. ATLa2 had a similar anti-inflammatory efficacy when compared to ZK-994 (Figure 2b).
Figure 3.
(a) Dose-dependent inhibition of leukocyte infiltration in zymosan A-induced peritonitis by intravenous ATLa2. ATLa2 was administered 5 min prior to i.p. administration of 1 mg zymosan A. After 2 h, the peritoneum was lavaged with 5 ml sterile phosphate-buffered saline and peritoneal exudate cells were enumerated. Values are expressed as the % inhibition of the leukocyte number in vehicle-treated mice (6.0±0.4 × 106 leukocytes). Values are mean±s.e.m., n=4–8 (* significantly different from vehicle, P⩽0.05; # indicates a significant difference among the treatment groups, P⩽0.05). (b) Inhibition of leukocyte infiltration in zymosan A-induced peritonitis by intragastric administration of ATLa2. ATLa2 was administered 45 min prior to i.p. administration of 1 mg zymosan A. After 2.5 h, the peritoneum was lavaged with 5 ml sterile phosphate-buffered saline and peritoneal exudate cells were enumerated. Values are expressed as the % inhibition of the leukocyte number in vehicle-treated mice (6.4±0.6 × 106 leukocytes). Values are means±s.e.m., n=3–4 (* significantly different from vehicle, P⩽0.05).
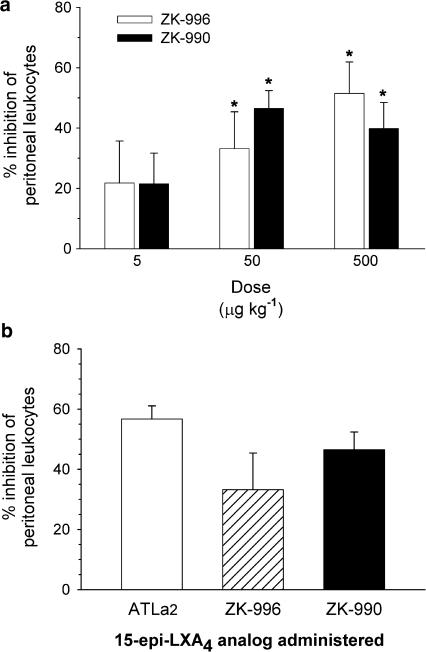
Intravenous administration of methyl esters ZK-990, ZK-996, and ATLa2 in zymosan A-induced peritonitis
Methyl esters ZK-990, ZK-996, and ATLa2 (Figure 1) were compared in the zymosan A-initiated peritonitis model with intravenous administration. In the case of ZK-990 and ZK-996, no statistically significant differences could be detected in leukocyte infiltration between compounds at any dose, and efficacy (P<0.05) for both compounds was shown at 50 and 500 μg kg−1 compared to vehicle alone (Figure 4a). Using a one-tailed analysis of variance, no statistically significant differences in anti-inflammatory activity could be determined among methyl esters ATLa2, ZK-996, and ZK-990 at the same intravenous dose (50 μg kg−1) (Figure 4b).
Figure 4.
(a) Dose-dependent inhibition of leukocyte infiltration in zymosan A-induced peritonitis by intravenous administration of 3-oxa-ATL analog ZK-996 (grey bars) and ZK-990 (black bars). ZK-996 and ZK-990 were administered by tail vein 5 min prior to i.p. administration of 1 mg zymosan A. After 2 h, the peritoneum was lavaged with 5 ml sterile phosphate-buffered saline and peritoneal exudate cells were enumerated. Values are expressed as the % inhibition of the leukocyte number in vehicle-treated mice (5.8±0.5 × 106 leukocytes). Values are means±s.e.m., n=3–7 (* significantly different from vehicle, P⩽0.05). (b) Inhibition of leukocyte infiltration in zymosan A-induced peritonitis by intravenous administration of (50 μg kg−1) ATLa2, and 3-oxa-ATL analogs ZK-996 and ZK-990. Values are expressed as the % inhibition of the leukocyte number in vehicle-treated mice (5.8±0.5 × 106 leukocytes). Values are means±s.e.m., n=3–5 (* significantly different from ATLa2; P⩽0.05).
The anti-inflammatory action of LX does not desensitize in vivo
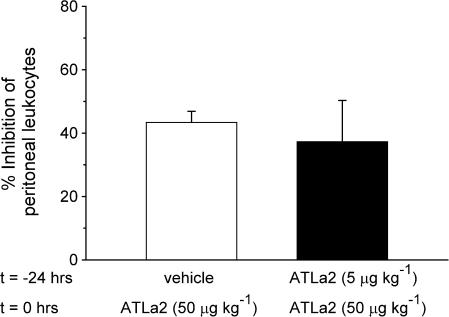
Stimulation of cells with LXA4 can induce homologous desensitization of LX signal transduction pathways to subsequent stimuli by LXA4 (Maddox et al., 1997). In the present experiment using the zymosan A-induced peritonitis model, we next determined if these desensitizing properties of ATLa2 are observed in vivo. The anti-inflammatory actions of ATLa2 were compared in the standard model, initiation of peritonitis and intravenous administration of 1 μg (50 μg kg−1) ATLa2, with and without treatment with 0.1 μg (5 μg kg−1) ATLa2 24 h prior to treatment. As shown in Figure 5, no significant difference was observed, which indicates that at this dose and timing in vivo, homologous desensitization to ATLa2 does not occur.
Figure 5.
The anti-inflammatory action of LX does not desensitize in vivo. At 24 h prior exposure to 0.1 μg (5 μg kg−1) ATLa2 or vehicle before a second intravenous dose of ATLa2 (5 μg kg−1) 5 min prior to induction of peritonitis by i.p zymosan A. Values are expressed as the % inhibition of the leukocyte number in mice that received vehicle alone before induction of peritonitis (6.6±0.9 × 106 leukocytes). Values are means±s.e.m., n=4–6.
Intravenous administration of methyl esters ZK-994, ZK-142, and ATLa2 in second-organ lung injury model
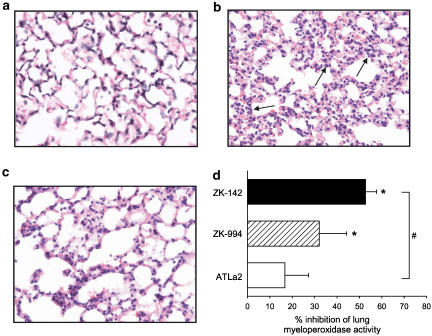
We next evaluated the ability of ZK-994, ZK-142, and ATLa2 (Figure 1) to protect against ischemia/reperfusion-induced second-organ lung injury by lowering PMN entry to the lung. Histological analysis of the lung shows the marked accumulation of PMN and thickening of alveolar septae after hind-limb ischemia/reperfusion (Figure 6, panel b), compared to lungs of mice that did not experience ischemia/reperfusion (Figure 6, panel a). In contrast to results observed with peritonitis (see Figure 4b), ZK-994 and ZK-142 (500 μg kg−1 prior to ischemia and 500 μg kg−1 prior to reperfusion) proved to be more efficacious than ATLa2 in reducing neutrophil accumulation in the lungs by 32±12% (n=3, P=0.04 vs vehicle) and 53±5% (n=3, P=0.0002 versus vehicle; P=0.04 compared to ATLa2), respectively (Figure 6, panel d). Intravenous administration of ATLa2 at these doses did not significantly reduce neutrophil accumulation in the lung. A clear reduction in ischemia/reperfusion-induced neutrophil accumulation by ZK-142 can also be observed in Figure 6, panel c.
Figure 6.
Inhibition of ischaemia–reperfusion-induced lung inflammation by intravenous administration of LX stable analogs ATLa2, ZK-994, and ZK-142. Analog (500 μg kg−1) or vehicle was administered i.v. 5 min prior to initiation of hind-limb ischemia and a second dose of analog (500 μg kg−1) or vehicle was administered i.v. 5 min prior to reperfusion (for detailed procedure, see Methods). Haematoxylin–eosin-stained sections of the right lung lobes were prepared from mice that did not experience ischemia–reperfusion (panel a), from mice that underwent 1 h ischemia followed by 1 h reperfusion (panel b; arrows indicate PMN), and from mice that received ZK-142 prior to ischemia and prior to reperfusion (panel c). Magnification 40 ×. Panel d. Inhibition of neutrophil accumulation in the lung by LX stable analogs as measured by myeloperoxidase activity in the left lung lobe. Values are expressed as the % inhibition of lung myeloperoxidase activity in vehicle-treated mice that underwent ischemia/reperfusion (4.1±0.7 mAU min−1 per mg tissue). Values are means±s.e.m., n=3–4 (* significantly different from vehicle, P⩽0.05; # significant differences among treatment groups, P⩽0.05).
Topical administration of methyl ester ATLa2 and acids ZK-142 and ZK-994 in models of inflammation
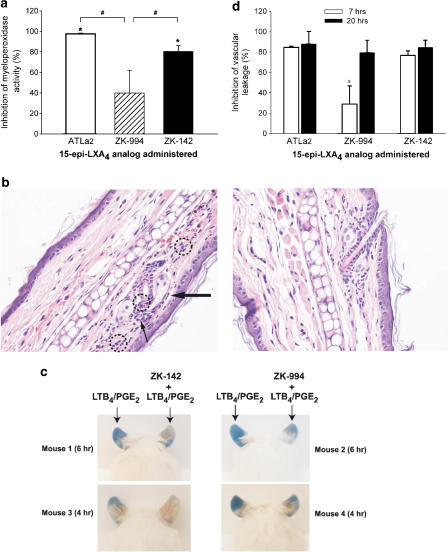
ATLa2, ZK-142, and ZK-994 display potent anti-inflammatory activity upon topical administration in experimental models of cutaneous inflammation in both mice and guinea pigs (Takano et al., 1997; Takano et al., 1998; Schottelius et al., 2002; Guilford et al., 2004). We tested ATLa2, the trienyne ZK-994, and the tetraene ZK-142 in a murine outer ear inflammation model induced by combined topical application of LTB4 and PGE2 (Takano et al., 1997). Topical treatment with 20 μg cm−2 ZK-142 reduced neutrophil infiltration by 80±6% (n=3, P<0.01), 20 h after application of the combined LTB4/PGE2 (Figure 7a), whereas ZK-994 did not reach statistical significance (39.8±22%; n=3, P=0.072). For purpose of direct comparison, 20 μg cm−2 ATLa2 reduced leukocyte infiltration by 98±0.1% (n=3, P<0.01). Histological examination demonstrated that ears, which received topical LTB4 plus PGE2 alone (Figure 7b, left panel), displayed a diffuse neutrophilic infiltrate in the papillary dermis. The neutrophils extend into the perifollicular squamous epithelium with a minute intraepithelial abscess formation. Ears that received 20 μg cm−2 ZK-142 (Figure 7b, right panel) demonstrated scant neutrophilic perifollicular infiltrate with some exocytosis into the squamous epithelium.
Figure 7.
(a) Inhibition of LTB4 plus PGE2-stimulated PMN infiltration in mouse outer ears by topical administration (20 μg per ear) of ZK-994, and ZK-142. Values are expressed as the % inhibition of ear myeloperoxidase activity in vehicle-treated mice that received LTB4 plus PGE2 (6.2±2.7 mAU min−1 per biopsy). Values are means±s.e.m., n=4 (* significantly different from vehicle, P⩽0.05; # significant differences among treatment groups, P⩽0.05). (b) Ear biopsies: inhibition of dermal inflammation. Sagittal sections of the mouse outer ear were stained with haematoxylin/eosin (magnification × 40). The representative photographs show ear sections with hair follicles and sebaceous glands. Left panel; LTB4 plus PGE2-stimulated inflammation. Note the prominent papillary dermal vascularity (large arrow), minute intraepithelial abscess (small arrow), and diffuse neutrophilic infiltrate (dashed circles). Right panel; inhibition of neutrophil infiltration by topical administration of compound ZK-142 (20 μg per ear) 5 min prior to LTB4 plus PGE2. (c) Photographs of mouse ears: inhibition of PMN-mediated vascular leakage in mouse outer ears by topical administration of ZK-994, and ZK-142 (20 μg per ear). (d) Inhibition of LTB4 plus PGE2-stimulated vascular leakage in mouse outer ear at 7 and 20 h after topical administration (20 μg per ear) of ATLa2, ZK-994, and ZK-142. Values are expressed as the % inhibition of Evans blue accumulation in ears of vehicle-treated mice that received LTB4 plus PGE2 (19.3±6.1 and 51.6±23.5 mAU min−1 per biopsy, at 7 and 20 h, respectively). Values are mean±s.e.m., n=3–5 (* significantly different from ATLa2 and ZK-142 at 7 h; P<0.05).
Leukocyte-mediated cell injury and increase in microvascular leakage can be visualized over time following the application of LTB4 plus PGE2 to mouse ears (Takano et al., 1997). Figure 7c is a photograph of four representative mice in which the left ear was treated with LTB4 plus PGE2 alone and the right ear was treated with either 20 μg cm−2 ZK-142 (left panels) or ZK-994 (right panels), 5–7 min prior to challenge with LTB4 plus PGE2. The progression of vascular leak at 4 h (lower panels) and 6 h (upper panels) is evident in the left ears that received LTB4 plus PGE2 and was clearly delayed in ear tissue treated with the 3-oxa 15-epi-lipoxin analogs. Dye accumulation was quantified in ear tissue punch biopsies at 7 and 20 h following LTB4 plus PGE2 (Figure 7d). At the 7 h time interval, topical administration of 20 μg cm−2 ZK-142 inhibited vascular leakage by 77±5% (n=3, P=0.005), whereas 20 μg cm−2 ZK-994 did not reach statistical significance (29±18%; n=3, P=0.090). In direct comparison, 20 μg cm−2 ATLa2 showed inhibition of 85±1% (n=3, P=0.003). By 20 h, ZK-994 and ZK-142 inhibited vascular leakage by 73±12% (n=4, P=0.04) and 84±7% (n=5, P=0.03), respectively (Figure 7d). ATLa2 inhibited vascular leakage by 88±12% (n=5, P=0.01). At 7 h, there was a significant difference between inhibition of vascular leakage by ZK-994 and ATLa2 (P=0.018), as well as with ZK-142 (P=0.030), whereas there was no such difference between the actions of the tetraenes (P=0.082). At 20 h, there were no statistically significant differences among the inhibitory actions of these three compounds. These results indicate that the tetraene ZK-142 had similar efficacy to ATLa2 at both time points, whereas the actions of the trienyne ZK-994 were more variable and somewhat delayed, being weaker at 7 h with more robust protection by 20 h.
Discussion
LX and aspirin-triggered LX are endogenously generated anti-inflammatory mediators. Their potent anti-inflammatory actions can be recapitulated in vitro with isolated cells, and in vivo by systemic and topical administration of LX and 15-epi-lipoxin analogs (Serhan & Oliw, 2001). The present study was undertaken to assess the extent to which LX and their analogs can be used as potential systemic anti-inflammatory agents after oral administration.
The present results indicate that ATLa2 and ZK-994, as well as native LXA4 and LXB4, are all potent, orally active inhibitors of acute inflammation in vivo. Systemic anti-inflammatory effects in the zymosan A-induced peritonitis model were observed with doses as low as 1–10 ng per mouse (50–500 ng kg−1), with increasing effects at higher doses (5–500 μg kg−1). Similar degrees of systemic anti-inflammatory efficacy of ATLa2 was observed in a rapidly developing inflammatory response (2 h) to intraperitoneal zymosan A, and this occurred whether ATLa2 was administered intravenously or orally via intragastric administration. The anti-inflammatory action of these related yet different structures such as LXA4, ATLa2, ZK-142, and ZK-994 likely reflect conserved key structural requirements for receptor recognition and activation. Results from earlier studies with transgenic mice that express human ALX indicate that the anti-inflammatory action of ATLa2 in acute inflammation is mediated by ALX (Devchand et al., 2003). Also, 15-epi-LXA4, 15(R/S)-methyl-LXA4, and 16-phenoxy analogs of LXA4 directly compete at the human ALX (Takano et al., 1997).
The activity of the tetraene analogs after oral administration is consistent with the pharmacokinetic results recently published on ATLa2 (Guilford et al., 2004), but is surprising because of the acid-labile nature of ATLa2, ZK-142, and LXA4 related to their tetraene pharmacophore (Serhan et al., 1986). ATLa2 survives acid-catalyzed degradation in the acidic gastric environment, possibly by being rapidly absorbed by a specific LX transporter for LX in the gastrointestinal tract. A LXA4 transporter is present in human neutrophils (Simchowitz et al., 1994). This transporter mediates H+/LXA4 cotransport, is sensitive to a range of typical organic anion transport inhibitors, and operates independently from membrane voltage and Na+. The presence and molecular characteristics of a putative LX transporter in the gastrointestinal tract remain to be established. Interestingly, in contrast to the dose-dependent actions observed after intravenous administration of ATLa2, the anti-inflammatory action observed after oral administration of ATLa2 was not dose-dependent in the dose range studied (50 ng kg−1–5 μg kg−1). The shallow dose response is consistent with a saturable transport system in the gastrointestinal tract. The oral bioavailability of LX has implications for understanding the results of other studies, such as the efficacy of ATLa2 in the acute phase of dextran sodium-sulfate-induced colitis in mice (Gewirtz et al., 2002). In the colitis study, ATLa2 was administered ad libitum at 10 μg ml−1 in the drinking water for an estimated dose of about 50 μg kg−1 per day ATLa2. Based on the present results in the peritonitis model, the anti-inflammatory or immunomodulatory effects of ATLa2 could be due to systemic rather than a topical application to the gastrointestinal tract. Hence, LX are potent mediators that display a surprising oral availability for systemic anti-inflammation. The oral availability of LX, 15-epi-LX and their stable analogs will facilitate further studies and provides a new noninvasive route of administration for these anti-inflammatory compounds.
A marked enhancement of inhibition of lung inflammation after hind-limb ischemia–reperfusion was found for novel 3-oxa-lipoxin analogs ZK-994 and ZK-142. While ATLa2 did not significantly reduce inflammation, the novel analogs demonstrated 32±12 and 53±5% inhibition, respectively. These results indicate that the 3-oxa substitution enhances the protective impact of LX analogs on the lung (Chiang et al., 1999). Histological examination of lung tissue indicated that compound ZK-142 reduced both leukocyte accumulation and septal wall thickening that may result from edema and tissue injury in the lung tissue.
The topical activity of ZK-142, ZK-994, and ATLa2 was confirmed in the blocking of dermal inflammation induced by LTB4 plus PGE2, as indicated by marked reductions in neutrophil infiltration and vascular leak. At 20 μg cm−2, ATLa2 appeared to have the most robust efficacy on both end points, being somewhat more effective than ZK-142. The trienyne ZK-994 appeared less effective than either tetraene compound, with a slower and less robust effect on vascular leak. These results suggest the absorption or penetration of ZK-994 into mouse ear skin tissue is slower than for the tetraene analogs. In line with earlier observations (Takano et al., 1997), neither ATLa2 (20 μg; 1 mg kg−1) nor ZK-142 (20 μg; 1 mg kg−1) gave statistically significant inhibitory effects with LTB4 plus PGE2-initiated vascular leakage in the outer ear when administered by tail vein injection (not shown). These results indicate specific local effects of LX in dermal inflammation that cannot be achieved by systemic delivery, and imply direct local actions of LX. The topical anti-inflammatory profile for the new 3-oxa LX analogs (ED50 <300 μg cm−2) was similar to that of ATLa2 in calcium ionophore-induced inflammation in mice (Guilford et al., 2004).
LX and 15-epi-LX appear to function as endogenous stop signals through the downregulation of neutrophil accumulation and activation of anti-inflammatory circuits that promote resolution (for recent reviews, see Gilroy et al. (2004) and Serhan & Oliw (2001)). Therapeutic doses of presently available nonsteroidal anti-inflammatory drugs range from approximately 0.3–1 mg kg−1 for, for example, indomethacin to 5–15 mg kg−1 for aspirin and acetaminophen (Roberts & Morrow, 2001). LX exert a similar degree of anti-inflammatory efficacy in certain models as NSAIDs, but are ∼100–1000-fold more potent on a molar and in vivo dose basis. For example, tumor necrosis factor-α-induced leukocyte trafficking into the murine air pouch was attenuated to the same extent by local delivery of 1 mg acetylsalicylic acid versus only 10 μg ATLa2 (Clish et al., 1999). Inhibiting carrageenan-induced leukocyte trafficking into the rat air pouch requires 3–10 mg kg−1 indomethacin given orally (Wallace et al., 1999), a much higher dose than shown in the present report for the oral doses of LX required to attenuate zymosan-induced peritonitis in mice. The direct activation of anti-inflammatory circuits by LX and stable LX analogs offers a rational therapeutic advantage over the use of selective and nonselective cyclooxygenase inhibitors, which act via inhibition of cyclooxygenase-derived proinflammatory eicosanoids but also inhibit the formation of endogenous anti-inflammatory cyclooxygenase-2-derived lipid mediators (Gilroy et al., 1999). A comparison to glucocorticoids has revealed equivalent anti-inflammatory actions of LXA4 analogs/ATLa2 and dexamethasone in the murine air pouch (Clish et al., 1999) and ear skin inflammation (Takano et al., 1997). More recently, a comparison of ATLa2 with a clinically relevant topical glucocorticoid, methyl prednisolone aceponate (MPA), in five diverse cutaneous reactions at multiple doses, demonstrated a similar degree of efficacy for leukocyte trafficking, edema, and epidermal hyperproliferation (Schottelius et al., 2002). Taken together, LX and stable analogs display a comparable efficacy to both NSAIDS and glucocorticoids, as well as in certain settings a considerable higher potency than NSAIDs. LX and stable analogs potentially offer an improved approach for anti-inflammatory therapy via a different manner of action, that is, direct activation of anti-inflammatory pathways, without interfering with endogenous anti-inflammation mechanisms and avoiding the severe side effects associated with prolonged steroid use. It is of interest to note that the anti-inflammatory pathways activated by glucocorticoid and aspirin, at least in part, converge at the level of the ATL/LX receptor, namely ALX, via formation of annexin 1 and ATL, respectively (Perretti et al., 2002; Fiorucci et al., 2003).
In the present study, LX and LX analogs were shown to have anti-inflammatory activity after oral administration in the zymosan A-induced peritonitis model. Similar anti-inflammatory activity was seen with LXA4 or ATLa2 and the novel β-oxidation resistant ZK-142/ZK-996 or the corresponding trienyne analog, ZK-990/ZK-994. The relative activity of the compounds proved to depend on the site and mode of induction of the inflammatory response (peritoneum, lung, skin). The present results indicate that the ability to administer 3-oxa-lipoxin analogs via oral, topical, and systemic routes will facilitate both preclinical and clinical research on the functions and therapeutic applications of LX in vivo.
Acknowledgments
We thank Eric Tjonahen and Jennifer Burg for technical assistance, and Mary H. Small for assistance in preparing the manuscript. The present work was supported by a Postdoctoral Fellowship to G. Bannenberg from the Arthritis Foundation. We also thank Richard Horuk (Director, Immunology) and Gary Phillips (Director, Medicinal Chemistry) for their support for lipoxin research at Berlex Biosciences.
Abbreviations
- ATL
aspirin-triggered lipoxin
- 3-oxa-ATL
aspirin-triggered lipoxins in which carbon-3 is replaced with oxygen
- LX
lipoxin
References
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- CHIANG N., GRONERT K., CLISH C.B., O'BRIEN J.A., FREEMAN M.W., SERHAN C.N. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J. Clin. Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIANG N., GRONERT K., QIU F.-H., SERHAN C.N.Lipoxin A4 receptor Cytokine Reference 2000London: Academic Press; 2219–2233.ed. OPPENHEIM, J.J. & FELDMANN, M. pp [Google Scholar]
- CLÀRIA J., SERHAN C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLISH C.B., O'BRIEN J.A., GRONERT K., STAHL G.L., PETASIS N.A., SERHAN C.N. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVCHAND P.R., ARITA M., HONG S., BANNENBERG G.L., MOUSSIGNAC R.-L., GRONERT K., SERHAN C.N. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host-defense. FASEB J. 2003;17:652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- EDENIUS C., KUMLIN M., BJÖRK T., ÄNGGÅRD E., LINDGREN J.A. Lipoxin formation in human nasal polyps and bronchial tissue. FEBS Lett. 1990;272:25–28. doi: 10.1016/0014-5793(90)80440-t. [DOI] [PubMed] [Google Scholar]
- FILEP J.G., ZOUKI C., PETASIS N.A., HACHICHA M., SERHAN C.N. Anti-inflammatory actions of lipoxin A4 stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94:4132–4142. [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., WALLACE J.L., SARDINA M., ROMANO M., DEL SOLDATO P., MORELLI A. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10937–10941. doi: 10.1073/pnas.1933204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIN J.L., SNYDERMAN R. Inflammation. Basic Principles and Clinical Correlates. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- GAVINS F.N., YONA S., KAMAL A.M., FLOWER R.J., PERRETTI M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- GEWIRTZ A.T., COLLIER-HYAMS L.S., YOUNG A.N., KUCHARZIK T., GUILFORD W.J., PARKINSON J.F., WILLIAMS I.R., NEISH A.S., MADARA J.L. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J. Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- GILROY D.W., COLVILLE-NASH P.R., WILLIS D., CHIVERS J., PAUL-CLARK M.J., WILLOUGHBY D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- GILROY D.W., LAWRENCE T., PERRETTI M., ROSSI A.G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug. Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- GUILFORD W.J., BAUMAN J.G., SKUBALLA W., BAUER S., WEI G.P., DAVEY D., SCHAEFER C., MALLARI C., TERKELSEN J., TSENG J.-L., SHEN J., SUBRAMANYAM B., SCHOTTELIUS A.J., PARKINSON J.F. Novel 3-oxa lipoxin A4 analogs with enhanced chemical and metabolic stability have anti-inflammatory activity in vivo. J. Med. Chem. 2004;47:2157–2165. doi: 10.1021/jm030569l. [DOI] [PubMed] [Google Scholar]
- LAWRENCE T., WILLOUGHBY D.A., GILROY D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- LEE T.H., CREA A.E., GANT V., SPUR B.W., MARRON B.E., NICOLAOU K.C., REARDON E., BREZINSKI M., SERHAN C.N. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am. Rev. Resp. Dis. 1990;141:1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- LEVY B.D., CLISH C.B., SCHMIDT B., GRONERT K., SERHAN C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- MADDOX J.F., COLGAN S.P., CLISH C.B., PETASIS N.A., FOKIN V.V., SERHAN C.N. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- MADDOX J.F., HACHICHA M., TAKANO T., PETASIS N.A., FOKIN V.V., SERHAN C.N. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J. Biol. Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- MIYASAKA C.K., MENDONCA J.R., NISHIYAMA A., ALVES DE SOUZA J.A., PIRES DE MELO M., PITHON-CURI T.C., CURI R. Comparative effects of fish oil given by gavage and fish oil-enriched diet in leukocytes. Life Sci. 2001;69:1739–1751. doi: 10.1016/s0024-3205(01)01253-x. [DOI] [PubMed] [Google Scholar]
- PACE-ASCIAK C.R. Mass Spectra of Prostaglandins and Related Products. New York: Raven Press; 1989. [PubMed] [Google Scholar]
- PERRETTI M., CHIANG N., LA M., FIERRO I.M., MARULLO S., GETTING S.J., SOLITO E., SERHAN C.N. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS L.J., II, MORROW J.D.Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout Goodman & Gilman's The Pharmacological Basis of Therapeutics 2001New York: McGraw-Hill; 687–731.ed. HARDMAN, J.G. & LIMBIRD, L.E. pp [Google Scholar]
- SCALIA R., GEFEN J., PETASIS N.A., SERHAN C.N., LEFER A.M. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOTTELIUS A.J., GIESEN C., ASADULLAH K., FIERRO I.M., COLGAN S.P., BAUMAN J., GUILFORD W., PEREZ H.D., PARKINSON J.F. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J. Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- SERHAN C.N. Advances in Rheumatology and Inflammation 1991Basel: Eular; 141–153.ed. HEDQVIST, P., KALDEN, J.R., MULLER-PEDDINGHAUS, R. & ROBINSON, D.R. pp [Google Scholar]
- SERHAN C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are endogenous components of antiinflammation: emergence of the counterregulatory side. Arch. Immunol. Ther. Exp. 2001;49:177–188. [PubMed] [Google Scholar]
- SERHAN C.N., FIORE S., BREZINSKI D.A., LYNCH S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry. 1993;32:6313–6319. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- SERHAN C.N., MADDOX J.F., PETASIS N.A., AKRITOPOULOU-ZANZE I., PAPAYIANNI A., BRADY H.R., COLGAN S.P., MADARA J.L. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- SERHAN C.N., NICOLAOU K.C., WEBBER S.E., VEALE C.A., DAHLÉN S.E., PUUSTINEN T.J., SAMUELSSON B. Lipoxin A. Stereochemistry and biosynthesis. J. Biol. Chem. 1986;261:16340–16345. [PubMed] [Google Scholar]
- SERHAN C.N., OLIW E. Unorthodox routes to prostanoid formation: new twists in cyclooxygenase-initiated pathways. J. Clin. Invest. 2001;107:1481–1489. doi: 10.1172/JCI13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMCHOWITZ L., FIORE S., SERHAN C.N. Carrier-mediated transport of lipoxin A4 in human neutrophils. Am. J. Physiol. 1994;267:C1525–C1534. doi: 10.1152/ajpcell.1994.267.6.C1525. [DOI] [PubMed] [Google Scholar]
- TAKANO T., CLISH C.B., GRONERT K., PETASIS N., SERHAN C.N. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogs. J. Clin. Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO T., FIORE S., MADDOX J.F., BRADY H.R., PETASIS N.A., SERHAN C.N. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogs are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J. Exp. Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., CHAPMAN K., MCKNIGHT W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br. J. Pharmacol. 1999;126:1200–1204. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]