Abstract
Kinins are a family of peptides implicated in several pathophysiological events. Most of their effects are likely mediated by the activation of two G-protein-coupled receptors: B1 and B2. Whereas B2 receptors are constitutive entities, B1 receptors behave as key inducible molecules that may be upregulated under some special circumstances. In this context, several recent reports have investigated the importance of B1 receptor activation in certain disease models. Furthermore, research on B1 receptors in the last years has been mainly focused in determining the mechanisms and pathways involved in the process of induction. This was essentially favoured by the advances obtained in molecular biology studies, as well as in the design of selective and stable peptide and nonpeptide kinin B1 receptor antagonists. Likewise, development of kinin B1 receptor knockout mice greatly helped to extend the evidence about the relevance of B1 receptors during pathological states. In the present review, we attempted to remark the main advances achieved in the last 5 years about the participation of kinin B1 receptors in painful and inflammatory disorders. We have also aimed to point out some groups of chronic diseases, such as diabetes, arthritis, cancer or neuropathic pain, in which the strategic development of nonpeptidic oral-available and selective B1 receptor antagonists could have a potential relevant therapeutic interest.
Keywords: Kinins, B1 receptors, inflsammation, pain, selective antagonists
Introduction
Kinins are a group of peptides involved in a series of pathophysiological processes. They are formed in plasma and tissues in response to infection, tissue trauma or inflammatory alterations. Once formed and released, kinins exert most of their biological effects by the activation of two G-protein-coupled receptors, denoted B1 and B2. B2 receptors are distributed in a constitutive manner throughout the central and peripheral tissues and present higher affinity for bradykinin (BK) and Lys-BK peptides. On the other hand, B1 receptors display high affinity for the metabolites des-Arg9-BK and Lys-des-Arg9-BK. It is worth noting that B1 receptors are not usually expressed under physiological conditions, but may be quickly upregulated under several inflammatory stimuli (see Calixto et al., 2000; 2001).
Lewis (1964) demonstrated for the first time that BK was able to evoke all classical signals of inflammation, such as increase in vascular permeability, oedema formation and pain. Soon after, many works pointed out that both B1 and B2 receptors are involved in the onset and maintenance of inflammatory and nociceptive alterations (Regoli & Barabé, 1980; Proud & Kaplan, 1988; Ahluwalia & Perretti, 1999; Calixto et al., 2000; 2001; Couture et al., 2001). For various reasons, research on the involvement of B2 receptors in nociceptive and inflammatory states has progressed more quickly than that on B1 receptors. Curiously, the first B1 receptor antagonist was developed almost 10 years before the first B2 receptor antagonist, but the characterization of B1 receptors was delayed, probably due to its complex ability to be induced. Thus, the role of B2, but not B1 receptors, in inflammatory and nociceptive processes was widely explored during the 1980s and 1990s. This was favoured by the systematic development of selective peptidic B2 receptor antagonists by the pharmaceutical companies, as at this time, the industry believed that B2 receptors were more attractive as pharmacological targets (in comparison to B1 receptors) for the possible treatment of several pathological states (Regoli et al., 1998; Stewart et al., 1999; Bock & Longmore, 2000). In addition, cloning studies with B2 receptors were initiated as early as 1991, whereas the B1 receptor was first cloned only in 1994 (see details below). Other factors have also largely contributed to the delay of the studies on B1 receptors. Firstly, B2 receptor knockout mice were generated in 1995 (Borkowski et al., 1995), while the generation of B1 receptor knockout mice was only achieved later in 2000 (Pesquero et al., 2000). In addition, the development of nonpeptidic selective B2 receptor antagonists was achieved almost 10 years before the synthesis of the first nonpeptidic selective B1 receptor antagonist (Table 1).
Table 1.
Selective kinin B1 receptor antagonists
| Name | Structure | Main characteristics | Reference |
|---|---|---|---|
| [Leu8]-des Arg9-BK | [Leu8]-des-Arg9-BK | Peptidic antagonist, first-generation, widely employed for studying the involvement of B1 receptors in inflammatory and nociceptive models. Mainly active in vivo when administered by topical or s.c. routes. | Regoli et al. (1977), Regoli & Barabé(1980) |
| Lys[Leu8]-des-Arg9-BK | Lys[Leu8]-des-Arg9-BK | Peptidic antagonist, first generation. Presents pharmacological actions similar to those described for [Leu8]-des-Arg9-BK. Has higher affinity for rabbit and human B1 receptors. | Regoli & Barabé (1980), Drapeau et al. (1991) |
| [des-Arg9]-HOE 140 | Des-Arg9-𝒟-Arg [Hyp3,Thi5, 𝒟-Tic7,Oic8] | Peptidic antagonist, second generation. Presents increased potency and stability in relation to first generation antagonists. | Wirth et al. (1991) |
| [des-Arg9]-NPC 17731 | 𝒟-Arg-Arg-Pro-Hyp-Gly-Phe-Ser-𝒟Hype-(Transpropyl-Oic) | Peptidic antagonist, second generation. Only a Few pharmacological studies have been conducted with this antagonist. | Cabrini & Calixto (1997), Vianna & Calixto (1998) |
| R-715 | AcLys[𝒟-βNal7,Ile8]des-Arg9-BK | Peptidic antagonist, third generation. Has less susceptibility to proteolytic degradation than second generation antagonists. Several recent works have employed this compound to evaluate the involvement of B1 receptors during pain and inflammation. Active when administered systemically by s.c., i.v. or i.p. routes. | Gobeil et al. (1996) |
| R-954 | AcOrn[Oic2, (αMe)Phe5,𝒟-βNal7, Ile8] des-Arg9-BK | Peptidic antagonist, third generation. Used at the same doses and schedules employed for R-715 | Gobeil et al. (1996), Neugebauer et al. (2002) |
| B-9858 | LysLys[Hyp3,Igl5,𝒟-Igl7,Oic8] des-Arg9-BK | Peptidic antagonist, third generation. Extremely potent and long-lasting B1 receptor antagonist. Some reports indicate that this compound may present B2 receptor antagonistic properties. | Stewart et al. (1996), Rhaleb et al. (1992), Larrivee et al. (2000) |
| B-9958 | Lys-Lys-Arg-Pro-Hyp-Gly-CpG-Ser-Dtic-CpG | Peptidic antagonist, fourth generation. A highly potent and long-acting B1 receptor antagonist, without any action on B2 receptors. | Stewart et al. (1996), Gobeil et al. (1997) |
| Benzodiazepine antagonist |  |
Nonpeptidic B1 receptor antagonist. Presents in vivo efficacy against inflammatory pain when administered by the i.p. route. | Wood et al. (2003) |
| Dihydroquinoxalinone antagonist |  |
Nonpeptidic antagonist. It presents higher affinity for human and rabbit B1 receptors, with low affinity for rat B1 receptor. In vivo, shows antinociceptive actions in a rabbit assay of hyperalgesia. | Su et al. (2003) |
| SSR240612 | (2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-{[(6-methoxy-2-naphtyl)sulfonyl]amino}propanoyl)amino]-3-(4-{[2R,6S)-2,6-dimethylpiperidinyl] methyl} phenyl)-N-isopropyl-N-methylpropanamide hydrochloride | Nonpeptidic B1 receptor antagonist. The first B1 receptor antagonist with proven efficacy when administered by oral route in several models of pain and inflammation. | Gougat et al. (2004) |
The former relevant in vivo studies regarding the role of B1 receptors in pain and inflammation were initiated only after B1 receptor cloning studies. At the same time, certain pioneer works provided convincing evidence that B1 receptors could be functionally upregulated in vivo following proinflammatory or trauma stimuli (Perkins et al., 1993; Perkins & Kelly, 1993; Davis & Perkins, 1994; Campos & Calixto, 1995; Khasar et al., 1995; Perkins et al., 1995; Campos et al., 1996). The great progress in molecular biology had also made it possible to demonstrate that both B1 receptor mRNA and protein expression could be markedly enhanced under many stressful conditions. Nowadays, signalling pathways underlying B1 receptor induction during inflammatory and/or nociceptive alterations are better understood, even though various points in the cascade of B1 receptor modulation still remain elusive. In addition, a major effort toward the development of selective B1 receptor antagonists has been undertaken, mainly by the pharmaceutical companies. Most of this effort entails the screening of large compound libraries in order to identify more potent and selective antagonists see (Table 1). In the present review, we will discuss the main advances obtained in the last 5 years on understanding the role of B1 receptors in pain and inflammation, with special emphasis on some pathophysiological alterations where B1 receptor induction and/or activation might be of pertinent clinical interest. We will also call attention to some mechanisms involved in the upregulation of B1 receptors during inflammatory and painful conditions.
The kinin B1 receptor as the special one
There are, so far, several reasons for describing kinin the B1 receptor as a special entity in relation to the classical well-known G protein-coupled receptors. Many particular characteristics draw the attention of researchers to B1 receptors. Without doubt, the most interesting feature regarding this receptor is its complex pattern of expression. As described above, B1 receptors are normally absent, but may be highly and rapidly upregulated following inflammatory stimuli. This is an unusual characteristic of G protein-coupled receptors. In fact, the pattern of induction of B1 receptors resembles more closely to tyrosine-kinase-linked receptors. Although some literature data has demonstrated that other G-protein-coupled receptors can be also upregulated – these include the platelet-activating factor (PAF), the neurokinin NK-1, the protease-activated receptors 1 and 4 and occasionally even the kinin B2 receptor – none of them present the same profile and complexity of induction observed for B1 receptors (Marceau, 1995; Donaldson et al., 1997; Calixto et al., 2000; 2001). Interestingly, kinin B1 receptors seem to be upregulated under the same conditions described for the inducible proinflammatory enzymes cyclooxygenase-2 (COX-2) and the inducible nitric oxide synthase (iNOS). In this way, the stimuli (and also the cellular signalling pathways) shown to be capable of increasing the expression of B1 receptors are the same as those recognized for stimulating the upregulation of COX-2 and iNOS (Calixto et al., 2000; 2001). Furthermore, transcriptional and post-transcriptional tools required for B1 receptors expression seem to be the same necessary for regulating COX-2 and iNOS enzymes (Zhou et al., 1998; 1999; Dixon et al., 2000; Haddad et al., 2000; Lasa et al., 2001; Dixon, 2004). At this moment, it is not easy to explain why a G-protein-coupled receptor behaves as a typical inducible enzyme and this constitutes a very interesting field of research that will probably receive much attention. Despite its inducible profile, it is important to mention that in some tissues, especially at the central nervous system, kinin B1 receptors can be found constitutively expressed, a feature that is shared with the enzyme COX-2.
Some recent reports have indicated the existence of a crosstalk between B1 and B2 receptors. Therefore, evidence has also shown that persistent stimulation of B2 receptors might result in B1 receptor upregulation (Campos & Calixto, 1995; Phagoo et al., 1999; 2000; Campos et al., 2001; Todorov et al., 2003; Rashid et al., 2004). It is worth noting that stimulation of B2 receptors leads to transient increases in Ca2+ concentration and to the fast desensitization of these receptors. Regarding B1 receptors, they do not seem to be susceptible to desensitization mechanisms and their stimulation results in sustained elevations of Ca2+ concentration (Faussner et al., 1999; Marceau et al., 2002). Recent studies conducted with fluorescent conjugated proteins have demonstrated that B1 receptors, in contrast to B2 receptors, normally do not internalize following agonist stimulation, but they seem to translocate and aggregate (by a caveolae-mediated mechanism) after agonist binding, probably to facilitate the amplification of B1 receptor-mediated responses. In addition, other relevant points are likely to contribute to the extension of this idea: (1) in some cases, B1 receptor upregulation appears to be secondary to the downregulation of B2 receptors; (2) some transduction pathways activated by B2 receptors are known to be capable of inducing B1 receptors; and (3) stimulation with both B1 and B2 receptor agonists may lead to B1 receptors induction (Campos et al., 1996; 2001; Hayashi et al., 1998; Modéer et al., 1998; Pan et al., 1998; Phagoo et al., 1999; 2000). Finally, it has been established that B1 and B2 receptor genes are both located at the same chromosome of humans (chromosome 14), rats (chromosome 6) and mice (chromosome 12) (Chai et al., 1996; Cayla et al., 2002). This feature might also contribute to facilitating a balanced regulation of B1 and B2 receptors.
Molecular characterization of B1 receptor
In the present section, we have remarked on some of the most important findings concerning the molecular biology studies carried out on kinin B1 receptors.
When, McEachern et al. (1991) cloned the B2 receptor-encoding cDNA from rats, Southern blot analysis indicated that if a distinct gene was responsible for encoding B1 receptor, it would not be highly homologous to the B2-enconding gene. Later, Webb et al. (1994) presented important evidence suggesting that mRNA-encoding B1 receptor was distinct in size and in coding sequence from that encoding B2 receptor. At the same time, Menke et al. (1994) reported the first cloning expression of kinin B1 receptor from the human embryonic lung fibroblast cDNA library. Afterwards, B1 receptor has been cloned from rabbit (MacNeil et al., 1995), mouse (Pesquero et al., 1996), rat (Ni et al., 1998a), dog (Hess et al., 2001) and monkey (Hess et al., 2002). Differences in the amino-acid sequence of B1 receptors from different species range between 69 and 97%. Greater degrees of homology are observed between mouse and rat (88%) and monkey and human (97%) receptors (Hess et al., 2002).
Pharmacological evidence has shown that human, rabbit, mouse and monkey receptors present higher affinity (until 1000 times) for Lys-des-Arg9-BK in comparison to des-Arg9-BK, whereas rat and mouse receptors present similar affinities for both B1 receptor agonists. This difference is also observed for the B1 receptor antagonists Lys-des-Arg9-[Leu8]-BK and des-Arg9-[Leu8]-BK (Regoli et al., 2001; Hess et al., 2002). Recent molecular studies have proposed that these differences in affinity rely on the heterogeneity of the extracellular domain IV of the B1 receptor. Therefore, it has been proposed that N-terminal lysine preferentially binds to the extracellular domain IV in the human B1 receptor (Fathy et al., 2000; Hess et al., 2002). From this, it is worth noting that B1 receptor agonists and antagonists might present significant differences of affinity and potency depending on the species studied. This is mainly important for the development of antagonists and also for the preclinical studies: selective B1 receptor antagonists with similar affinities for all species would be preferable.
Molecular biology studies have widely contributed for understanding the inducible characteristic of B1 receptors. It has demonstrated that 5′-flanking region of B1 receptor gene (located upstream from the initiation site) holds several putative transcriptional regulatory sequences, including the TATA box and positive and negative control elements, with all characteristics of a core promoter (Bachvarov et al., 1996; Yang & Polgar, 1996; Ni et al., 1998b; Schanstra et al., 1998; Angers et al., 2000). Ni et al. (1998a, b) and Schanstra et al. (1998) have reported an NF-κB-binding domain, although the precise position remains elusive. Ni et al. (1998b) have further suggested putative sequences for AP-1 and CREB regulation. These pieces of evidence have largely contributed to the recent advances obtained in the comprehension of the mechanisms and signalling pathways involved in the upregulation of B1 receptors (see the next section of this review).
Other relevant molecular studies need to be mentioned. For instance, footprint analysis of the promoter region has revealed that it is possibly bound by several sequence-specific DNA binding proteins, like GATA-1, PEA3, AP-1, CAAT, Sp1, Pit-1a, Oct-1 and CREB (Angers et al., 2000). With the construction of a minigene (1.8 kb promoter plus exon 1, 1.5 kb intron 1, exon 2 and intron 2), it has been verified that motifs not included in the promoter sequence, such as 5′-UTR and intronic regions, are also required for induction of this gene (Yang et al., 2001b). The cotransfection of c-Jun with the minigene causes an increase of the promoter activity in a concentration-dependent manner, probably through interaction with multiple AP-1 sites. On the other hand, the tumour suppressor protein p53 has been demonstrated to suppress B1 receptor promoter activity in a concentration-dependent manner (Yang et al., 2001a). Finally, it has been suggested that the 3′-untranslated region (3′-UTR) containing a polyadenylation signal in the B1 receptor gene might be involved in the mRNA stability processes and its ultimate expression, since its absence directly reflects on the half-life of B1 receptor mRNA (Zhou et al., 1999). These data further extends the notion that the kinin B1 receptor represents an unusual protein-G-coupled receptor which can be modulated by the activation of highly specialized mechanisms.
Main mechanisms involved in the upregulation of B1 receptors
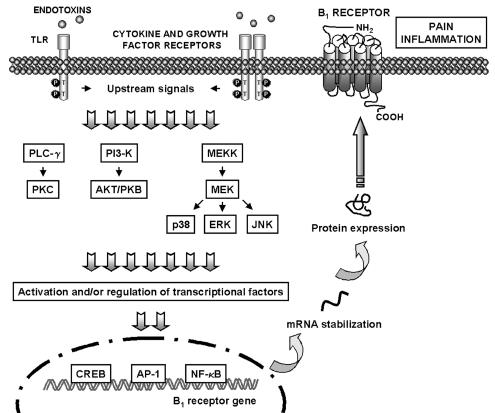
The induction of B1 receptors has been associated with the production of inflammatory mediators, stimulation of inflammatory cells, and, finally, activation of several intracellular signalling pathways. Understanding which intracellular signalling pathways mediate B1 receptor induction is of high interest in the discovery of therapeutic targets. In this section, we have summarized the recent advances made on the mechanisms involved in B1 receptor upregulation in several inflammatory models (Figure 1).
Figure 1.
Possible mechanisms underlying B1 receptor upregulation. Stressful stimuli (such as infection and inflammation) are able to activate several signalling pathways including MAPKs, PKC and PI3-K, which in turn might modulate transcriptional factors (e.g. NF-κB, AP-1 and CREB), and are supposed to be crucial for the regulation of B1 receptor expression. AP-1, activating protein-1; AKT/PKB, protein kinase B; CREB, c-AMP response element-binding; ERK, extracellular signal-regulated kinase; JNK, c-jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; MEKK, mitogen-activated protein kinase kinase kinase; MEK, mitogen-activated protein kinase kinase; NF-κB, nuclear factor-κB; PI3-K, phosphatidylinositol-3 kinase; PLC, phospholipase C; PKC, protein kinase C; TLR, Toll-like receptors.
Primary studies concerning B1 receptor expression have reported the importance of in vitro incubation time in the increase of functional responses to B1 receptor agonists in many isolated organ preparations. These studies have also shown that the time-dependent B1 receptor upregulation is sensitive to RNA and protein synthesis inhibitors, suggesting its dependence on de novo protein synthesis (see Regoli & Barabé, 1980; Marceau et al., 1998). Although the elucidation of the pivotal molecular mechanisms implicated in the regulation of these processes has only just begun, recent literature data has already shown that B1 receptor upregulation can be modulated at different levels. The main pharmacological strategies applied to the study of the mechanisms involved in the upregulation of B1 receptors are listed in Table 2 .
Table 2.
Some possible pathways for pharmacological modulation of B1 receptor upregulation
| Target | Pharmacological modulation |
|---|---|
| mRNA transcription | Actinomycin D |
| Translocation | Brefeldin A |
| Translation/ N-glycosylation | Cycloheximide |
| Tunicamycin | |
| Inhibition of ribonuclease | MAPK inhibitors |
| Interference with mRNA unstable regions (AUUUA) | Dexamethasonea |
| NF-κB activation | Inhibitors of IκB kinase (PDTC, BAY 117082) |
| Proteasome activity inhibitors (TLCK, MG 132) | |
| Cytokine modulation | Inhibitors of cytokines synthesis, antibodies, recombinant receptors |
| Protein kinase inhibitors | Blockers of TKs, PKC and MAPKs, etc. |
For further details, refer to the following reviews: Marceau et al. (1998), Calixto et al. (2000; 2001), Couture et al. (2001).
Dexamethasone is able to block B1 receptor induction at several points.
The determination of distinct signalling pathways involved in the regulation of B1 receptor expression has been accomplished by several approaches. Gene sequence analysis has revealed that the B1 receptor promoter presents numerous sequence-specific motifs for different DNA binding proteins, which indicates that B1 receptor expression could be modulated by several transcriptional factors (Bachvarov et al., 1996; Yang & Polgar, 1996; Ni et al., 1998b; Schanstra et al., 1998; Angers et al., 2000). In this context, there is now a considerable amount of experimental evidence indicating that NF-κB, a transcriptional factor that governs the expression of gene-encoding cytokines, chemokines, growth factors, cell adhesion molecules, and some acute phase proteins in health and various pathological states, plays a pivotal role in the regulation of B1 receptor induction. It has been demonstrated that specific NF-κB blockers are able to prevent B1 receptor upregulation both in vitro (Sardi et al., 2000; 2002; Medeiros et al., 2001; 2004; Phagoo et al., 2001; Sabourin et al., 2002) and in vivo (Campos et al., 1999; Fernandes et al., 2003; Passos et al., 2004). Indeed, studies conducted by Ni et al. (1998b) have revealed the existence of an NF-κB-like binding site on the human B1 receptor promoter, which seems to be essential for the control of receptor transcription following exposure to certain inflammatory agents such as IL-1β, Tumour necrosis factor α (TNFα) or lipopolysaccharide (LPS). Furthermore, it has been demonstrated that in cultured human lung fibroblasts, B1 receptor upregulation induced by IL-1β is modulated at the transcriptional level, in a process mediated by the activation of NF-κB (Schanstra et al., 1998). Recently, we have verified that tissue damage in the rat portal vein is also able to induce the expression of B1 receptor, in a process involving IκBα degradation and the subsequent translocation of NF-κB into the nucleus with an increase in NF-κB DNA-binding activity (Medeiros et al., 2004).
Protein phosphorylation has also been reported to exert an important role in the regulation of many cellular processes, including gene expression and protein synthesis (Hunter, 1995). Thus, it has been suggested that the activation of some classes of protein kinases is essential for the induction of the B1 receptor. In fact, Larriveé et al. (1998) were the first to show that both spontaneous and IL-1β-induced B1 receptor upregulation in rabbit aorta are greatly sensitive to inhibitors of tyrosine kinase, extracellular signal-regulated kinase (ERK) or p38 MAPK pathway. In addition, it has been demonstrated that protein kinase C (PKC) activation also critically regulates the B1 receptor mRNA expression in cultured human lung fibroblasts (Schanstra et al., 1998). Other studies have confirmed and extended these previous data. It has been shown that in vivo B1 receptor upregulation induced by inflammatory cytokines IL-1β or TNFα involves the activation of PKC, tyrosine kinase and ERK (Campos et al., 1999). Moreover, PKC is also involved in the B1 receptor upregulation in different isolated tissue preparations (Medeiros et al., 2001; Ueno et al., 2002). Of interest is the recent data demonstrating that p38 MAPK and c-Jun NH2-terminal kinase (JNK) are critically involved in the regulation of B1 receptor mRNA expression induced by vascular tissue trauma (Medeiros et al., 2004). Altogether, these data clearly demonstrates the importance of different classes of protein kinases in the modulation of B1 receptor induction. However, the precise mechanisms through which this regulation occurs is currently a matter of debate. Recently, several studies have suggested that protein kinases may regulate the expression of certain inflammatory genes, acting at both transcriptional and post-transcriptional levels. In addition, the activation of protein kinases has been associated to the increased activation of different transcriptional factors and to the regulation of the half-lives of many inflammatory mediator mRNAs (e.g. COX-2, TNFα and IL-1β) (Dunn et al., 2002; Clark et al., 2003; Lahti et al., 2003; Yang et al., 2003). Following this, a recent study has suggested that B1 receptor expression could also be regulated through the enhancement of mRNA stabilization (Zhou et al., 1999). This notion has been extended further by Haddad et al. (2000), who demonstrated that B1 receptor induction in human lung fibroblasts (HEL 299), following incubation with IL-1β and TNFα, is largely dependent on post-transcriptional mechanisms, being this effect greatly sensitive to dexamethasone and p38 MAPK inhibitors. Therefore, it is tempting to suggest that the regulation of B1 receptor might be controlled both transcriptionally and post-transcriptionally. Therefore, the activation of protein kinases could certainly be involved in the enhancement of both transcriptional factor activity and B1 receptor mRNA stability. However, additional studies are still required to better understand and confirm this hypothesis.
The B1 receptor and its role in development and maintenance of inflammatory diseases
Several pieces of evidence indicate that B1 receptor induction plays a critical role in inflammatory pathological states. In the next sections, we have highlighted the main recent findings, which implicate B1 receptors in inflammatory models. Data described herein is summarized in Table 3.
Table 3.
Inflammatory stimuli associated with B1 receptors up-regulation
B1 receptors and infection
The effects of treatment with bacterial endotoxin on B1 receptor upregulation (both functional and molecular) have been extensively reported (see Marceau et al., 1998; Calixto et al., 2000; 2001). Likewise, infection seems to constitute a classical signal known for its ability to induce B1 receptors. A very interesting study conducted by DeBlois & Horlick (2001) suggested that the haemodynamic and inflammatory responses observed after systemic LPS treatment in green monkeys are largely mediated by B1 activation. The authors reported that the selective B1 receptor agonist des-Arg9-BK caused a marked fall in blood pressure, accompanied by an increase in heart rate only in monkeys treated with LPS. In the same way, des-Arg9-BK-induced skin oedema formation was observed solely in monkeys treated with LPS. The work of Deblois & Horlick (2001) was the first experimental evidence indicating the upregulation of B1 receptors in non-human primates. In addition, it has been shown that gene deletion of B1 receptors prevents the endotoxic shock in mice (Pesquero et al., 2000). Extending this idea, Ni et al. (2003) demonstrated that transgenic overexpression of B1 receptors renders mice more susceptible to endotoxic shock and greatly potentializes the oedematogenic responses caused by the inflammatory agent carrageenan. Based on these results, it is tempting to speculate that the new generation of nonpeptidic B1 receptor antagonists see (Table 1) could represent a valuable tool for the control/treatment of situations associated with disseminated infection, such as sepsis.
Studies conducted with rodents confirm and further extend the proposition that endotoxins classically induce B1 receptors. For instance, B1 receptors seem to exert an important role in vascular changes and hypotension induced by LPS from Escherichia coli in rats (McLean et al., 1999). LPS has also been found to markedly increase plasma extravasation induced by the selective B1 receptor agonist des-Arg9-BK in the duodenum, ileum and trachea of rats (Wille et al., 2001). More recently, Passos et al. (2004) have demonstrated the occurrence of kinin B1 receptor upregulation after the local administration of LPS into the rat paw, in a process involving activation of the transcriptional factor NF-κB, neutrophil migration, and release of inflammatory mediators (e.g. IL-1β, TNFα and PAF). Another recent and relevant publication has shown that incubation of tracheal organ culture for 1 or 4 days with the bacterial products LPS and polyinosininc polycytidylic acid enhances BK- and des-Arg9-BK-mediated contractile responses by mechanisms largely dependent on protein synthesis, MAPK activation and NF-κB activation (Bachar et al., 2004).
More recently, it has been widely demonstrated that LPS and other endotoxins induce several inflammatory alterations by binding to a family of receptors denoted toll-like receptors (TLR). In the case of LPS, it has been reported that their actions are mediated by the activation of TLR4 receptors and the consequent stimulation of NF-κB and several protein kinases, associated with the release of proinflammatory cytokines (e.g. IL-1β or TNFα) (for more details see Beg, 2002; Dobrovolskaia & Vogel, 2002; Akira et al., 2003). In this regard, all these signalling pathways have now been demonstrated to be involved in the process of B1 receptor upregulation.
Another aspect which deserves attention is the recent evidence implicating the B1 receptor as an adjuvant for Trypanosoma cruzi infection. Recent data have shown that intradermal injection of trypomastigotes into the mouse paw results in a long-lasting oedema formation: the first phase of oedematogenic response (3 h) is blocked by the B2 receptor antagonist Hoe 140, whereas the late phase (24 h) can be prevented by treatment with the selective B1 receptor antagonist B9858. Late-phase oedema has also been abolished in B1 receptor knockout mice (Scharfstein et al., 2000; Todorov et al., 2003). These results have led authors to conclude that B1 receptors are upregulated following local infection with T. cruzi and to point out the importance of B1 receptors for the progress of Chagas disease. Also of interest are the results from the same group showing that B1 receptors (as well as B2 receptors) seem to facilitate the invasion of T. cruzi to the host cells. These conclusions are based on evidence indicating that both B1 and B2 receptor antagonists are effective in preventing the parasitic infection of Chinese Hamster ovary cells which coexpress B1 and B2 receptors (Todorov et al., 2003).
Ischaemia–reperfusion lesions
Literature data have suggested that B1 receptors might exert a pivotal role in postischaemic situations. Thus, it has been shown that B1 receptors can be induced in the endothelial cells of rabbits (Mazenot et al., 2001) or in the hearts of mice (Lagneux et al., 2002) submitted to myocardial ischaemia–reperfusion. In the rabbit model, the vascular effects evoked by ischemia–reperfusion can be significantly reduced by a selective B1 receptor antagonist – [Leu8]-des-Arg9-BK (Mazenot et al., 2001). In mice, either pharmacological blocking with selective B1 antagonist [Leu8]-des-Arg9-BK or gene deletion of B1 receptors largely diminishes the infarction's extension, an effect that further reinforces the relevance of B1 receptors under these conditions. Kuebler et al. (2003) have affirmed that B1 receptors also seem relevant in the ischaemia–reperfusion of rat pancreas. The authors have demonstrated, by means of binding studies, that B1 receptor expression is augmented by about 22-fold after pancreatic ischaemia–reperfusion injury. They have also shown that the B1 receptor antagonist CP-0298 (alone or in combination with the B2 antagonist CP-0597) significantly reduces the number of adherent leukocytes in postcapillary venules (Kuebler et al., 2003). Another recent contribution has demonstrated that intestinal ischaemia–reperfusion injury results in a striking increase of B1 receptor expression in rats (Souza et al., 2004). In this model, B1 receptor upregulation is probably secondary to the activation of B2 receptors, as it can be significantly blocked by selective B2 receptor antagonists. It should be noted that all the main inflammatory parameters such as, changes of vascular permeability, neutrophil migration and cytokines production, are completely abolished in B1 receptor knockout mice.
Ischaemia–reperfusion lesions are associated with a marked migration of inflammatory cells, cytokine generation and activation of kinases and transcriptional factors (e.g. p38 MAPK and NF-κB, respectively) (Jones et al., 2003; Lien et al., 2003). It is noteworthy that all these signalling pathways have been correlated with the process of B1 receptor upregulation. Taking into account these considerations, it is tempting to suggest that B1 receptors modulation could be a very interesting target for the control and/or treatment of the alterations observed after ischaemia–reperfusion injury.
Inflammation of airways
Kinins seem to exert a central role in the control and maintenance of the inflammatory states of the airways. The relevance of B2 receptors has been widely investigated, but only a few publications have remarked on the role of B1 receptors in the airways. Thus, some recent reports have pointed out the critical relevance of kinin B1 receptors in allergen-induced bronchial hyper-responsiveness. Huang et al. (1999) have demonstrated that challenge with ovalbumin (OVA) results in a marked increase of B1 receptor mRNA expression in the lungs of previously sensitized rats. This increase peaks between 2 and 6 h and remains elevated up to 24 h following the challenge with OVA. Another recent report has shown that allergic lung inflammation in ovalbumin-sensitized mice is significantly diminished by the systemic treatment of animals with the selective B1 receptor antagonist R-954 (Landgraf et al., 2003). The same effect of R-954 has been observed in immunocomplex-induced lung inflammation (Landgraf et al., 2004). These findings were extended and confirmed by Eric et al. (2003), who has demonstrated that treatment with B1 receptor antagonists R-715 or R-954 significantly reduces lung eosinophilia evoked by OVA in sensitized mice. Importantly, allergen-evoked hyperalgesia in OVA-sensitized rats has been found to be markedly reduced by local treatment with [Leu8]-des-Arg9-BK (Lavich et al., 2003). In addition, B1 receptor mRNA levels are consistently increased in carrageenan-induced pleurisy at 3 h, remaining elevated up to 5 h after challenge in rats (Hayashi et al., 2002). The same authors have also reported that administration of either of B1 receptor antagonists [Leu8]-des-Arg9-BK or des-Arg9-D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-BK is able to prevent carrageenan-induced exudation. Furthermore, Pesquero et al. (2000) have reported that disruption of the B1R gene in mice results in a marked reduction of the inflammatory responses induced by carrageenan in the pleurisy model in mice. Interestingly, the relevance of B1 receptors in human airway inflammation has recently been reported by Christiansen et al. (2002). These authors have shown that nasal tissue samples from allergic rhinitis present a significantly higher B1 receptor mRNA expression in comparison to tissue samples obtained from normal subjects.
Some in vitro studies conducted with cell lines from airways confirm the data obtained in vivo and reinforce the notion that B1 receptors are critical for the pathological conditions affecting the airways. For instance, B1 receptors are upregulated by the incubation of IL-1β and TNFα in human embryonic lung fibroblasts HEL 299 (Haddad et al., 2000). Likewise, Phagoo et al. (2001) have shown that stimulation of human lung fibroblasts with IL-1β or des-Arg9-bradykinin results in a marked upregulation of B1 receptors. Moreover, in pulmonary A549 and human bronchial epithelial cells, treatment with the proinflammatory cytokines IL-1β or TNFα causes a remarkable enhancement of B1R mRNA expression (Newton et al., 2002). Very recently, an interesting study conducted by Zhang et al. (2004) has demonstrated that the incubation of mouse tracheal segments (for at least 4 days) results in both functional and molecular upregulation of B1 receptors, these responses being further enhanced by the continuous exposure to the proinflammatory cytokine TNFα. This work also demonstrates that the upregulation of B1 receptors following TNFα exposure is largely dependent on the activation of MAPKs JNK and ERK 1/2.
Diabetes and B1 receptor modulation
Type I diabetes constitutes an autoimmune disorder in which insulin production is affected by the destruction of pancreatic β-cells. It has been well documented that inflammatory alterations exert a critical role in the development of diabetes – a massive infiltration of inflammatory cells, the elevated production of proinflammatory cytokines (e.g. IL-1β TNFα) and the generation of free radicals are the main features related to the destruction of β-cells in the pancreas (see Couture et al., 2001; Hohmeier et al., 2003). The progress of the diabetes and the constant fluctuation of glucose levels are associated with changes in vascular structure (mainly related to the endothelium) and the production of vasoactive substances. In this context, the kinin system has often been implicated in the pathophysiology of diabetes (Garcia Leme et al., 1973; Couture et al., 2001). Moreover, the extent to which diabetes may influence the modulation of B1 receptors has been the matter of several recent publications. Campos et al. (2001) have shown that the induction of diabetes with the nitrosamine streptozotocin (STZ) results in a long-lasting (8 weeks) functional upregulation of B1 receptors in the rat paw, as indicated by a marked increase in the oedematogenic response induced by the selective B1 receptor agonist des-Arg9-BK in diabetic rats. This effect is associated with a significant reduction of B2 receptor-mediated paw oedema. Based on these results, it is possible to assume that during diabetes development, inducible B1 receptors may be overexpressed, whereas constitutive B2 receptors are downregulated. The functional induction of B1 receptors has also been described in the pleural cavity of rats following STZ treatment (Vianna et al., 2003). In this model, both mononuclear and neutrophil influx in response to intrapleural injection of des-Arg9-BK has been found to be markedly enhanced in diabetic rats. In addition, autoradiographic B1 receptors binding specific sites are significantly augmented in the lungs of STZ (4 days)-treated rats, as compared with control animals (Couture et al., 2001; Vianna et al., 2003). It has further been demonstrated that intrathecal injection of des-Arg9-BK results in a biphasic nociceptive response following acute (24 h) administration of STZ (Couture et al., 2001). The importance of B1 receptors in diabetes has also been shown by a series of studies indicating that systemic treatment with the highly selective B1 receptor antagonists R-715 and R-954 consistently prevents thermal hyperalgesia induced by STZ (Gabra & Sirois, 2002; 2003a, 2003b). Recently, Abdouh et al. (2003) have suggested a potential role for B1 receptors in diabetes-associated retinopathies: a marked increase in autoradiographic B1 receptor-specific binding sites, associated with the enhancement of des-Arg9-BK-induced vasodilatation, has been observed in the retinas of STZ (4–21 days)-treated rats. In this regard, it is possible to propose that selective B1 receptor antagonists would constitute a very attractive strategy for the control of some of the symptoms associated with diabetes, namely inflammatory and neuropathic complications.
Kinin B1 receptors and cancer
An increasing body of evidence has emerged indicating that kinins and their receptors appear to be involved in cancer (Mahabeer & Bhoola, 2000; Stewart, 2003). The known mitogenic properties of kinins and their ability to activate tyrosine and MAP-kinase cascades could explain, at least in part, the effects of kinins in tumour growth and migration (Mahabeer & Bhoola, 2000; Bhoola et al., 2001; Stewart, 2003). In addition, as cancer growth and metastasis are critically dependent on the activation of inflammatory pathways, it is possible to conclude that B1 receptor upregulation could play an important role in this scenario. Most publications regarding the participation of kinin receptors in cancer indicate a prevalent role for B2 receptors. Thus, immunohistochemical and autoradiographic studies have pointed out a marked distribution of B2 receptors in several human and mouse tumour cells (Wang et al., 2001; Wu et al., 2002). Some studies conducted with MG63 human osteosarcoma cells and mice bearing sarcoma 180 cells have discarded a role for B1 receptors in cancer (Wang et al., 2001; Ishihara et al., 2001; 2002). On the other hand, B1 receptors immunoreactivity has been found to be markedly increased in astrocytes and endothelial cells, as well as in the stromal blood vessels from human astrocytic tumour biopsies (Raidoo et al., 1999). In addition, Barki-Harrington et al. (2003) have reported that both B1 and B2 receptors activation seem to be linked with the mitogenic signalling in androgen-insensitive prostate cancer PC3 cells. This evidence has been further extended by recent results showing that B1 receptors are present in prostatic intraepithelial neoplasia and malignant lesions, but not in benign prostate tissues (Taub et al., 2003). These data permit us to suggest that kinin B1 receptor antagonists might be potentially useful as adjuvant therapies in some types of cancer. The mechanisms underlying the precise role of B1 receptors in cancer remain to be investigated and constitute a promising and growing area of research.
Participation of B1 receptors in other inflammatory states
The modulation of B1 receptors and their participation in other inflammatory alterations has been demonstrated by several additional contributions. Thus, in the model of cystitis induced by cyclophosphamide in rats, B1 receptor selective agonists cause a marked contractile response of the urinary bladder, an effect that is not observed in naïve animals (Bélichard et al., 1999). Also, a consistent and progressive increase of B1R mRNA expression in the urinary bladder for up to 48 h following cyclophosphamide treatment has been reported (Bélichard et al., 1999). These data suggest that B1 receptor induction and activation could constitute an important event during the development of cystitis.
In the acute and severe synovitis induced by intra-articular injection of carrageenan and kaolin, a significant increase in B1R mRNA expression has been observed in the rat knee synovia (Seegers et al., 2004). Sainz et al. (2004) have reported that in peptidoglycan–polysaccharide-induced arthritis in rats, the selective B1 receptor antagonist B9858 causes a significant decrease in lymphocyte homing and leukocyte transmigration.
Kinin-induced rat paw oedema is a classical in vivo model of inflammation that has been widely used for studying B1 receptor upregulation. In this model, B2 receptor agonists induce a marked oedema formation in naïve animals, while the injection of selective B1 agonists evokes only slight alterations in paw volume. However, systemic or local treatment with several distinct inflammatory stimuli evokes a considerable increase in B1 receptor-mediated paw oedema. Of note is data showing that acute local treatment with the proinflammatory cytokines IL-1β and TNFα (15–120 min) results in a rapid- onset and time-dependent increase in rat paw oedema caused by the selective B1 receptor agonists des-Arg9-BK and des-Arg10-kallidin (Campos et al., 1998). The effects of IL-1β and TNFα in the functional upregulation of B1 receptors in the rat paw have been found to be modulated by the secondary production of inflammatory cytokines, by the activation of several protein kinases, and also by the transcriptional factor NF-κB (Campos et al., 1998; 1999). Participation of NF-κB activation in the upregulation of des-Arg9-BK-induced paw oedema has also been evidenced 7 days after the surgical removal of adrenal glands (Cabrini et al., 2001). Additional evidence has indicated that intraplantar injection of IL-1β induces a striking augmentation of B1 receptor mRNA expression in the rat paw (Campos et al., 2002). In the same publication, it was proposed that B1 receptor upregulation was directly linked to the activation of chemotatic mediators and neutrophil migration. Likewise, Fernandes et al. (2003) have recently shown that PAF, a known chemotatic agent, was capable of inducing both functional and molecular upregulation of B1 receptors when administered locally (6 h prior) into the rat paw. The mechanisms involved in PAF effects are largely related to the stimulation of PAF receptors, NF-κB activation, and neutrophil migration. Similar findings have been described by Passos et al. (2004) following treatment (12 h) with LPS in the rat paw. Recently, Gougat et al. (2004) have demonstrated that des-Arg9-BK produced a marked oedema formation in mice locally pretreated with IL-1β (5 ng/paw, 40 min before). Interestingly, they report that des-Arg9-BK-induced mouse oedema was dose-dependently inhibited by the systemic treatment (by oral or i.p. routes) with the new nonpeptidic B1 receptor antagonist SSR240612.
Participation of kinin B1 receptors in painful processes
Pain may be defined as an unpleasant sensorial and emotional experience associated with actual or perceived tissue damage by a noxious (damaging) stimulus (Watkins & Maier, 2003). As the emotional component of pain is very difficult to measure, especially in experimental animals, the sensorial component of pain (nociception) is more commonly assessed in preclinical and clinical pain studies. BK is among the most potent endogenous algogen substances, and its role in nociceptive processes has been extensively reviewed (Dray & Perkins, 1997; Calixto et al., 2000; 2001; Couture et al., 2001). BK, acting at B2 kinin receptors, is able to produce excitation and sensitization of the free endings of C- and Aδ-primary afferent fibres (nociceptors) leading to the production of overt nociception (stimuli-independent pain), hyperalgesia (exaggerated response to a painful stimulus) and allodynia (pain produced by a previously innocuous stimulus) in experimental animals and humans. The activation of B2 receptors triggers several well-known signalling pathways to produce depolarization and lowering of the threshold of the nociceptors, including phospholipase C and A2, protein kinase C, formation of COX and lypooxygenase metabolites from arachidonic acid, and activation of vanilloid receptors and tetrodotoxin-resistant sodium currents (Dray & Perkins, 1997; Premkumar & Ahern, 2000; Ferreira et al., 2004). On the other hand, the participation of kinin B1 receptors in pain transmission is a relatively new area of research and is even less explored. B1 receptors have generally been implicated in the modulation of the persistent and chronic inflammatory hyperalgesia induced by different agents, including cytokines (IL-1β, IL-2 and IL-8, but not TNFα and IL-6), bacterial components (LPS, CFA and Mycobacterium bovis bacillus Calmette–Guerin – BCG), irritants (carrageenan and capsaicin), ultra-violet irradiation, and substance P (Perkins & Kelly, 1993; Perkins et al., 1993; Davis & Perkins, 1994; Khasar et al., 1995; Rupniak et al., 1997; De Campos et al., 1998; Poole et al., 1999; Ganju et al., 2001; see for review: Dray & Perkins, 1997; Calixto et al., 2000; 2001). However, recent data regarding the constitutive expression of the B1 receptor in sensory neurones and the participation of B1 receptors in chronic models of inflammatory and neuropathic pain (especially using knockout mice and selective nonpeptide receptor antagonists) has dramatically improved our comprehension about the critical role of B1 receptor activation during painful processes. Thus, in the next section, we will focus our attention on these novel ideas and we will try to demonstrate the potential of the B1 receptor as a new and relevant target for the development of a new class of analgesic drugs.
Expression of B1 receptors in pain-transmitting neurones
Pain is produced by the stimulation of small-diameter primary afferent fibres that innervate regions of the head and body and arise from cell bodies in trigeminal and dorsal root ganglia (DRG), respectively (Julius & Basbaum, 2001). In a first attempt to identify B1 receptors in sensory neurons, Davis et al. (1996) were unable to detect specific any binding to [3H]-des-Arg10-kallidin rat DRG cultured for 7–8 days. Afterwards, expression of B1 receptor mRNA was detected in freshly isolated mouse and rat DRG by means of RT–PCR (Seabrook et al., 1997; Levy & Zochodne, 2000; Yamaguchi-Sase et al., 2003). The constitutive expression of B1 receptors in rat and mouse sensory neurones was further confirmed by the use of immunohistochemical staining (Ma et al., 2000; Wotherspoon & Winter, 2000). Furthermore, peripheral B1-containing neurons were found in peripheral nerve terminals, such as those that innervate rat urinary bladder (Wotherspoon & Winter, 2000). B1 receptor staining was also localized in rat, mouse and monkey trigeminal and DRG ganglia (Ma et al., 2000; Wotherspoon & Winter, 2000; Shughrue et al., 2003; Rashid et al., 2004). B1 receptors were predominantly expressed by small diameter DRG neurones, colocalized with calcitonin gene-related peptide or isolectin B4 (Ma, 2001).
It has been well demonstrated that both B1 receptor mRNA and protein are constitutively present in the spinal cord of rats, mice, monkeys and humans (Couture & Lindsey, 2000; Wotherspoon & Winter, 2000; Ma & Heavens, 2001; Shughrue et al., 2003). It is worth noting that B1 receptor immunoreactivity has been identified in the superficial layers of the dorsal horn, confined mainly to the spinal terminals of primary afferent fibres (Couture & Lindsey, 2000; Wotherspoon & Winter, 2000; Ma & Heavens, 2001). Subsets of dorsal horn neurons project axons and transmit pain messages to higher brain structures related by the somatic, affective and autonomic responses to pain (Hunt & Mantyh, 2001). Of interest is the fact that basal B1 receptor expression has been described in several structures related to pain transmission and modulation, including the somatosensory cortex and thalamus (Ongali et al., 2003; Shughrue et al., 2003). However, the function of B1 receptors in these regions remains obscure.
Role of kinin B1 receptor in acute and chronic painful processes
Much about the earlier studies on the role of B1 receptors in physiological and pathological processes has been elucidated on the basis of functional studies or the use of selective receptor antagonists. However, because of the problems of selectivity and agonist activity, and the rapid degradation of some of the antagonists, advances in understanding the role played by B1 receptors in most physiological and pathological processes has been hampered. The generation of the B1 knockout mouse has made it possible to expand our current knowledge regarding the contribution of this receptor in nociceptive processes (Pesquero et al., 2000). B1 receptor-deficient mice present hypoalgesia against the acute overt nociception induced by capsaicin or formalin and by high intensity heat stimuli (Pesquero et al., 2000). This evidence confirms and extends previous data that demonstrates the role of B1 receptor in acute pain. For instance, intraplantar or systemic treatment with the selective B1 receptor antagonist des-Arg9-Leu8-BK was found to be capable of reducing capsaicin, glutamate, and first-phase of formalin-induced pain (Shibata et al., 1989; Corrêa & Calixto, 1993; Sufka & Roach, 1996; Beirith et al., 2003; J.B. Calixto, unpublished results). Since the nociceptive behaviours of the former tests are very short (lasting up to 10 min), it seems improbable that B1 receptor expression depends on de novo protein synthesis (see details above) and the involvement of constitutively expressed receptors is indicated. However, the peripheral injection of B1 receptor agonists rarely induces nociception in naïve animals (Perkins & Kelly, 1994; Khasar et al., 1995; Ganju et al., 2001; Fox et al., 2003; Ferreira et al., 2004). Moreover, so far, there is no functional evidence showing that B1 receptor agonists directly activate peripheral terminals or the cell bodies of sensory neurones (Dray et al., 1992; Davis et al., 1996; Seabrook et al., 1997; Brand et al., 2001). Thus, it has been suggested that B1 receptors on the peripheral sensory nerve terminals need some stimulation to prime their nociceptive action (Ma, 2001). In fact, des-Arg9-BK is able to produce overt nociception when intraplantarly coadministered with formalin (Campos et al., 1995; De Campos et al., 1998). The mechanisms involved in this short-lasting functional induction of B1 receptor remain elusive and require further study.
Studies carried out with B1 receptor knockout mice have also confirmed the important role played by B1 receptors in the development and maintenance of chronic pain. Chronic pain differs substantially from acute pain, not only in terms of the persistence, but also in relation to the maladaptive neuroplasticity described at various levels of the nervous system (Woolf & Mannion, 1999). Peripheral injection of CFA has been used as an experimental animal model of arthritis, causing persistent hyperalgesia and allodynia to mechanical and thermal stimuli that is developed as early as 2 h after its administration and persists for weeks. As observed in humans, this nociceptive behaviour takes place on both ipsilateral and contralateral sides of the injection, an effect which is mediated by local nociceptor sensitization and systemic neuronal (such as central sensitization) and immune (such as increase in cytokine serum level) mechanisms (Shenker et al., 2001). Gene deletion of the B1 receptor reduces ipsilateral, and in particular contralateral, thermal hyperalgesia and mechanical allodynia induced by CFA (Ferreira et al., 2001). These findings confirm previous studies which have indicated that the B1 receptor antagonists des-Arg9-Leu8-BK, des-Arg10-Hoe 140 and B9858 are capable of inhibiting CFA-induced nociception in rats, mice and rabbits, respectively (Perkins et al., 1993; Panesar et al., 1998; Mason et al., 2002). Moreover, intraplantar injection of des-Arg9-BK induces contralateral mechanical hyperalgesia and allodynia in rats pretreated with intraplantar CFA (Khasar et al., 1995; Fox et al., 2003). Interestingly, in the experiment of Fox et al. (2003), B1 receptor-immunoreactivity was significantly increased 24 h after CFA administration in both ipsilateral and contralateral small DRG neurons. It is worth noting that the nonpeptidic B1 receptor antagonist derived from dihydroquinoxalinone produces potent antinociceptive action in the model of CFA-induced hyperalgesia in rabbits (Su et al., 2003).
Painful neuropathies may result from nerve injury, chronic treatment with certain drugs and metabolic disorders (Woolf & Mannion, 1999). As the mechanisms underlying these syndromes are not fully understood, available therapy does not provide satisfactory pain relief and patients suffer from chronic intractable pain. Several studies have demonstrated the important role played by kinins and their receptors in neuropathic pain induction. Increased levels of B1 receptor mRNA or protein have been found in dorsal root ganglion after sciatic nerve constriction injury in rats and mice (Petersen et al., 1998; Eckert et al., 1999; Levy & Zochodne, 2000; Yamaguchi-Sase et al., 2003; Rashid et al., 2004). Very recently, it has been reported that B1 receptors are newly expressed after nerve injury, mainly in myelinated DRG neurons, whereas B2 receptor expression drastically decreases in DRG (Rashid et al., 2004). Importantly, systemic administration of B1 receptor antagonist des-Arg9-Leu8-BK is able to reduce the thermal hyperalgesia and mechanical allodynia produced by sciatic nerve constriction in rats (Levy & Zochodne, 2000; Yamaguchi-Sase et al., 2003). Also, the gene deletion of B1 receptors practically abolishes the nociceptive hypersensitivity produced by sciatic nerve injury in mice (J.B. Calixto, unpublished results). This effect appears as early as 1 day after lesion and remains significant up to 28 days, suggesting that the B1 receptor is involved in both development and maintenance of neuropathic pain symptoms. Corroborating these data, intraplantar administration of Lys-des-Arg9-BK 7 days after sciatic nerve injury in mice is able to induce both nociception and activation of ERK in DRG neurones (Rashid et al., 2004). Interestingly, oral treatment with the newly developed nonpeptide B1 receptor antagonist SSR240612 is capable of reducing the thermal hyperalgesia produced by sciatic nerve injury in rats (Gougat et al., 2004).
Painful neuropathy may also be developed in relation to diabetes (Woolf & Mannion, 1999). As discussed earlier, the B1 receptor seems to be implicated in type I diabetes complications (for review see: Couture et al., 2001; Gabra & Sirois, 2003a, 2003b). In fact, thermal hyperalgesia produced by STZ in mice is blocked by the systemic treatment of the selective B1 receptor antagonists R715 or R954 (Gabra & Sirois, 2003a, 2003b). In addition, acute administration of des-Arg9-BK significantly potentiates diabetes-induced hyperalgesia. Considering these data, it is possible to suggest that the use of selective B1 receptor antagonists could represent a novel approach for the treatment of chronic pain of inflammatory and neuropathic origin.
Other recent evidence for the involvement of B1 receptors in painful processes
Other relevant studies have addressed the involvement of B1 receptors in various models of acute and chronic pain. For instance, intraplantar injection of zymozam produces local increase in the B1 receptor mRNA expression and mechanical hyperalgesia that has been reversed by the B1 receptor antagonists des-Arg9-Leu8-BK and R715 (Bélichard et al., 2000). Moreover, intraplantar injection of the B1 receptor agonist des-Arg9-BK is able to produce hyperalgesia 1 h following administration of IL1-β at the same site, a response that seems to be dependent on p38 MAPK activation (Ganju et al., 2001). A recent report has demonstrated that peripheral B1 receptor is also involved in the orofacial nociception caused by formalin in rats (Chichorro et al., 2004). B1 receptors seem likely to be involved in visceral pain production, since the antagonism of the B1 receptor reduces the late viscero-visceral hyper-reflexia induced by turpentine inflammation. Another recent study has shown that the benzodiazepine-derived nonpeptide B1 receptor antagonists are able to reduce carrageenan-induced hyperalgesia in rats, apart from their poor bioavailability after systemic treatment (Wood et al., 2003). Moreover, oral treatment with the nonpeptide B1 receptor antagonist SSR240612 is capable of reducing the nociception produced by formalin and ultra-violet application (Gougat et al., 2004). Based on these data, it is possible to infer that B1 receptors (expressed constitutively or upregulated) might be related to acute and chronic pain of somatic and visceral origin.
The spinal cord is an important site for B1 receptor nociceptive action
As previously demonstrated, B1 receptors have been identified in the spinal cord (Couture & Lindsey, 2000; Wotherspoon & Winter, 2000; Ma & Heavens, 2001; Shughrue et al., 2003). Using an in vitro spinal cord preparation, Pesquero et al. (2000) have shown that B1 receptor stimulation increases the C fibre component, but not the Aβ fibre-component, of the ventral root potential (VRP) produced by electrical excitation of naïve mouse dorsal root. This indicates that the B1 receptor functions specifically in nociceptive synaptic pathways and that it may be involved with some forms of central sensitization. However, B1 receptor activation is not able to produce direct ongoing activation of VRP in rats (Dunn & Rang, 1990). Thus, it is possible to suggest that B1 receptor activity is not sufficient for the depolarization of unstimulated spinal cord, but that it produces excitation only following C-fibre activation. In fact, intrathecal administration of B1 receptor agonists induces hyperalgesia after peripheral thermal or mechanical stimulation in mice and rats (Ferreira et al., 2002; Fox et al., 2003). In addition, the B1 receptor seems to be involved in the late component of the hyperalgesia induced by bradykinin, a potent activator of C fibres (Ferreira et al., 2002; Sot et al., 2002). Moreover, repetitive electrical stimulation of the dorsal root produces an increase in the VRP, a use-dependent facilitation plasticity of the spinal cord neurones named wind-up. Interestingly, in an experiment on B1 receptor knockout mice conducted by Pesquero et al. (2000), wind-up was significantly reduced (by about 50%) in comparison with the wild-type littermates. These data indicate that the nociceptive impairment observed in knockout B1 receptor mice might be attributed, at least in part, to a deficit in the pathological plasticity of the spinal neurones. Indeed, it has been shown that spinal B1 receptor activation greatly contributes to the inflammatory phase of formalin-induced pain and to the chronic inflammatory pain caused by CFA or sciatic nerve injury in mice and rats (McNair et al., 2001; Ferreira et al., 2002; Fox et al., 2003). Moreover, intrathecal administration of B1 receptor agonist produces thermal hyperalgesia in hyperglycaemic rats (Couture et al., 2001). The data described above strongly supports the notion that the spinal cord represents a relevant site of action for kinins acting at B1 receptors to produce nociception in both acute and chronic painful processes.
Possible role of B1 receptors expressed in nonsensory neurons in pain production
Apart from their expression in DRG neurones, B1 receptors might be expressed and induced in other cells that are involved in pain production, especially in chronic situations. For example, B1 receptor immunoreactivity has been found in non-neuronal DRG satellite cells after nerve injury in mice (Rashid et al., 2004). Satellite cells (such as fibroblast-like cells and Schwann cells) are closely associated with neurons and may regulate the function of nociceptors (Heblich et al., 2001). However, the role of B1 receptors in these cells continues to be elusive.
Some painful processes are mediated by sympathetic activity (Woolf & Mannion, 1999). Interestingly, functional B1 receptors are expressed in sympathetic ganglia, since their activation is able to depolarize superior cervical ganglia neurones in vitro (Seabrook et al., 1995; 1997). Postganglionic sympathetic terminals have been demonstrated to be involved in B1 receptor agonist-induced hyperalgesia (Khasar et al., 1995). Thus, sympathetic fibres seem to be important to the nociceptive action of B1 receptor agonists. Glial cells are also involved in pain production, as their activation results in the release of several pronociceptive mediators (including prostaglandins, glutamate and cytokines) (Watkins & Maier, 2003). Furthermore, B1 receptor upregulation has been reported in rat primary cultured microglia after BK stimulation (Noda et al., 2003). Moreover, the B1 receptor agonist des-Arg9-BK elicits outward membrane current and increases in intracellular calcium in cultured rat astrocytes (Gimpl et al., 1992). Apart from its participation in nociception, the role of glial cell activation by B1 receptors during painful processes is so far unknown.
Concluding remarks
In the present review, we have described the main advances in the involvement of kinin B1 receptors in painful and inflammatory processes. The most recent studies are supported by evidence obtained with selective B1 receptor agonists or antagonists in various distinct models of pain and inflammation. Nevertheless, greater progress achieved in relation to B1 receptors implies a better understanding of the mechanisms related to their induction. As discussed previously, although pharmacological data suggested early on that B1 receptors were inducible molecules, only recently has this hypothesis been confirmed by molecular biology studies. In fact, combined functional and molecular studies have permitted a step forward in identifying the signalling pathways implicated in the upregulation of B1 receptors. These studies have demonstrated that B1 receptor induction is the result of a strictly regulated cascade of events which involves an interaction among transcriptional factors, protein kinases, cytokines and inflammatory/neuronal cells. Understanding these pathways brings new possibilities for the control of B1 receptor expression during pathological states. In addition, the efforts to generate mice lacking B1 receptor gene, together with the recent development of selective peptidic and nonpeptidic B1 receptor antagonists, has opened up an important option for the development of clinically relevant drugs for the treatment of inflammatory and painful conditions.
With basis on the literature data presented in this review, it is clear that B1 receptors occupy a relevant place in the inflammatory scenario. In addition, without doubt, B1 receptors seem to be deeply involved in both acute and chronic nociceptive processes. Given the large number of diseases in which B1 receptors are expected to be involved, there is much hope that orally active and selective B1 receptor antagonists could be clinically tested and proved useful in the management of important pathologies, especially in relation to inflammation and pain. In spite of that, the real effectiveness of selective kinin B1 receptors antagonists for treating inflammatory/nocipetive alterations remains to be investigated by future studies.
Acknowledgments
R.M. and J.F. are Ph.D. students holding grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). E.S.F. is a Ph.D. student receiving a grant from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) D.A.C. and M.M.C. are holders of post-doctoral fellowships from CNPq and CAPES, respectively. Works from our laboratory were supported by grants of CNPq, CAPES and PRONEX.
Abbreviations
- AP-1
activating protein-1
- AKT/PKB
protein kinase B
- BCG
Mycobacterium bovis bacillus Calmette – Guerin
- BK
bradykinin
- CFA
complete Freund's adjuvant
- COX-2
cyclooxygenase-2
- CREB
c-AMP response element-binding
- DRG
dorsal root ganglia
- ERK
extracellular signal-regulated kinase
- IL
interleukin
- JNK
c-Jun NH2-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- MEKK
mitogen-activated protein kinase kinase kinase
- iNOS
inducible nitric oxide synthase
- NF-κB
nuclear factor-κB
- OVA
ovalbumin
- PAF
platelet-activating factor
- PI3-K
phosphatydilinositol-3 kinase
- PLC
phospholipase C
- PKC
protein kinase C
- STZ
streptozotocin
- TLR
Toll-like receptors
- TNFα
tumour necrosis factor α
- VRP
ventral root potential
References
- ABDOUH M., KHANJARI A., ABDELAZZIZ N., ONGALI B., COUTURE R., HASSÉSSIAN H.M. Early upregulation of kinin B1 receptors in retinal microvessels of the streptozotocin-diabetic rats. Br. J. Pharmacol. 2003;140:33–40. doi: 10.1038/sj.bjp.0705210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHLUWALIA A., PERRETTI M. B1 receptors as a new inflammatory target. Could this B the 1. Trends Pharmacol. Sci. 1999;20:100–104. [Google Scholar]
- AKIRA S., YAMAMOTO M., TAKEDA K. Role of adapters in Toll-like receptor signalling. Biochem. Soc. Trans. 2003;31:637–642. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- ANGERS M., DROUIN R., BACHVAROVA M., PARADIS I., MARCEAU F., BACHVAROV D.R. In vivo protein-DNA interactions at the kinin B1 receptor gene promoter: no modification on interleukin-1 beta or lypopolysaccharide induction. J. Cell. Biochem. 2000;78:278–296. doi: 10.1002/(sici)1097-4644(20000801)78:2<278::aid-jcb10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- BACHAR O., ADNER M., UDDMAN R., CARDELL L.O. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, an effect mediated by JNK and NF-kappaB signaling pathways. Eur. J. Immunol. 2004;34:1196–1207. doi: 10.1002/eji.200324569. [DOI] [PubMed] [Google Scholar]
- BACHVAROV D.R., HESS J.F., MENKE J.G., LARRIVEE J.F., MARCEAU F. Structure and genomic organization of the human B1 receptor gene for kinins (BDKRB1) Genomics. 1996;33:374–381. doi: 10.1006/geno.1996.0213. [DOI] [PubMed] [Google Scholar]
- BARKI-HARRINGTON L., BOOKOUT A.L., WANG G., LAMB M.E., LEEB-LUNDBERG L.M., DAAKA Y. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem. J. 2003;371:581–587. doi: 10.1042/BJ20021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEG A.A. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- BEIRITH A., SANTOS A.R., CALIXTO J.B. The role of neuropeptides and capsaicin-sensitive fibres in glutamate-induced nociception and paw oedema in mice. Brain Res. 2003;969:110–116. doi: 10.1016/s0006-8993(03)02286-8. [DOI] [PubMed] [Google Scholar]
- BÉLICHARD P., LANDRY M., FAYE P., BACHVAROV D.R., BOUTHILLIER J., PRUNEAU D., MARCEAU F. Inflammatory hyperalgesia induced by zymosan in the plantar tissue of the rat: effect of kinin receptor antagonists. Immunopharmacology. 2000;46:139–147. doi: 10.1016/s0162-3109(99)00165-4. [DOI] [PubMed] [Google Scholar]
- BÉLICHARD P., LUCCARINI JM., DEFRENE E., FAYE P., FRANCK R.M., DUCOS H., PAQUET J.L., PRUNEAU D. Pharmacological and molecular evidence for kinin expression in urinary bladder of cyclophosphamide-treated rats. Br. J. Pharmacol. 1999;128:213–219. doi: 10.1038/sj.bjp.0702769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHOOLA K.D., RAMSAROOP R., PLENDL J., CASSIM B., DLAMINI Z., NAICKER S. Kallikrein and kinin receptor expression in inflammation and cancer. Biol. Chem. 2001;382:77–89. doi: 10.1515/BC.2001.013. [DOI] [PubMed] [Google Scholar]
- BOCK M.G., LONGMORE J. Bradykinin antagonists: new opportunities. Curr. Opin. Chem. Biol. 2000;4:401–406. doi: 10.1016/s1367-5931(00)00107-1. [DOI] [PubMed] [Google Scholar]
- BORKOWSKI J.A., RANSOM R.W., SEABROOK G.R., TRUMBAUER M., CHEN H., HILL R.G., STRADER C.D., HESS J.F. Targeted disruption of a B2 bradykinin receptor gene in mice eliminates bradykinin action in smooth muscle and neurons. J. Biol. Chem. 1995;270:13706–13710. doi: 10.1074/jbc.270.23.13706. [DOI] [PubMed] [Google Scholar]
- BRAND M., KLUSCH A., KURZAI O., VALDEOLMILLOS M., SCHMIDT R.F., PETERSEN M. No evidence for bradykinin B1 receptors in rat dorsal root ganglion neurons. NeuroReport. 2001;12:3165–3168. doi: 10.1097/00001756-200110080-00036. [DOI] [PubMed] [Google Scholar]
- CABRINI D.A., CALIXTO J.B. Characterization of des-Arg9-bradykinin-induced contraction in guinea-pig gallbladder in vitro. Eur. J. Pharmacol. 1997;331:31–38. doi: 10.1016/s0014-2999(97)01010-8. [DOI] [PubMed] [Google Scholar]
- CABRINI D.A., CAMPOS M.M., TRATSK K.S., MERINO V.F., SILVA J.A., JR, SOUZA G.E., AVELLAR M.C., PESQUERO J.B., CALIXTO J.B. Molecular and pharmacological evidence for modulation of kinin B1 receptor expression by endogenous glucocorticoids hormones in rats. Br. J. Pharmacol. 2001;132:567–577. doi: 10.1038/sj.bjp.0703846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Inflammatory pain: kinins and antagonists. Curr. Opin. Anaesthesiol. 2001;14:519–526. doi: 10.1097/00001503-200110000-00010. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., CABRINI D.A., CARDOZO A.H., RAE G.A., TORO J.H., CALIXTO J.B. Changes in paw oedema triggered via bradykinin B(1) and B(2) receptors in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2001;416:169–177. doi: 10.1016/s0014-2999(01)00883-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., CALIXTO J.B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br. J. Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., MATA L.V., CALIXTO J.B. Expression of B1 kinin receptors mediating paw edema and formalin-induced nociception. Modulation by glucocorticoids. Can. J. Physiol. Pharmacol. 1995;73:812–819. doi: 10.1139/y95-110. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E., CALIXTO J.B. Upregulation of B1 receptor mediating des-Arg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br. J. Pharmacol. 1996;117:793–798. doi: 10.1111/j.1476-5381.1996.tb15262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E., CALIXTO J.B. Modulation of kinin B1 but not B2 receptors-mediated rat paw edema by IL-1beta and TNFalpha. Peptides. 1998;19:1269–1276. doi: 10.1016/s0196-9781(98)00087-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E., CALIXTO J.B. In vivo B1 kinin-receptor upregulation. Evidence for involvement of protein kinases and nuclear factor kappaB pathways. Br. J. Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E., RICCI N.D., PESQUERO J.L., TEIXEIRA M.M., CALIXTO J.B. The role of migrating leukocytes in IL-1 beta-induced up-regulation of kinin B1 receptors in rats. Br. J. Pharmacol. 2002;135:1107–1114. doi: 10.1038/sj.bjp.0704488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAYLA C., MERINO V.F., CABRINI D.A., SILVA J.A., JR, PESQUERO J.B., BADER M. Structure of the mammalian kinin receptor gene locus. Int. Immunopharmacol. 2002;2:1721–1727. doi: 10.1016/s1567-5769(02)00175-3. [DOI] [PubMed] [Google Scholar]
- CHAI K.X., NI A., WANG D., WARD D.C., CHAO J., CHAO L. Genomic DNA sequence, expression, and chromosomal localization of the human B1 bradykinin receptor gene BDKRB1. Genomics. 1996;31:51–57. doi: 10.1006/geno.1996.0008. [DOI] [PubMed] [Google Scholar]
- CHICHORRO J.G., LORENZETTI B.B., ZAMPRONIO A.R. Involvement of bradykinin, cytokines, sympathetic amines and prostaglandins in formalin-induced orofacial nociception in rats. Br. J. Pharmacol. 2004;141:1175–1184. doi: 10.1038/sj.bjp.0705724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN S.C., EDDLESTON J., WOESSNER K.M., CHAMBERS S.S., YE R., PAN Z.K., ZURAW B.L. Up-regulation of functional kinin B1 receptors in allergic airway inflammation. J. Immunol. 2002;169:2054–2060. doi: 10.4049/jimmunol.169.4.2054. [DOI] [PubMed] [Google Scholar]
- CLARK A.R., DEAN J.L., SAKLATVALA J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546:37–44. doi: 10.1016/s0014-5793(03)00439-3. [DOI] [PubMed] [Google Scholar]
- CORRÊA C.R., CALIXTO J.B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br. J. Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTURE R., LINDSEY C.J.Brain kallikrein–kinin system: from receptors to neuronal pathways and physiological functions Handbook of Chemical Anatomy: Peptide Receptors 2000The Netherlands: Elsevier; 241–298.ed. QUIRION, R., BJ, A. & H, T. pp [Google Scholar]
- COUTURE R., HARRISSON M., VIANNA R.M., CLOUTIER F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- DAVIS A.J., PERKINS M.N. Induction of B1 receptors in vivo in a model of persistent inflammatory mechanical hyperalgesia in the rat. Neuropharmacology. 1994;33:127–133. doi: 10.1016/0028-3908(94)90107-4. [DOI] [PubMed] [Google Scholar]
- DAVIS C.J., NAEEM S., PHAGOO S.B., CAMPBELL E.A., URBAN L., BURGESS G.M. B1 bradykinin receptors and sensory neurones. Br. J. Pharmacol. 1996;118:1469–1476. doi: 10.1111/j.1476-5381.1996.tb15562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CAMPOS R.O., HENRIQUES M.G., CALIXTO J.B. Systemic treatment with Mycobacterium bovis bacillus Calmette–Guerin (BCG) potentiates kinin B1 receptor agonist-induced nociception and oedema formation in the formalin test in mice. Neuropeptides. 1998;32:393–403. doi: 10.1016/s0143-4179(98)90062-2. [DOI] [PubMed] [Google Scholar]
- DEBLOIS D., HORLICK R.A. Endotoxin sensitization to kinin B1 receptor agonist in a non-human primate model: haemodynamic and pro-inflammatory effects. Br. J. Pharmacol. 2001;132:327–335. doi: 10.1038/sj.bjp.0703748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON D.A. Dysregulated post-transcriptional control of COX-2 gene expression in cancer. Curr. Pharm. Des. 2004;10:635–646. doi: 10.2174/1381612043453171. [DOI] [PubMed] [Google Scholar]
- DIXON D.A., KAPLAN C.D., MCINTYRE T.M., ZIMMERMAN G.A., PRESCOTT S.M. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J. Biol. Chem. 2000;275:11750–11757. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- DOBROVOLSKAIA M.A., VOGEL S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- DONALDSON L.F., HANLEY M.R., VILLABRANCA A.C. Inducible receptors. Trends Pharmacol. Sci. 1997;18:171–181. doi: 10.1016/s0165-6147(97)01048-1. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G., DEBLOIS D., MARCEAU F. Hypotensive effects of Lys-des-Arg9-Bradykinin and metabolically protected agonists of b1 receptors for kinins. J. Pharmacol. Exp. Ther. 1991;259:997–1003. [PubMed] [Google Scholar]
- DRAY A., PATEL I.A., PERKINS M.N., RUEFF A. Bradykinin-induced activation of nociceptors: receptor and mechanistic studies on the neonatal rat spinal cord-tail preparation in vitro. Br. J. Pharmacol. 1992;107:1129–1134. doi: 10.1111/j.1476-5381.1992.tb13418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAY A., PERKINS M.Kinins and pain The Handbook of Immunopharmacology: the Kinin System 1997London: Academic Press; 157–172.ed. Farmer, S.G. pp [Google Scholar]
- DUNN P.M., RANG H.P. Bradykinin-induced depolarization of primary afferent terminals in the neonatal rat spinal cord in vitro. Br. J. Pharmacol. 1990;100:656–660. doi: 10.1111/j.1476-5381.1990.tb15863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN C., WILTSHIRE C., MACLAREN A., GILLESPIE D.A. Molecular mechanism and biological functions of c-Jun N-terminal kinase signalling via the c-Jun transcription factor. Cell. Signal. 2002;14:585–593. doi: 10.1016/s0898-6568(01)00275-3. [DOI] [PubMed] [Google Scholar]
- ECKERT A., VON BANCHET G.S., SOPPER S., PETERSEN M. Spatio-temporal pattern of induction of bradykinin receptors and inflammation in rat dorsal root ganglia after unilateral nerve ligation. Pain. 1999;83:487–497. doi: 10.1016/S0304-3959(99)00152-9. [DOI] [PubMed] [Google Scholar]
- ERIC J., BKAILY G., BKAILY G.B., VOLKOV L., GABRA B.H., SIROIS P. Des-Arg9-bradykinin increases intracellular Ca2+ in bronchoalveolar eosinophils from ovalbumin-sensitized and -challenged mice. Eur. J. Pharmacol. 2003;475:129–137. doi: 10.1016/s0014-2999(03)02108-3. [DOI] [PubMed] [Google Scholar]
- FATHY D.B., KYLE D.J., LEEB-LUNDBERG L.M. High-affinity binding of peptide agonists to the human B1 bradykinin receptor depends on interaction between the peptide N-terminal L-lysine and the fourth extracellular domain of the receptor. Mol. Pharmacol. 2000;57:171–179. [PubMed] [Google Scholar]
- FAUSSNER A., BATHON J.M., PROUD D. Comparison of the responses of B1 and B2 kinin receptors to agonist stimulation. Immunopharmacology. 1999;45:13–20. doi: 10.1016/s0162-3109(99)00052-1. [DOI] [PubMed] [Google Scholar]
- FERNANDES E.S., PASSOS G.F., CAMPOS M.M., ARAÚJO J.G., PESQUERO J.L., AVELLLAR M.C., TEIXEIRA M.M., CALIXTO J.B. Mechanisms underlying the modulatory action of platelet activating factor (PAF) on the upregulation of kinin B1 receptors in the rat paw. Br. J. Pharmacol. 2003;139:973–981. doi: 10.1038/sj.bjp.0705314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA J., CAMPOS M.M., ARAÚJO R., BADER M., PESQUERO J.B., CALIXTO J.B. The use of kinin B1 and B2 receptor knockout mice and selective antagonists to characterize the nociceptive responses caused by kinins at the spinal cord. Neuropharmacology. 2002;43:1188–1197. doi: 10.1016/s0028-3908(02)00311-8. [DOI] [PubMed] [Google Scholar]
- FERREIRA J., CAMPOS M.M., PESQUERO J.B., ARAÚJO R.C., BADER M., CALIXTO J.B. Evidence for the participation of kinins in Freund's adjuvant-induced inflammatory and nociceptive responses in kinin B1 and B2 receptor knockout mice. Neuropharmacology. 2001;41:1006–1012. doi: 10.1016/s0028-3908(01)00142-3. [DOI] [PubMed] [Google Scholar]
- FERREIRA J., SILVA G.L., CALIXTO J.B. Involvement of vanilloid receptors in the overt nociception induced by B2 kinin receptor activation in mice. Br. J. Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX A., WOTHERSPOON G., MCNAIR K., HUDSON L., PATEL S., GENTRY C., WINTER J. Regulation and function of spinal and peripheral neuronal B1 bradykinin receptors in inflammatory mechanical hyperalgesia. Pain. 2003;104:683–691. doi: 10.1016/S0304-3959(03)00141-6. [DOI] [PubMed] [Google Scholar]
- GABRA B.H., SIROIS P. Role of bradykinin B1 receptors in diabetes-induced hyperalgesia in streptozotocin-treated mice. Eur. J. Pharmacol. 2002;457:115–124. doi: 10.1016/s0014-2999(02)02658-4. [DOI] [PubMed] [Google Scholar]
- GABRA B.H., SIROIS P. Kinin B1 receptor antagonists inhibit diabetes-induced hyperalgesia in mice. Neuropeptides. 2003a;37:36–44. doi: 10.1016/s0143-4179(02)00148-8. [DOI] [PubMed] [Google Scholar]
- GABRA B.H., SIROIS P. Beneficial effect of chronic treatment with the selective bradykinin B1 receptor antagonists, R-715 and R-954, in attenuating streptozotocin-diabetic thermal hyperalgesia in mice. Peptides. 2003b;24:1131–1139. doi: 10.1016/j.peptides.2003.06.003. [DOI] [PubMed] [Google Scholar]
- GANJU P., DAVIS A., PATEL S., NUNEZ X., FOX A. p38 stress-activated protein kinase inhibitor reverses bradykinin B(1) receptor-mediated component of inflammatory hyperalgesia. Eur. J. Pharmacol. 2001;421:191–199. doi: 10.1016/s0014-2999(01)01048-2. [DOI] [PubMed] [Google Scholar]
- GARCIA LEME J., HAMAMURA L., MIGLIORINI R.H., LEITE M.P. Experimental diabetes and inflammatory reactions in the rat. Agents Actions. 1973;3:380–381. doi: 10.1007/BF01986499. [DOI] [PubMed] [Google Scholar]
- GIMPL G., WALZ W., OHLEMEYER C., KETTENMANN H. Bradykinin receptors in cultures astrocytes from neonatal brain are linked to physiological responses. Neurosci. Lett. 1992;144:139–142. doi: 10.1016/0304-3940(92)90735-p. [DOI] [PubMed] [Google Scholar]
- GOBEIL F., NEUGEBAUER W., FILTEAU C., JUKIC D., ALLOGHO S.N., PHENG L.H., NGUYEN-LE X.K., BLOUIN D., REGOLI D. Structure–activity studies of B1 receptor-related peptides. Antagonists Hypertension. 1996;28:833–839. doi: 10.1161/01.hyp.28.5.833. [DOI] [PubMed] [Google Scholar]
- GOBEIL F., NEUGBAUER W., NGUEYEN-LE X.K., NSA ALLOGHO S., PHENG L.H., BLOUIN D., WHALLEY E.T., REGOLI D. Pharmacological profiles of the human and rabbit B1 receptors. Can. J. Physiol. Pharmacol. 1997;75:591–595. [PubMed] [Google Scholar]
- GOUGAT J., FERRARI B., SARRAN L., PLANCHENAULT C., PONCELET M., MARUANI J., ALONSO R., CUDENNEC A., CROCI T., GUAGNINI F., URBAN-SZABO K., MARTINOLLE J.P., SOUBRIE P., FINANCE O., LE FUR G. SSR240612 [(2R)- 2-[((3R)- 3-(1,3-benzodioxol-5-yl)- 3-{[(6- methoxy-2-naphthyl) sulfonyl] amino} propanoyl) amino]-3- (4-{[2R,6S)-2,6-dimethylpiperidinyl] methyl}phenyl)-N-isopropyl-N-methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin B1 receptor: Biochemical and pharmacological characterization. J. Pharmacol. Exp. Ther. 2004;309:661–669. doi: 10.1124/jpet.103.059527. [DOI] [PubMed] [Google Scholar]
- HADDAD E.B., FOX A.J., ROUSELL J., BURGESS G., MCINTYRE P., BARNES P.J., CHUNG K.F. Post-transcriptional regulation of bradykinin B1 and B2 receptor gene expression in human lung fibroblasts by tumor necrosis factor-alpha: modulation by dexamethasone. Mol. Pharmacol. 2000;57:1123–1131. [PubMed] [Google Scholar]
- HAYASHI I., AMANO H., ISHIHARA K., KUMAGAI Y., YOSHIMURA H., MAJIMA M. The role of kinin B1 in the plasma extravasation of carragenin-induced pleurisy. Life Sci. 2002;70:937–949. doi: 10.1016/s0024-3205(01)01460-6. [DOI] [PubMed] [Google Scholar]
- HAYASHI R., YAMASHITA N., MATSUI S., MARUYAMA M., SUGIYAMA E., SUGIYAMA S. Bradykinin stimulates interleukin-8 production by human lung fibroblasts. Immunology. 1998;95:507–511. doi: 10.1046/j.1365-2567.1998.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBLICH F., ENGLAND S., DOCHERTY R.J. Indirect actions of bradykinin on neonatal rat dorsal root ganglion neurones: a role for non-neuronal cells as nociceptors. J. Physiol. 2001;536:111–121. doi: 10.1111/j.1469-7793.2001.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS J.F., HEY P.J., CHEN T.B., O'BRIEN J., OMALLEY S.S., PETTIBONE D.J., CHANG R.S. Molecular cloning and pharmacological characterization of the canine B1 and B2 bradykinin receptors. Biol. Chem. 2001;382:123–129. doi: 10.1515/BC.2001.018. [DOI] [PubMed] [Google Scholar]
- HESS J.F., HEY P.J., CHEN T.B., PETTIBONE D.J., CHANG R.S. Molecular and pharmacological diversity of the kinin B1 receptor. Int. Immunopharmacol. 2002;2:1747–1754. doi: 10.1016/s1567-5769(02)00147-9. [DOI] [PubMed] [Google Scholar]
- HOHMEIER H.E., TRAN V.V., CHEN G., GASA R., NEWGARD C.B. Inflammatory mechanisms in diabetes; lessons form the beta-cell. Int. J. Obes. Relat. Metab. Disord. 2003;27:S12–S16. doi: 10.1038/sj.ijo.0802493. [DOI] [PubMed] [Google Scholar]
- HUANG T.J., HADDAD E.B., FOX A.J., SALMON M., JONES C., BURGESS G., CHUNG K.F. Contribution of bradykinin B1 and B2 receptors in allergen-induced bronchial hyperresponsiveness. Am. J. Respir. Crit. Care Med. 1999;160:1717–1723. doi: 10.1164/ajrccm.160.5.9901029. [DOI] [PubMed] [Google Scholar]
- HUNT S.P., MANTYH P.W. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- HUNTER T. Protein kinases and phosphatases: the Yin and Yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- ISHIHARA K., HAYASH I., YAMASHINA S., MAJIMA M. A potential role of bradykinin in angiogenesis and growth of S-180 mouse tumors. Jpn. J. Pharmacol. 2001;87:318–326. doi: 10.1254/jjp.87.318. [DOI] [PubMed] [Google Scholar]
- ISHIHARA K., KAMATA M., HAYASHI I., YAMASHINA S., MAJIMA M. Roles of bradykinin in vascular permeability and angiogenesis in solid tumor. Int. Immunopharmacol. 2002;2:499–509. doi: 10.1016/s1567-5769(01)00193-x. [DOI] [PubMed] [Google Scholar]
- JONES W.K., BROWN M., REN X., HE S., MCGUINNESS M. NF-kappaB as an integrator of diverse signaling pathways: the heart of myocardial signaling. Cardiovasc. Toxicol. 2003;3:229–254. doi: 10.1385/ct:3:3:229. [DOI] [PubMed] [Google Scholar]
- JULIUS D., BASBAUM A.I. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- KHASAR S.G., MIAO F.J., LEVINE J.D. Inflammation modulates the contribution of receptor-subtypes to bradykinin-induced hyperalgesia in the rat. Neuroscience. 1995;69:685–690. doi: 10.1016/0306-4522(95)00280-v. [DOI] [PubMed] [Google Scholar]
- KUEBLER J.F., SCHREMMER-DANNINGER E., BHOOLA K.D., ROSCHER A.A., MESSMER K., HOFFMANN T.F. Kinin-B1 receptors in ischaemia-induced pancreatitis: functional importance and cellular localisation. Biol. Chem. 2003;384:1311–1319. doi: 10.1515/BC.2003.147. [DOI] [PubMed] [Google Scholar]
- LAGNEUX C., BADER M., PESQUERO J.B., DEMENGE P., RIBUOT C. Detrimental implication of B1 receptors in myocardial ischemia: evidence from pharmacological blockade and gene knockout mice. Int. Immunopharmacol. 2002;2:815–822. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- LAHTI A., JALONEN U., KANKAANRANTA H., MOILANEN E. c-Jun NH2-terminal kinase inhibitor anthra(1,9-cd)pyrazol-6(2H)-one reduces inducible nitric-oxide synthase expression by destabilizing mRNA in activated macrophages. Mol. Pharmacol. 2003;64:308–315. doi: 10.1124/mol.64.2.308. [DOI] [PubMed] [Google Scholar]
- LANDGRAF R.G., JANCAR S., STEIL A.A., SIROIS P. Modulation of allergic and immune complex-induced lung inflammation by bradykinin receptor antagonists. Inflamm. Res. 2004;53:78–83. doi: 10.1007/s00011-003-1226-0. [DOI] [PubMed] [Google Scholar]
- LANDGRAF R.G., SIROIS P., JANCAR S. Differential modulation of murine lung inflammation by bradykinin B1 and B2 selective receptor antagonists. Eur. J. Pharmacol. 2003;460:75–83. doi: 10.1016/s0014-2999(02)02880-7. [DOI] [PubMed] [Google Scholar]
- LARRIVÉE J.-F., BACHVAROV D.R., HOULE F., LANDRY J., HUOT J., MARCEAU F. Role of the mitogen-activated protein kinases in the expression of the kinin B1 receptors induced by tissue injury. J. Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- LARRIVEÉ J.F., GERA L., HOULE S., BOUTHILLIER J., BACHVAROV D.R., STEWART J.M., MARCEAU F. Non-competitive pharmacological antagonism at the rabbit B1 receptor. Br. J. Pharmacol. 2000;131:885–892. doi: 10.1038/sj.bjp.0703656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASA M., BROOK M., SAKLATVALA J., CLARK A.R. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol. 2001;21:771–780. doi: 10.1128/MCB.21.3.771-780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVICH T.R., CORDEIRO R.S., CALIXTO J.B., SILVA P.M., MARTINS M.A. Combined action of vasoactive amines and bradykinin mediates allergen-evoked thermal hyperalgesia in rats. Eur. J. Pharmacol. 2003;462:185–192. doi: 10.1016/s0014-2999(02)02947-3. [DOI] [PubMed] [Google Scholar]
- LEVY D., ZOCHODNE D.W. Increased mRNA expression of the B1 and B2 bradykinin receptors and antinociceptive effects of their antagonists in an animal model of neuropathic pain. Pain. 2000;86:265–271. doi: 10.1016/S0304-3959(00)00256-6. [DOI] [PubMed] [Google Scholar]
- LEWIS G.P. Plasma kinins and inflammation. Metabolism. 1964;13:1256–1263. doi: 10.1016/s0026-0495(64)80042-1. [DOI] [PubMed] [Google Scholar]
- LIEN Y.H., LAI L.W., SILVA A.L. Pathogenesis of renal ischemia/reperfusion injury: lessons from knockout mice. Life Sci. 2003;74:543–552. doi: 10.1016/j.lfs.2003.08.001. [DOI] [PubMed] [Google Scholar]
- MA Q.-P. The expression of bradykinin B1 receptors on primary sensory neurones that give rise to small calibre sciatic nerve fibres in rats. Neuroscience. 2001;107:665–673. doi: 10.1016/s0306-4522(01)00387-6. [DOI] [PubMed] [Google Scholar]
- MA Q.-P., HEAVENS R. Basal expression of bradykinin B(1) receptor in the spinal cord in humans and rats. NeuroReport. 2001;12:2311–2314. doi: 10.1097/00001756-200108080-00006. [DOI] [PubMed] [Google Scholar]
- MA Q.-P., HILL R., SIRINATHSINGHJI D. Basal expression of bradykinin B1 receptor in peripheral sensory ganglia in the rat. NeuroReport. 2000;11:4003–4005. doi: 10.1097/00001756-200012180-00020. [DOI] [PubMed] [Google Scholar]
- MACNEIL T., BIERILO K.K., MENKE J.G., HESS J.F. Cloning and pharmacological characterization of a rabbit bradykinin B1 receptor. Biochim. Biophys. Acta. 1995;1264:223–228. doi: 10.1016/0167-4781(95)00152-7. [DOI] [PubMed] [Google Scholar]
- MAHABEER R., BHOOLA K.D. Kallikrein and kinin receptor genes. Pharmacol. Ther. 2000;88:77–89. doi: 10.1016/s0163-7258(00)00080-2. [DOI] [PubMed] [Google Scholar]
- MARCEAU F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MARCEAU F., SABOURIN T., HOULE S., FORTIN J.P., PETITCLERC E., MOLINARO G., ADAM A. Kinin receptors: functional aspects. Int. Immunopharmacol. 2002;2:1729–1739. doi: 10.1016/s1567-5769(02)00189-3. [DOI] [PubMed] [Google Scholar]
- MASON G.S., CUMBERBATCH M.J., HILL R.G., RUPNIAK N.M.J. The bradykinin B1 receptor antagonist B9858 inhibits a nociceptive spinal reflex in rabbits. Can. J. Physiol. Pharmacol. 2002;80:264–268. doi: 10.1139/y02-049. [DOI] [PubMed] [Google Scholar]
- MAZENOT C., LOUFRANI L., HENRION D., RIBUOT C., MULLER-ESTERL W., GODIN-RIBUOT D. Endothelial kinin B1-receptors are induced by myocardial ischaemia–reperfusion in the rabbit. J. Physiol. 2001;530:69–78. doi: 10.1111/j.1469-7793.2001.0069m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCEACHERN A.E., SHELTON E.R., BHAJTA S., OBERNOLT R., BACH C., ZUPPAN P., FUJISAKI J., ALDRISH R.W., JARNAGIN K. Expression cloning of rat B2 bradykinin receptor. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P.G., PERRETTI M., AHLUWALIA A. Inducible expression of the kinin B1 receptor in the endotoxemic heart: mechanisms of des-Arg9-bradykinin-induced coronary vasodilation. Br. J. Pharmacol. 1999;128:275–282. doi: 10.1038/sj.bjp.0702743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAIR K., KESINGLAND A., URBAN L., FOX A. The role of central and peripheral bradykinin B1 receptors in models of neuropathic and inflammatory pain in the rat. Br. J.Pharmacol. 2001;134:p158. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- MEDEIROS R., CABRINI D.A., CALIXTO J.B. The in vivo and ex vivo roles of cyclooxygenase-2, nuclear factor-κB and protein kinases pathways in the up-regulation of B1 receptor mediated contraction of the rabbit aorta. Regul. Pept. 2001;97:121–130. doi: 10.1016/s0167-0115(00)00186-5. [DOI] [PubMed] [Google Scholar]
- MEDEIROS R., CABRINI D.A., FERREIRA J., FERNANDES E.S., MORI M.A., PESQUERO J.B., BADER M., AVELLAR M.C., CAMPOS M.M., CALIXTO J.B. Bradykinin B1 receptor expression induced by tissue damage in the rat portal vein. A critical role for mitogen-activated protein kinase and nuclear factor-κB signaling pathways. Circ. Res. 2004;94:1375–1382. doi: 10.1161/01.RES.0000128404.65887.08. [DOI] [PubMed] [Google Scholar]
- MENKE J.G., BORKOWSKI J.A., BIERILO K.K., MACNEIL T., DERRICK A.W., SCHNECK K.A., RANSOM R.W., STRADER C.D., LINEMEYER D.L., HESS J.F. Expression cloning of a human B1 bradykinin receptor. J. Biol. Chem. 1994;269:21583–21586. [PubMed] [Google Scholar]
- MODÉER T., ANDUREN I., YUCEL-LINDBERG T. Bradykinin synergistically stimulates interleukin 6 production in human gingival fibroblasts challenged with interleukin 1 or tumour necrosis factor alpha. Cytokine. 1998;10:26–31. doi: 10.1006/cyto.1997.0255. [DOI] [PubMed] [Google Scholar]
- NEUGEBAUER W., BLAIS P.A., HALLE S., FILTEAU C., REGOLI D., GOBEIL F., JR Kinin B1 receptor antagonists with multi-enzymatic resistance properties. Can. J. Physiol. Pharmacol. 2002;80:287–292. doi: 10.1139/y02-053. [DOI] [PubMed] [Google Scholar]
- NEWTON R., EDDLESTON J., HADDAD E.-B., HAWISA S., MAK J., LIM S., FOX A.J., DONNELY L.E., CHUNG K.F. Regulation of kinin receptors in airway epithelial cells by inflammatory cytokines and dexamethasone. Eur. J. Pharmacol. 2002;441:193–202. doi: 10.1016/s0014-2999(01)01624-7. [DOI] [PubMed] [Google Scholar]
- NI A., CHAI K.X., CHAO L., CHAO J. Molecular cloning and expression of rat bradykinin B1 receptor. Biochim. Biophys. Acta. 1998a;1442:177–185. doi: 10.1016/s0167-4781(98)00163-8. [DOI] [PubMed] [Google Scholar]
- NI A., CHAO L., CHAO J. Transcription factor nuclear factor κB regulates the inducible expression of the human B1 receptor gene in inflammation. J. Biol. Chem. 1998b;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- NI A., YIN H., AGATA J., YANG Z., CHAO L., CHAO J. Overexpression of kinin B1 receptors induces hypertensive response to des-Arg9-bradykinin and susceptibility to inflammation. J. Biol. Chem. 2003;278:219–225. doi: 10.1074/jbc.M209490200. [DOI] [PubMed] [Google Scholar]
- NODA M., KARIURA Y., AMANO T., MANAGO Y., NISHIKAWA K., AOKI S., WADA K. Expression and function of bradykinin receptors in microglia. Life Sci. 2003;72:1573–1581. doi: 10.1016/s0024-3205(02)02449-9. [DOI] [PubMed] [Google Scholar]
- ONGALI B., CAMPOS M.M., BREGOLA G., RODI D., REGOLI D., THIBAULT G., SIMONATO M., COUTURE R. Autoradiographic analysis of rat brain kinin B1 and B2 receptors: normal distribution and alterations induced by epilepsy. J. Comp. Neurol. 2003;461:506–519. doi: 10.1002/cne.10706. [DOI] [PubMed] [Google Scholar]
- PAN Z.K., YE R.D., CHRISTIANSEN S.C., JAGELS M.A., BOKOCH G.M., ZURAW B.L. Role of the Rho GTPase in bradykinin-stimulated nuclear factor-κB activation and IL-1β gene expression in cultured human epithelial cells. J. Immunol. 1998;160:3038–3045. [PubMed] [Google Scholar]
- PANESAR M.S., GENTRY C.T., URBAN L., WALKER K., FOX A. A model of persistent, inflammation-induced mechanical hyperalgesia in the mouse. Br. J. Pharmacol. 1998;125 Suppl:122. [Google Scholar]
- PASSOS G.F., FERNANDES E.S., CAMPOS M.M., ARAÚJO J.G., PESQUERO J.L., SOUZA G.E., AVELLAR M.C., TEIXEIRA M.M., CALIXTO J.B. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J. Immunol. 2004;172:1839–1847. doi: 10.4049/jimmunol.172.3.1839. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., KELLY D. Induction of bradykinin-B1 receptors ‘in vivo' in a model of ultra-violet irradiation-induced thermal hyperalgesia in the rat. Br. J. Pharmacol. 1993;110:1441–1444. doi: 10.1111/j.1476-5381.1993.tb13982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS M.N., KELLY D. Interleukin-1β induced-desArg9bradykinin-mediated thermal hyperalgesia in the rat. Neuropharmacology. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., CAMPBELL E., DRAY A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9,[Leu8]-BK and Hoe 140, in two models of persistent hyperalgesia in the rat. Pain. 1993;53:191–197. doi: 10.1016/0304-3959(93)90080-9. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., KELLY D., DAVIS A.J. Bradykinin B1 and B2 receptor mechanisms and cytokine-induced hyperalgesia in the rat. Can. J. Physiol. Pharmacol. 1995;73:832–836. doi: 10.1139/y95-113. [DOI] [PubMed] [Google Scholar]
- PESQUERO J.B., ARAUJO R.C., HEPPENSTALL P.A., STUCKY C.L., SILVA JR., JA, WALTHER T., OLIVEIRA S.M., PESQUERO J.L., PAIVA A.C., CALIXTO J.B., LEWIN G.R., BADER M. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PESQUERO J.B., PESQUERO J.L., OLIVEIRA S.M., ROSCHER A.A., METZGER R., GANTEN D., BADER M. Molecular cloning and functional characterization of a mouse bradykinin B1 receptor gene. Biochem. Biophys. Res. Commun. 1996;224:281. doi: 10.1006/bbrc.1996.0384. [DOI] [PubMed] [Google Scholar]
- PETERSEN M., ECKERT A.S., VON BANCHET G.S., HEPPELMANN B., KLUSCH A., KNIFFKI K.D. Plasticity in the expression of bradykinin binding sites in sensory neurons after mechanical nerve injury. Neuroscience. 1998;83:949–959. doi: 10.1016/s0306-4522(97)00465-x. [DOI] [PubMed] [Google Scholar]
- PHAGOO S.B., POOLE S., LEEB-LUNDBERG L.M. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1beta shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol. Pharmacol. 1999;56:325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- PHAGOO S.B., REDDI K., ANDERSON K.D., LEEB-LUNDBERG L.M., WARBURTON D. Bradykinin B1 receptor up-regulation by interleukin-1β agonist occurs through independent and synergistic intracellular signaling mechanisms in human lung fibroblasts. J. Pharmacol. Exp. Ther. 2001;298:77–85. [PubMed] [Google Scholar]
- PHAGOO S.B., YAQOOB M., HERRERA-MARTINEZ E., MCINTYRE P., JONES C., BURGESS G.M. Regulation of bradykinin receptor gene expression in human lung fibroblasts. Eur. J. Pharmacol. 2000;397:237–246. doi: 10.1016/s0014-2999(00)00323-x. [DOI] [PubMed] [Google Scholar]
- POOLE S., LORENZETTI B.B., CUNHA J.M., CUNHA F.Q., FERREIRA S.H. Bradykinin B1 and B2 receptors, tumour necrosis factor alpha and inflammatory hyperalgesia. Br. J. Pharmacol. 1999;126:649–656. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREMKUMAR L.S., AHERN G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- PROUD D., KAPLAN A.P. Kinin formation: mechanisms and role in inflammatory disorders. Annu. Rev. Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- RAIDOO D.M., SWANT S., MAHABEER R., BHOOLA K.D. Kinin receptors are expressed in human astrocytic tumour cells. Immunopharmacology. 1999;43:255–263. doi: 10.1016/s0162-3109(99)00097-1. [DOI] [PubMed] [Google Scholar]
- RASHID M.H., INOUE M., MATSUMOTO M., UEDA H. Switching of bradykinin-mediated nociception following partial sciatic nerve injury in mice. J. Pharmacol. Exp. Ther. 2004;308:1158–1164. doi: 10.1124/jpet.103.060335. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABE J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J., PARK W.K. Receptors for bradykinin in rabbit aortae. Can. J. Physiol. Pharmacol. 1977;55:855–867. doi: 10.1139/y77-115. [DOI] [PubMed] [Google Scholar]
- REGOLI D., NSA ALLOGHO S., RIZZI A., GOBEIL F.J. Bradykinin receptors and their antagonists. Eur. J. Pharmacol. 1998;348:1–10. doi: 10.1016/s0014-2999(98)00165-4. [DOI] [PubMed] [Google Scholar]
- REGOLI D., RIZZI A., PERRON S.L., GOBEIL F. Classification of kinin receptors. Biol. Chem. 2001;382:31–35. doi: 10.1515/BC.2001.005. [DOI] [PubMed] [Google Scholar]
- RHALEB N.E., ROUISSI N., JUKIC D., REGOLI D., HENKE S., BREIPOHL G., KNOLLE J. Pharmacological characterization of a new potent B2 receptor antagonist (HOE 140): D-Arg[Hyp3, Thi5, D Tic7, Oic8]BK. Eur. J. Pharmacol. 1992;163:263–272. doi: 10.1016/0014-2999(92)90661-m. [DOI] [PubMed] [Google Scholar]
- RUPNIAK N.M.J., BOYCE S., WEBB J.K., WILLIAMS A.R., CARLSON E.J., HILL R.G., BORKOWSKI J.A., HESS J.F. Effects of the bradykinin B1 receptor antagonist des-Arg9-[Leu8]-bradykinin and genetic disruption of the B2 receptor on nociception in rats and mice. Pain. 1997;71:89–97. doi: 10.1016/s0304-3959(97)03343-5. [DOI] [PubMed] [Google Scholar]
- SABOURIN T., MORISSETTE G., BOUTHILLIER J., LEVESQUE L., MARCEAU F. Expression of kinin B1 receptor in fresh or cultured rabbit aortic smooth muscle: role of NF-κB. Am. J. Physiol. Heart. Circ. Physiol. 2002;283:H227–H237. doi: 10.1152/ajpheart.00978.2001. [DOI] [PubMed] [Google Scholar]
- SAINZ I.M., UKNIS A.B., ISORDIA-SALAS I., DELA CADENA R.A., PIXLEY R.A., COLMAN R.W. Interactions between bradykinin (BK) and cell adhesion molecule (CAM) expression in peptidoglycan–polysaccharide (PG-PS)-induced arthritis. FASEB J., Published online March. 2004;4:2004. doi: 10.1096/fj.03-0835fje. [DOI] [PubMed] [Google Scholar]
- SARDI S.P., ERRASTI A.E., REY-ARES V., ROGINES-VELO M.P., ROTHLIN R.P. Bradykinin B1 receptor in isolated human umbilical vein: an experimental model of the in vitro up-regulation. Acta Pharmacol. Sinica. 2000;21:105–110. [PubMed] [Google Scholar]
- SARDI S.P., REY-ARES V., PUJOL-LEREIS V.A., SERRANO S.A., ROTHLIN R.P. Further pharmacological evidence of nuclear factor-κB pathway involvement in bradykinin B1 receptor-sensitized responses in human umbilical vein. J. Pharmacol. Exp. Ther. 2002;301:975–980. doi: 10.1124/jpet.301.3.975. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLÉ E., CASTAÑO M.E.M., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.-P., BASCANDS J.-L. The B1 -agonist [des-Arg10]-kallidin activates transcription factor NF-κB and induces homologous up-regulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARFSTEIN J., SCHMITZ V., MORANDI V., CAPELLA M.M., LIMA A.P., MORROT A., JULIANO L., MULLER-ESTERL W. Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B2 receptors. J. Exp. Med. 2000;192:1289–1300. doi: 10.1084/jem.192.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEABROOK G.R., BOWERY B.J., HEAVENS R., BROWN N., FORD H., SIRINATHSINGHJI D.J.S., BORKOWSKI J.A., HESS J.F., STRADER C.D., HILL R.G. Expression of B1 and B2 bradykinin receptor mRNA and their function roles in sympathetic ganglia and sensory root ganglia neurones from wild-type and B2 receptor knockout mice. Neuropharmacology. 1997;36:1009–1017. doi: 10.1016/s0028-3908(97)00065-8. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., BOWERY B.J., HILL R.G. Bradykinin receptors in mouse and rat isolated superior cervical ganglia. Br. J. Pharmacol. 1995;115:368–372. doi: 10.1111/j.1476-5381.1995.tb15887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEEGERS H.C., AVERY P.S., MCWILLIAMS D.F., HAYWOOD L., WALSH D.A. Combined effect of bradykinin B2 and neurokinin-1 receptor activation on endothelial cell proliferation in acute synovitis. FASEB J. 2004;18:762–764. doi: 10.1096/fj.03-0727fje. [DOI] [PubMed] [Google Scholar]
- SHENKER N., HAIGH R., ROBERTS E., MAPP P., HARRIS A., BLAKE D. Pain, neurogenic inflammation and symmetry in medical practice. Pain Rev. 2001;8:27–34. [Google Scholar]
- SHIBATA M., OHKUBO T., TAKAHASHI H., INOKI R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- SHUGHRUE P.J., KY B., AUSTIN C.P. Localization of B1 bradykinin receptor mRNA in the primate brain and spinal cord: an in situ hybridization study. J. Comp. Neurol. 2003;465:372–384. doi: 10.1002/cne.10846. [DOI] [PubMed] [Google Scholar]
- SOT U., MISTEREK K., GUMULKA S.W., DOROCIAK A. Intrathecal bradykinin administration: opposite effects on nociceptive transmission. Pharmacology. 2002;66:76–80. doi: 10.1159/000065629. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., LOMEZ E.S.L., PINHO V., PESQUERO J.B., BADER M., PESQUERO J.L., TEIXEIRA M.M. Role of bradykinin B2 and B1 receptors in the local, remote, and systemic inflammatory responses that follow intestinal ischemia and reperfusion injury. J. Immunol. 2004;172:2542–2548. doi: 10.4049/jimmunol.172.4.2542. [DOI] [PubMed] [Google Scholar]
- STEWART J.M. Bradykinin antagonists as anti-cancer agents. Curr. Pharm. Design. 2003;9:2036–2042. doi: 10.2174/1381612033454171. [DOI] [PubMed] [Google Scholar]
- STEWART J.M., GERA L., HANSON W., ZUZACK J.S., BUCKARD M., MCCULLOUGH R., WHALLEY E.T. A new generation of bradykinin antagonists. Immunopharmacology. 1996;33:51–60. doi: 10.1016/0162-3109(96)00084-7. [DOI] [PubMed] [Google Scholar]
- STEWART J.M., GERA L., YORK E.J., CHAN D.C., BUNN P. Bradykinin antagonists: present progress and future prospects. Immunopharmacology. 1999;43:155–161. doi: 10.1016/s0162-3109(99)00102-2. [DOI] [PubMed] [Google Scholar]
- SU D.S., MARKOWITZ M.K., DIPARDO R.M., MURPHY K.L., HARRELL C.M., O'MALLEY S.S., RANSOM R.W., CHANG R.S., HA S., HESS F.J., PETTIBONE D.J., MASON G.S., BOYCE S., FREIDINGER R.M., BOCK M.G. Discovery of a potent, non-peptide bradykinin B1 receptor antagonist. J. Am. Chem. Soc. 2003;125:7516–7517. doi: 10.1021/ja0353457. [DOI] [PubMed] [Google Scholar]
- SUFKA K.J., ROACH J.T. Stimulus properties and antinociceptive effects of selective bradykinin B1 and B2 receptor antagonists in rats. Pain. 1996;66:99–103. doi: 10.1016/0304-3959(96)02990-9. [DOI] [PubMed] [Google Scholar]
- TAUB J.S., GUO R., LEEB-LUNDBERG L.M., MADDEN J.F., DAAKA Y. Bradykinin receptor subtype 1 expression and function in prostate cancer. Cancer Res. 2003;63:2037–2041. [PubMed] [Google Scholar]
- TODOROV A.G., ANDRADE D., PESQUERO J.B., ARAÚJO R.DEC., BADER M., STEWART J., GERA L., MULLER-ESTERL W., MORANDI V., GOLDENBERG R.C., NETO H.C., SCHARFSTEIN J. Trypanosoma cruzi induces edematogenic responses in mice and invades cardiomyocytes and endothelial cells in vitro by activating distinct kinin receptor (B1/B2) subtypes. FASEB J. 2003;17:73–75. doi: 10.1096/fj.02-0477fje. [DOI] [PubMed] [Google Scholar]
- UENO A., DEKURA E., KOSUGI Y., YOSHIMURA M., NARABA H., KOJIMA F., OH-ISHI S. Effects of dexamethasone and protein kinase C inhibitors on the induction of bradykinin B1 mRNA and the bradykinin B1 receptor-mediated contractile response in isolated rat ileum. Biochem. Pharmacol. 2002;63:2043–2053. doi: 10.1016/s0006-2952(02)00905-x. [DOI] [PubMed] [Google Scholar]
- VIANNA R.M., CALIXTO J.B. Characterization of the receptor and the mechanisms underlying the inflammatory response induced by des-Arg9-BK in mouse pleurisy. Br. J. Pharmacol. 1998;123:281–291. doi: 10.1038/sj.bjp.0701590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIANNA R.M., ONGALI B., REGOLI D., CALIXTO J.B., COUTURE R. Up-regulation of kinin B1 receptor in the lung of streptozotocin-diabetic rat: autoradiographic and functional evidence. Br. J. Pharmacol. 2003;138:13–22. doi: 10.1038/sj.bjp.0704999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J.W., SU W., LAW Y.P., LU C.H., CHEN Y.C., WANG J.L., CHANG H.J., CHEN W.C., JAN C.R. Mechanism of bradykinin-induced Ca(2+) mobilization in MG63 human osteosarcoma cells. Horm. Res. 2001;55:265–270. doi: 10.1159/000050011. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., MAIER S.F. Glia: a novel drug discovery target for clinical pain. Nat. Rev. Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- WEBB M., MCINTYRE P., PHILLIPS E. B1 and B2 bradykinin receptors encoded by distinct mRNAs. J. Neurochem. 1994;62:1247–1253. doi: 10.1046/j.1471-4159.1994.62041247.x. [DOI] [PubMed] [Google Scholar]
- WILLE P.R., VITOR R., GABILAN N.H., NICOLAU M. Plasma extravasation mediated by lipopolysaccharide-induction of kinin B1 receptors in rat tissues. Mediat. Inflamm. 2001;10:163–167. doi: 10.1080/09629350124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIRTH K., BREIPOHL G., STECHL J., KNOLLE J., HENKE S., SCHOLKENS B. DesArg9-D-Arg[Hyp3,Thi5,D-Tic7,Oic8]bradykinin (desArg10-[Hoe140]) is a potent bradykinin B1 receptor antagonist. Eur. J. Pharmacol. 1991;205:217–218. doi: 10.1016/0014-2999(91)90824-a. [DOI] [PubMed] [Google Scholar]
- WOOD M.R., KIM J.J., HAN W., DORSEY B.D., HOMNICK C.F., DIPARDO R.M., KUDUK S.D., MACNEIL T., MURPHY K.L., LIS E.V., RANSOM R.W., STUMP G.L., LYNCH J.J., O'MALLEY S.S., MILLER P.J., CHEN T.B., HARRELL C.M., CHANG R.S., SANDHU P., ELLIS J.D., BONDISKEY P.J., PETTIBONE D.J., FREIDINGER R.M., BOCK M.G. Benzodiazepines as potent and selective bradykinin B1 antagonists. J. Med. Chem. 2003;46:1803–1806. doi: 10.1021/jm034020y. [DOI] [PubMed] [Google Scholar]
- WOOLF C.J., MANNION R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- WOTHERSPOON G., WINTER J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci. Lett. 2000;294:175–178. doi: 10.1016/s0304-3940(00)01561-5. [DOI] [PubMed] [Google Scholar]
- WU J., AKAIKE T., HAYASHIDA K., MIYAMOTO Y., NAKAGAWA T., MIYAKAWA K., MULLER-ESTERL W., MAEDA H. Identification of bradykinin receptors in clinical cancer specimens and murine tumor tissues. Int. J. Cancer. 2002;98:29–35. doi: 10.1002/ijc.10142. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI-SASE S., HAYASHI I., OKAMOTO H., NARA Y., MATSUZAKI S., HOKA S., MAJIMA M. Amelioration of hyperalgesia by kinin receptor antagonists or kininogen deficiency in chronic constriction nerve injury in rats. Inflamm. Res. 2003;52:164–169. doi: 10.1007/s000110300067. [DOI] [PubMed] [Google Scholar]
- YANG X., POLGAR P. Genomic structure of the human bradykinin B1 receptor gene and preliminary characterization of its regulatory regions. Biochem. Biophys. Res. Commun. 1996;222:18–25. doi: 10.1006/bbrc.1996.0810. [DOI] [PubMed] [Google Scholar]
- YANG S.H., SHARROCKS A.D., WHITMARSH A.J. Transcriptional regulation by the MAP-kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- YANG X., TAYLOR L., POLGAR P. p53 down-regulates human bradykinin B1 receptor gene expression. J. Cell. Biochem. 2001a;82:38–45. doi: 10.1002/jcb.1117. [DOI] [PubMed] [Google Scholar]
- YANG X., TAYLOR L., YU J., FENTON M.J., POLGAR P. Mediator caused induction of a human bradykinin B1 receptor minigene: participation of c-jun in the process. J. Cell. Biochem. 2001b;82:163–170. doi: 10.1002/jcb.1116. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., ADNER M., CARDELL L.O. Up-regulation of bradykinin receptors in a murine in-vitro model of chronic airway inflammation. Eur. J. Pharmacol. 2004;489:117–126. doi: 10.1016/j.ejphar.2004.02.033. [DOI] [PubMed] [Google Scholar]
- ZHOU X., POLGAR P., TAYLOR L. Roles for interleukin-1beta, phorbol ester and a post-transcriptional regulator in the control of bradykinin B1 receptor gene expression. Biochem. J. 1998;330:361–366. doi: 10.1042/bj3300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X., PRADO G.N., CHAI M., YANG X., TAYLOR L., POLGAR P. Posttranscriptional destabilization of the bradykinin B1 receptor messenger RNA: cloning and functional characterization of the 3′-untranslated region. Mol. Cell. Biol. Res. Commun. 1999;1:29–35. doi: 10.1006/mcbr.1999.0105. [DOI] [PubMed] [Google Scholar]