Abstract
α1-Adrenoceptor (AR) subtypes in mouse carotid arteries were characterised using a combination of agonist/antagonist pharmacology and knockout (KO) mice.
Phenylephrine (PE) was most potent in the α1B-KO (pEC50=6.9±0.2) followed by control (pEC50=6.3±0.06) and α1D-KO (pEC50=5.5±0.07). Both N-[5-(4,5-dihydro-1H-imidazol-2yl)-2-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl] methanesulphonamide hydrobromide (A-61603) and 5-hydroxytryptamine (5-HT) were more potent in the α1D-KO (pEC50=7.4±0.27 and 7.4±0.05, respectively) than the control (pEC50=6.9±0.09 and 6.9±0.08, respectively) and equipotent with the control in the α1B-KO (pEC50=6.7±0.07 and 6.8±0.04). Maximum responses to PE and A-61603 were reduced in the α1D-KO compared to control; there was no difference in maximum responses to 5-HT.
In control arteries, prazosin and 5-methylurapidil acted competitively with pA2 of 9.6 and 7.5, respectively. BMY7378 produced antagonism only at the highest concentration used (100 nM; pKB 8.3).
Prazosin, 5-methylurapidil and BMY7378 acted competitively in α1B-KO carotid arteries with pA2 of 10.3, 7.6 and 9.6, respectively.
In the α1D-KO, against PE, 5-methylurapidil produced a pA2 of 8.1. pKB values were calculated for prazosin (10.6) and BMY7378 (7.0). Against A-61603, 5-methylurapidil had a pA2 of 8.5, prazosin 8.6, while BMY7378 had no effect.
In conclusion, the α1B-KO mediates contraction solely through α1D-ARs and the α1D-KO through α1A-ARs. Extrapolating back to the control from the knockout data suggests that all three subtypes could be involved in the responses, but we propose that the α1D-AR causes the contractile response and that the role of the α1B-AR is mainly regulatory.
Keywords: α1-Adrenoceptor, subtyping, myography, mouse carotid artery, α1B-knockout mouse, α1D-knockout mouse
Introduction
Three native α1-adrenoceptor (AR) subtypes, defined by ligand binding and functional pharmacology, α1A, α1B and α1D, correspond to three cloned subtypes, α1a, α1b and α1d (Bylund et al., 1994). It is not known whether the three subtypes have different biological roles. Several tissues, including arteries, express more than one subtype. The mRNA and protein for all three α1-AR subtypes are expressed in the major blood vessels of the rat (Piascik et al., 1995; Scofield et al., 1995; Piascik et al., 1997; Hrometz et al., 1999). However, separating the responses mediated by these subtypes has proved difficult, due to the limitations of selectivity of antagonists between the three receptors and the proposition that they might all be involved in the same type of response, namely contraction of vascular smooth muscle.
A handful of α1A-AR-selective antagonists are available, such as 5-methylurapidil, WB4101 and RS100329 (Gross et al., 1988; Schwinn et al., 1995; Williams et al., 1999), while BMY7378 is the only widely accepted α1D-AR-selective antagonist (Saussy et al., 1994; Goetz et al., 1995; Kenny et al., 1995). A major pharmacological complication when attempting to subtype α1-ARs is the lack of a selective competitive antagonist for the α1B-AR. This appears to be a situation in which receptor knockouts might simplify the pharmacological analysis.
Most studies of vascular α1-ARs, either as an undivided class or as subtypes, have been carried out in rats, rabbits and dogs and until recently little data has been available for the mouse. However, α1-AR knockout (KO) mice are now available for the α1B- and α1D-ARs (Cavalli et al., 1997; Tanoue et al., 2002). These provide novel environments to study and subtype the remaining two possible α1-ARs.
We have chosen to study the carotid artery since this vessel has greater potential as an experimental model, being accessible to surgical manipulation in vivo and amenable to perfusion studies in vitro. There is also controversy over whether contraction is mediated by the α1B- or α1D-ARs according to species (dog: α1B (Muramatsu et al., 1991; Kohno et al., 1994); rabbit: α1B (Muramatsu et al., 1995); rat: α1D (Villalobos-Molina & Ibarra, 1996; de Oliveira et al., 1998)). Theoretically, this presents a relatively straightforward scenario for observing the consequences of knocking out each of these subtypes. Previous work has shown that (1) knockout of the α1B-AR produces little change in the size or sensitivity of responses to phenylephrine (PE) in the aorta and carotid arteries; the antagonist pharmacology is more consistent with α1D-AR pharmacology, suggesting a major role for the α1D-AR and a minor one for the α1B-AR (Daly et al., 2002), and (2) knockout of the α1D-AR produced a significant reduction in sensitivity and maximum response to PE in the aorta, consistent with the loss of the major contractile α1-AR (Tanoue et al., 2002).
The objective of the present study was to apply a consistent antagonist analysis using the two knockouts to allow us to explore the functional relationship between α1-AR subtypes; for example, what are the consequences of deleting each receptor? Does this show how they interact? Do other subtypes upregulate to compensate? We used the ‘definitive' antagonists (prazosin, 5-methylurapidil and BMY7378) and the α1-AR-selective agonist PE (eliminates possible complications from α2- and β-ARs). We also used the α1A-AR-selective agonist N-[5-(4,5-dihydro-1H-imidazol-2yl)-2-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl] methanesulphonamide hydrobromide more commonly known as A-61603 (Knepper et al., 1995) to reinforce the antagonist analysis.
Latterly, we applied the knowledge obtained from mouse carotid arteries to data collected from our laboratory a number of years ago on the rat carotid artery, which at the time were difficult to interpret.
Methods
Animals used and set-up procedure
All transgenic mice (C57 Black genetic background; for a full description of genetic background see Cavalli et al. (1997) and Tanoue et al. (2002)) were bred at the University of Glasgow. Mice were killed by lethal overdose of carbon dioxide. The common carotid arteries were removed, placed in cold oxygenated Krebs and dissected free of connective tissue with the aid of a dissecting microscope.
Experiments were carried out in a four-chamber wire myograph (J.P. Trading, Aarhus, Denmark). Arteries were cut into approximately 2 mm lengths and mounted on two 40 μm wires. One wire was attached to a fixed head, while the other was attached to a head connected to a force transducer. The force transducer was in turn connected to a Linseis pen recorder to allow recordings of the force achieved.
Vessels were allowed to equilibrate in Krebs (37°C and gassed with 95% O2, 5% CO2) for 15 min after which time the vessels were set under their optimal resting length tensions: previously calculated to be 250 mg for the control, α1B- and α1D-KO mouse carotid arteries (Deighan, 2001). The vessels were left to equilibrate at this tension for 30–45 min with washes every 15 min. Prior to the start of each experiment, vessels were challenged with a sensitising concentration of 0.3 μM PE (control and α1B-KO mouse), 10 μM PE (α1D-KO), 10 μM A-61603 (α1D-KO) or 1 μM 5-hydroxytryptamine (5-HT) (all mice strains). The contraction was allowed to plateau and then washed with Krebs. This was repeated three times to minimise changes in sensitivity to further challenges with agonists. Cumulative concentration–response curves (CRC) were carried out to either PE (1 nM–1 mM), A-61603 (1 nM–300 μM) or 5-HT (1 nM–30 μM). Subsequent CRCs to PE or A-61603 were carried out in the presence of antagonists (prazosin, 5-methylurapidil and BMY7378), which were equilibrated with the tissue for 30 min prior to beginning the CRC. Time controls were carried out in parallel with antagonist curves.
A similar analysis was carried out on carotid artery rings from male Wistar rats (320–400 g) suspended between two wire hooks and recorded isometrically. The protocol was identical to that in mice except that the vessels were equilibrated under 2.5 g of tension and noradrenaline (NA; 1 nM–10 μM) was used as the agonist.
Data analysis
Responses to agonists are expressed as tension in grams or as a percentage of the maximum response of the first CRC. The pEC50 was calculated as the negative logarithm of the concentration of agonist that produces half the maximal response. pEC50 values for PE, A-61603 and 5-HT in control, α1B- and α1D-KO mice were analysed using a one-way analysis of variance (ANOVA) followed by a Bonferroni post test.
The pEC50 values, Hill slopes and maximum responses calculated from the antagonist data in mouse carotid arteries were analysed using a two-way ANOVA followed by a Bonferroni post test. For both one- and two-way ANOVA, a P-value of less than 0.05 was considered significant. The agonist concentration ratios (CRs) were determined from the ratio of the EC50 of the agonist in the presence and absence of the antagonist and used for Schild analysis where the log[antagonist] is plotted against log(CR−1) (Arunlakshana & Schild, 1959). Linear regression produces an x-intercept that is equal to the pA2 of the antagonist. If the slope of the Schild plot is equal to 1, then pA2=pKB and is indicative of competitive binding. Where a pA2 value could not be calculated (e.g. where there is only a small shift with antagonist), a pKB value was calculated instead using the equation
where pKB is the negative logarithm of the dissociation constant KB and [B] is the concentration of antagonist.
Solutions and drugs
The Krebs–Henseleit solution was of the following composition (mM): NaCl (119), KCl (4.7), MgCl2 (1.2), CaCl2 (2.5), NaHCO3 (25), NaHPO4 (1.2), glucose (11.5) and Na2EDTA (0.023).
The following compounds were used: A-61603 hydrobromide (Tocris, U.K.), BMY 7378 dihydrochloride (8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4,5]decane-7,9-dione; Research Biochemicals International, U.K.), 5-HT (Sigma, U.K.), 5-methylurapidil (Research Biochemicals International, U.K.), noradrenaline hydrochloride (Sigma, U.K.), phenylephrine hydrochloride (Sigma, U.K.) and prazosin hydrochloride (Sigma, U.K.).
All drugs were dissolved in deionised water and then diluted (1 : 10) to give the concentrations used for the CRCs.
Results
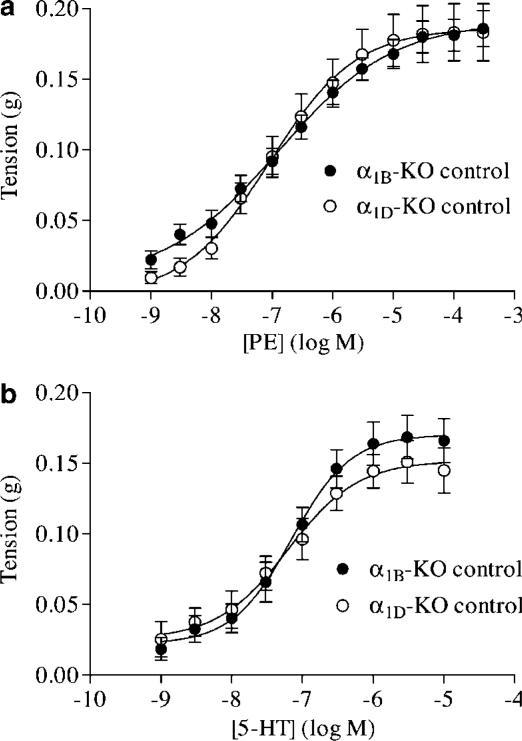
The genetic controls for the α1B- and α1D-KO were found to be pharmacologically similar in their sensitivities and maximum responses to PE and 5-HT (Figure 1). Therefore, only one set of control experiments was required.
Figure 1.
Mean concentration response data to (a) PE and (b) 5-HT in α1B-KO and α1D-KO control mouse carotid arteries expressed as tension in grams. Both agonists produced similar responses in the two strains of control mice. Mean curves were generated using nonlinear regression upon which the mean±s.e.m. data have been superimposed (n>9).
Control, α1B-KO and α1D-KO mouse carotid arteries
All agonist data (pEC50 values, maximum responses, Hill slopes and statistical comparisons) are presented in Table 1.
Table 1.
Comparison of pEC50 values, maximum responses and Hill slopes for agonists producing contractions in (a) control, (b) α1B-KO and (c) α1D-KO mouse carotid arteries
| Agonist | pEC50 | Max. response (g) | Hill slope (95% CI) |
|---|---|---|---|
| (a) Control | |||
| PE | 6.3±0.06 | 0.37±0.01 | 0.5 (0.4–0.6)# |
| (R)-A-61603 | 6.9±0.09 | 0.27±0.01 | 0.65 (0.5–0.8)# |
| 5-HT | 6.9±0.08 | 0.27±0.04 | 1.15 (0.6–1.7) |
| (b) α1B-KO | |||
| PE | 6.9±0.22* | 0.33±0.01 | 0.4 (0.2–0.6)# |
| (R)-A-61603 | 6.7±0.07 | 0.28±0.01 | 0.65 (0.5–0.8)# |
| 5-HT | 6.8±0.04 | 0.27±0.05 | 1.3 (0.9–1.7) |
| (c) α1D-KO | |||
| PE | 5.5±0.07* | 0.21±0.02* | 0.85 (0.6–1.1) |
| (R)-A-61603 | 7.4±0.27* | 0.11±0.01* | 0.4 (0.1–0.7)# |
| 5-HT | 7.4±0.05* | 0.26±0.06 | 1.0 (0.7–1.3) |
pEC50 values and maximum responses are expressed as mean±s.e.m. and the Hill slopes are given along with their 95% confidence intervals (95% CI).
P<0.05 compared to control;
Hill slope significantly different from unity.
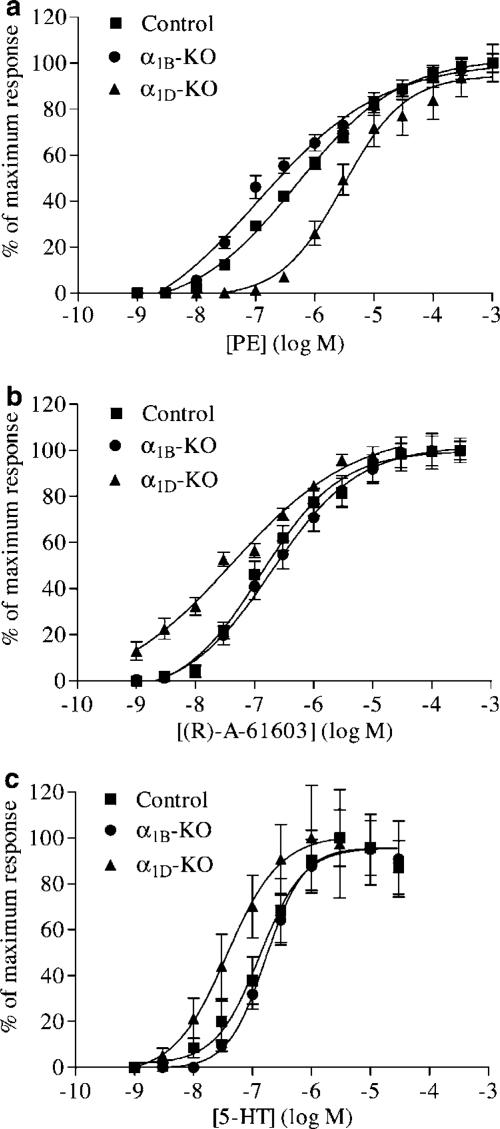
PE produced concentration-dependent contractions in carotid arteries from all three strains of mice. Sensitivity to PE was found to vary between mouse strains (Figure 2a). The α1B-KO was the most sensitive to PE, followed by the control carotid artery and finally the least sensitive was the α1D-KO. All pEC50 values were significantly different between the three mouse strains. Control and α1B-KO carotid arteries produced similar maximum responses, whereas the maximum response from the α1D-KO carotid artery was significantly smaller. CRCs to PE demonstrated shallow Hill slopes significantly different from unity in both control and α1B-KO carotid arteries. This was not the case in α1D-KO carotid arteries; the PE CRC had a Hill slope that was not significantly different from unity.
Figure 2.
Mean concentration response data to (a) PE, (b) A-61603 and (c) 5-HT in carotid arteries from control, α1B-KO and α1D-KO mice expressed as a percentage of their own maximum response. Mean curves were generated using nonlinear regression upon which the mean±s.e.m. data have been superimposed (n>9).
A-61603 produced concentration-dependent contractions in all three mouse strains (Figure 2b). The α1D-KO was more sensitive to A-61603 than the control or the α1B-KO carotid arteries. However, the efficacy of A-61603 in the α1D-KO was reduced compared to the other two mouse strains. The maximum responses produced by the control and α1B-KO carotid arteries were similar, while the α1D-KO response was smaller. All three strains of mice produced shallow Hill slopes significantly different from unity.
5-HT produced concentration-dependent contractions in all three mouse strains (Figure 2c). Desensitisation occurred at the higher concentrations of 5-HT; therefore, CRCs were stopped as soon as the maximum response began to decline. All three mouse strains produced similar responses to 5-HT, with no differences observed in maximum responses or Hill slopes. However, α1D-KO carotid arteries were found to be more sensitive to 5-HT than either control or α1B-KO arteries.
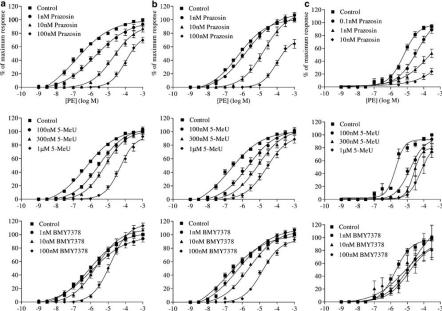
In control, α1B-KO and α1D-KO carotid arteries, the subtype-selective antagonists produced a rightward displacement of the PE curve without a depression in the maximum response (Figure 3). Prazosin was found to cause a decrease in the maximum response at 1 and 10 nM in α1D-KO arteries and at 100 nM in control and α1B-KO arteries (Figure 3). The pA2 values for prazosin and 5-methylurapidil in control tissue were calculated to be 9.6 and 7.5, respectively, with slopes that were not significantly different from unity, indicating competitive antagonism (Table 2). Only the highest concentration of BMY7378 (100 nM) produced a significant shift in the CRC to PE. Therefore, a pA2 value could not be calculated. Instead, a pKB value was calculated at 100 nM BMY7378 and was found to be 8.3. In the α1B-KO carotid artery, all antagonists acted competitively. The pA2 values for prazosin, 5-methylurapidil and BMY7378 were found to be 10.3, 7.6 and 9.6, respectively (Table 2). In the α1D-KO, it was possible to calculate a pA2 value only for 5-methylurapidil, which was 8.1. Prazosin antagonised the contractions to PE with such potency that we had to dilute the concentrations used in control and α1B-KO by a factor of 10 to allow us to obtain four consecutive CRCs. Despite this, it was still possible to calculate EC50 values only for the smallest concentration used (0.1 nM). This was the concentration used to calculate a pKB for prazosin, which was 10.6. In contrast BMY7378 could only weakly antagonise the contraction to PE in α1D-KO carotid arteries. A pKB value could be calculated only at 100 nM, which was 7.0 (Table 2).
Figure 3.
Mean concentration response data to PE in the presence of increasing concentrations of antagonists in (a) control, (b) α1B-KO and (c) α1D-KO mouse carotid arteries. Mean curves were generated using nonlinear regression upon which the mean±s.e.m. data have been superimposed (n>6).
Table 2.
pA2 or pKB values and slope parameters of antagonists in control, α1B-KO or α1D-KO mouse carotid arteries
| Control | α1B-KO | α1D-KO | ||||
|---|---|---|---|---|---|---|
| Antagonist | pA2/pKB | Slope | pA2/pKB | Slope | pA2/pKB | Slope |
| Prazosin | 9.6 | 0.93 (0.77–1.08) | 10.3 | 0.92 (0.68–1.2) | 10.6 | NA |
| 5-MeU | 7.5 | 1.1 (0.73–1.5) | 7.6 | 1.1 (0.77–1.5) | 8.1 | 0.82 (0.4–1.3) |
| BMY7378 | 8.3 | NA | 9.6 | 0.9 | 7.0 | NA |
CRCs were constructed to PE. Values in parentheses are the 95% confidence limits for the slope value. 5-MeU, 5-methylurapidil; NA, not applicable.
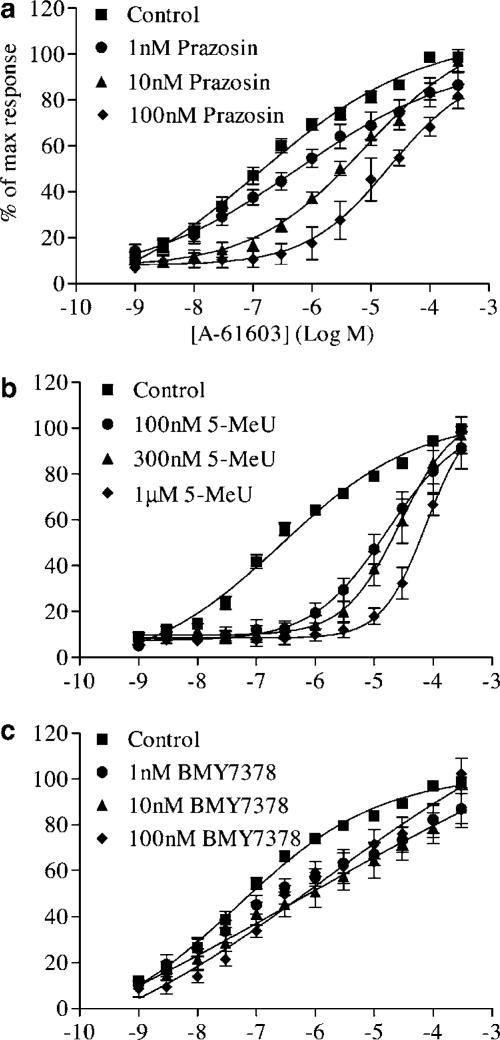
In addition to the antagonist data obtained to PE, we repeated these experiments with A-61603 as the agonist in the α1D-KO (Figure 4). As with PE, prazosin produced a rightward displacement of the curve. However, unlike PE, there was no depression in the maximum response. A pA2 was calculated and found to be 8.6 with a slope not significantly different from unity, indicating competitive antagonism. 5-Methylurapidil potently inhibited contractions to A-61603 in the α1D-KO with a higher pA2 than had been previously calculated for any of the strains of mice used, including the pA2 obtained against PE contractions in the α1D-KO. As with prazosin, 5-methylurapidil acted competitively. Relative to the time control, no significant shift occurred with BMY7378. Therefore, no pA2 or pKB could be calculated. pA2 values and slope parameters are presented in Table 3.
Figure 4.
Mean concentration response data to A-61603 in the presence of increasing concentrations of antagonists in α1D-KO mouse carotid arteries. Mean curves were generated using nonlinear regression upon which the mean±s.e.m. data have been superimposed (n>6).
Table 3.
pA2 values and slope parameters of antagonists in α1D-KO mouse carotid arteries
| Antagonist | pA2 | Slope |
|---|---|---|
| Prazosin | 8.6 | 1.2 (0.7–1.8) |
| 5-MeU | 8.5 | 0.9 (0.2–1.8) |
| BMY7378 | ND | ND |
CRCs were constructed to A-61603. Values in parentheses are the 95% confidence limits for the slope value. ND, not determined.
Time controls for control, α1B-KO and α1D-KO carotid arteries showed no significant change in sensitivity or maximum response to PE or A-61603 (data not shown).
Rat carotid arteries
NA produced concentration-dependent contractions in rat carotid arteries with a pEC50 of 7.9±0.06 and a maximum response of 1.1±0.06 g (n=6) (graphs not shown).
All antagonists used shifted the CRC to NA to the right in a concentration-dependent manner. There was no decrease in the maximum response for any of the antagonists. Schild regression produced pA2 values of 10.0 for prazosin with a Schild slope indicative of competitive antagonism. 5-Methylurapidil and BMY7378 had pA2 values of 9.1 and 9.2, respectively, accompanied by shallow Schild slopes, significantly different from unity (Table 4). Time controls showed no significant change in sensitivity or maximum response to NA (data not shown).
Table 4.
pA2 values and slope parameters of antagonists in rat carotid artery
| Antagonist | pA2 | Slope |
|---|---|---|
| Prazosin | 10.7 | 1.1 (0.4–1.4) |
| 5-MeU | 9.0 | 0.6 (0.3–0.8) |
| BMY7378 | 9.8 | 0.5 (0.3–0.7) |
Values in parentheses are the 95% confidence limits for the slope value.
Discussion
This study demonstrates that when the α1B-KO and α1D-KO strains of mice are used in conjunction with antagonists, a different pharmacological situation emerges relative to control mice and to each other. However, the pharmacological differences between strains cannot simply be explained in terms of the effects of removing one of the subtypes. Interpreting the pharmacology of the control remains complex and suggests interactions between the subtypes beyond their effects on smooth muscle contraction.
In the α1B-KO, the α1D-ARs were apparently isolated, producing robust vasoconstrictor responses that were amenable to classical pharmacological analysis. In contrast, the α1D-KO responses were less sensitive to PE (though not the α1A-AR-selective A61603), had a smaller maximum response and responded to selective antagonists with the characteristics of an α1A-AR. This raises a few related questions: (1) why is there no evidence for an α1A-AR-mediated response in the control or α1B-KO? (2) is the α1A-AR-mediated response present in these arteries but has not been identified with the antagonists used or (3) has it been upregulated?
Receptor subtypes as revealed by agonists
The pattern of the relative potency of agonists for the three mouse strains was inverted for the two agonists tested. For PE, the order of potency was α1B-KO>control>α1D-KO and, for A-61603, it was α1D-KO>control>α1B-KO (Figure 2 and Table 1). Knepper et al. (1995) have shown that A-61603 is much more potent than PE at α1A-AR and less potent than PE at α1D- and α1B-ARs. In these terms, the responses in the α1D-KO are strongly correlated with α1A-AR, while the control and the α1B-KO correlate better with either α1B- or α1D-ARs.
The α1D-KO does not produce such large contractions as the control or α1B-KO in response to the α1-AR agonists PE or A-61603. The maximum response achieved is approximately half of the response produced by the control and the α1B-KO, yet the responses to 5-HT produced by the three strains of mice are not significantly different. This suggests that the α1-AR(s) mediating contraction in control and α1B-KO carotid arteries are either more efficiently coupled to contraction than the α1-AR mediating contraction in the α1D-KO or that there are fewer receptors present in the α1D-KO to mediate a response.
The maximum to 5-HT was not significantly different between the three mouse strains, indicating that the decreased response to PE and A-61603 in the α1D-KO is not due to a general decline in agonist-mediated responses (indeed sensitivity to 5-HT was enhanced) but seems likely to be a consequence of deleting the α1D-AR. This deserves closer analysis to determine whether it represents engagement of a subpopulation of smooth muscle cells or a submaximal excitation of each cell; however, the data presented here seem to show that the remaining α1A- and/or α1B-ARs are not as efficient as the α1D-AR when it comes to mediating contraction in the carotid artery, perhaps consistent with their different physiological roles as discussed below.
The increase in sensitivity to 5-HT in the α1D-KO suggests heterologous upregulation in response to the loss of sensitivity to catecholamines via α1-ARs. A similar observation has been made in the aorta of the α1D-KO mouse by Tanoue et al. (2002). Both 5-HT1A receptors and α1-ARs are coupled to Gq/11 (Alexander et al., 2004). Therefore, it seems likely that they will share common pathways that are subject to feedback modulation and may be capable of compensating for one another in a KO mouse.
Receptor subtypes as revealed by agonist–antagonist interactions
In the α1B-KO, the estimated affinity for BMY7378 increased compared with controls. This would be expected if the primary response in the control is α1D-AR mediated and a secondary (α1A- or α1B-AR) component is present. There is no positive evidence for the presence of α1A-ARs in the control; 5-methylurapidil has lower affinity than in vessels believed to utilise α1A-AR (Jarajapu et al., 2001a, 2001b; Daly et al., 2002) and the control shows a relatively low sensitivity to A-61603 (Knepper et al., 1995). The analysis of control and α1B-KO data together suggest that control carotid arteries mediate contraction through α1D-ARs (primary response) and α1B-ARs (secondary response) while α1B-KO carotid arteries mediate contraction solely through α1D-ARs.
In the α1D-KO, where only α1A- or α1B-AR can be present, the affinity of 5-methylurapidil increases compared with the control, pointing to the presence of α1A-ARs. To test this hypothesis, 5-methylurapidil was tested against the α1A-AR agonist A-61603 in the α1D-KO. 5-Methylurapidil showed still higher affinity, suggesting that α1A-ARs were indeed contributing to contraction.
There is no positive evidence from control or α1B-KO data to suggest an α1A-AR component to their contractions. Therefore, if the α1A-AR is involved in the functional response of the α1D-KO, it may be as a result of upregulation of the α1A-AR from a subfunctional level as a consequence of losing the preferred, dominant receptor, the α1D-AR.
Comparison with published studies
Evidence has been presented for and against the presence of functional α1A-ARs in large arteries. Several analyses have favoured α1D- and/or α1B-ARs over α1A-ARs (Muramatsu et al., 1991; 1995; Aboud et al., 1993; Kohno et al., 1994; Testa et al., 1995a, 1995b; Villalobos-Molina & Ibarra, 1996; de Oliveira et al., 1998; Martinez et al., 1999). Furthermore, Rokosh & Simpson (2002) created an α1A-KO mouse and showed histochemically that Lac-Z, whose gene substituted for the α1A-ARs gene, was not detected in the major conducting arteries, including the carotid artery. However, there is some evidence in favour of an α1A-AR response in rat conducting arteries. Gisbert et al. (2003) showed in rat aorta that there was an α1A-AR-mediated component to the production of inositol phosphates by NA. In addition, our own data from the rat carotid artery show that it is difficult to define the subtype involved in the contractile response to NA. The subtype-selective antagonists BMY7378 and 5-methylurapidil both produced high pA2 values, although low slopes indicate a complex response consistent with multiple subtypes (Table 4). In the light of the mouse data, we now propose that the rat carotid artery expresses a mixture of α1A- and α1D-ARs. The high sensitivity of antagonists to both α1A- and α1D-ARs implies not only the presence of both of these subtypes but that they may act synergistically, allowing all antagonists to be effective.
Overall, the present data suggest that large conducting arteries have the potential to express and employ all three α1-AR subtypes. There is positive evidence for the α1D-AR in the conducting arteries of mice and rats. There is more controversial evidence that it can be accompanied by an α1B-AR in normal mice and by either or both of the α1B-AR and the α1A-AR in rats. In the α1B-KO mouse, the α1D-AR component becomes dominant as expected from simple removal of the α1B-AR. However, in the α1D-KO, the remaining response shows characteristics of the α1A-AR. This suggests that the vessel can withstand the loss of its minor receptor without compensation, but that when its major receptor is lost it compensates by upregulating the α1A-AR. This is not the first time we have observed this phenomenon. The α1A-AR is upregulated in the liver of the α1B-KO mouse (Deighan et al., 2004), which in control mice expresses only the α1B-AR (Garcia-Sainz et al., 1994; Cavalli et al., 1997; Deighan et al., 2004). Compensatory upregulation of another α1-AR subtype may be a general response to loss of the major subtype in any particular tissue, be it α1B- or α1D-ARs.
Physiological relevance
In general, there seems to be a reciprocal presence of the α1A-AR and the α1D-AR in blood vessels. Large noninnervated conductance arteries are associated with expressing the α1D-AR (Kenny et al., 1995; de Oliveira et al., 1998; Daly et al., 2002; Tanoue et al., 2002), while small innervated resistance vessels are associated with the α1A-AR (Stassen et al., 1998; Jarajapu et al., 2001b; Daly et al., 2002). The innervated vessels, on the whole, are less sensitive to agonists. This suggests a physiological basis for the relative balance of subtypes based on the balance of humoral and neurogenic control; that is, developmentally, when vessels become innervated they lose α1D-ARs and gain α1A-ARs, making them less sensitive to circulating catecholamines but gaining a graded sensitivity to locally released NA, at a higher concentration range. Conducting arteries do not become innervated, so remain sensitive to catecholamines through their α1D-AR. This would explain why α1-AR agonists, such as NA or PE, are more potent at α1D-ARs than at the other subtypes (Minneman et al., 1994; Knepper et al., 1995; Yang et al., 1997). The present work shows that if they are deprived of this natural selection process by deletion of the preferred receptor's gene, they upregulate the alternative catecholamine contractile-signalling receptor that is best able to contract vascular smooth muscle, the α1A-AR. However, the lower efficiency of the α1A-AR can only partly compensate in functional terms, as witnessed by the weak submaximal contractions elicited to both PE and A-61603 in the α1D-KO carotid artery.
Our data seem to suggest the presence of α1B-ARs in the control; yet, when it is conclusively absent in the α1B-KO, this has little effect on the artery's contractile ability and in the α1D-KO there is no evidence for its presence. If it is indeed present in the control, then what functional role does the α1B-AR have in these blood vessels? Regulatory interactions between the subtypes involving heterodimerisation of α1B-ARs and the other two subtypes have been proposed from studies of fluorescent-labelled recombinant receptors. The α1B-AR, which of the three subtypes is the most susceptible to agonist-mediated endocytosis (Chalthorn et al., 2002), can form heterodimers with the other subtypes that can affect their cellular location and expression levels (Uberti et al., 2003; Stanasila et al., 2003). The formation of the α1A/α1B heterodimer allowed the α1A- and α1B-ARs to cointernalise and consequently increased the extent of agonist-mediated internalisation of the α1A-AR (Stanasila et al., 2003). In the case of the α1B/α1D heterodimer, the presence of the α1B-AR was found to relocate the α1D-AR from intracellular sites to the plasma membrane (Uberti et al., 2003; Hague et al., 2004). The present data are the first to show that the functions of α1-ARs are influenced by the presence of the other subtypes.
The heterodimersation studies may show potential interactions that play a part in long-term receptor regulation involving other factors. The absence of the α1B-AR seems to cause some sensitisation of the mainly α1D-AR-mediated contraction of mouse carotid, which might indicate the loss of a regulation of receptor expression or of some other essentially negative effects of the α1B-AR. The apparent absence of an α1B-AR-mediated contraction in the α1D-KO, where the α1A-AR has taken over function, may indicate an adaptation to counteract the negative influence of the α1B-AR since α1-AR function is already compromised. Overall, it seems probable that the α1B-AR will emerge as a regulatory receptor capable of fine-tuning the properties of the α1A- and α1D-ARs.
To summarise, normal mouse carotid arteries have antagonist absolute affinity values that are not consistent with a single α1-AR subtype but correspond to those expected from a mixed population of at least two and possibly all three subtypes. The α1B-KO mouse presents a straightforward picture of contractile α1D-ARs, while the α1D-KO mouse utilises α1A-ARs. The emergence of α1A-ARs when the major subtype, α1D-AR, is knocked out suggests compensatory upregulation of the α1A-AR. The α1B-AR may have a regulatory role to play in control carotid artery by influencing the expression and cellular location of the other subtypes.
Acknowledgments
We thank Mr Simon McGrory for his expert technical assistance and Professor Susanna Cotecchia for kindly gifting us a colony of α1B-KO and their genetic controls. We also thank the British Heart Foundation, which funded this project.
Abbreviations
- AR
adrenoceptor
- CRC
concentration–response curve
- 5-HT
5-hydroxytryptamine
- KO
knockout
- NA
noradrenaline
- PE
phenylephrine
- A-61603
N-[5-(4,5-dihydro-1H-imidazol-2yl)-2-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl] methanesulphonamide hydrobromide
References
- ABOUD R., SHAFI M., DOCHERTY J.R. Investigation of the subtypes of α1-adrenoceptor mediating contractions of rat aorta, vas deferens and spleen. Br. J. Pharmacol. 1993;109:80–87. doi: 10.1111/j.1476-5381.1993.tb13534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., MATHIE A., PETERS J.A. Guide to receptors and channels. Br. J. Pharmacol. 2004;141:S1–S126. doi: 10.1038/sj.bjp.0705672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBERG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLLINOF P.B., RUFFOLO R.R., JR, TRENDELENBURG U. International Union of Pharmacology: nomenclature of ARs. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- CAVALLI A., LATTION A.-L., HUMMLER E., NENNIGER M., PEDRAZZINI T., AUBERT J.-F., MICHEL M.C., YANG M., LEMBO G., VECCHIONE C., MOSTARDININ M., SCMIDT A., BEERMANN F., COTECCHIA S. Decreased blood pressure response in mice deficient of the α1b-AR. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHALTHORN D., MCCUNE D.F., EDELMANN S.E., GARCIA-CAZARIN M.L., TSUJIMOTO G., PIASCIK M.T. Difference in the cellular localization and agonist-mediated internalisation properties of the α1-AR subtypes. Mol. Pharmacol. 2002;61:1008–1016. doi: 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- DALY C.J., DEIGHAN C., MCGEE A., MENNIE D., ALI Z., MCBRIDE M., MCGRATH J.C. A knockout approach indicates a minor vasoconstrictor role for vascular α1b-ARs in mouse. Physiol. Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- DE OLIVEIRA A.M., CAMPOS-MELLO C., LEITAO M.C., CORREA F.M.A. Maturation and ageing-related differences in responsiveness of rat aorta and carotid arteries to α1-adrenoceptor stimulation. Pharmacology. 1998;57:305–313. doi: 10.1159/000028256. [DOI] [PubMed] [Google Scholar]
- DEIGHAN C.A combined pharmacological/knockout approach to subtyping α1-ARs in murine tissues 2001. PhD thesis, University of Glasgow
- DEIGHAN C., WOOLLHEAD A.M., COLSTON J.F., MCGRATH J.C. Hepatocytes from α1B-adrenoceptor knockout mice reveal compensatory adrenoceptor subtype substitution. Br. J. Pharmacol. 2004;142:1031–1037. doi: 10.1038/sj.bjp.0705872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-SAINZ J.A., CASAS-GONZALEZ P., ROMERO-AVILA M.T., GONZALEZ-ESPINOSA C. Characterization of the hepatic α1B-adrenoceptors of rats, mice and hamsters. Life Sci. 1994;52:1995–2003. doi: 10.1016/0024-3205(94)90134-1. [DOI] [PubMed] [Google Scholar]
- GISBERT R., MADRERO Y., SABINO V., NOGUERA M.A., IVORRA M.D., D'OCON P. Functional characterization of alpha 1-adrenoceptor subtypes in vascular tissues using different experimental approaches: a comparative study. Br. J. Pharmacol. 2003;138:359–368. doi: 10.1038/sj.bjp.0705033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D.C., TRUE T.A., RIMELE T.J., SAUSSY D.L. BMY7378 is a selective antagonist of the D subtype of α1-ARs. Eur. J. Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- GROSS G., HANFT G., RUGEVICS C. 5-Methylurapidil discriminates between subtypes of the α1-AR. Eur. J. Pharmacol. 1988;151:333–335. doi: 10.1016/0014-2999(88)90819-9. [DOI] [PubMed] [Google Scholar]
- HAGUE C., UBERTI M.A., CHEN Z., HALL R.A., MINNEMAN K.P. Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J. Biol. Chem. 2004;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- HROMETZ S.L., EDELMANN S.E., MCCUNE D.F., OLGES J.R., HADLEY R.W., PEREZ D.M., PIASCIK M.T. Expression of multiple α1-ARs on vascular smooth muscle: correlation with the regulation of contraction. J. Pharmacol. Exp. Ther. 1999;290:452–463. [PubMed] [Google Scholar]
- JARAJAPU Y.P.R., COATS P., MCGRATH J.C., HILLIER C., MACDONALD A. Functional characterization of α1-AR subtypes in human skeletal muscle resistance arteries. Br. J. Pharmacol. 2001a;133:679–686. doi: 10.1038/sj.bjp.0704130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAJAPU Y.P.R., HILLIER C., MACDONALD A. The α1A-AR subtype mediates contraction in rat femoral resistance arteries. Eur. J. Pharmacol. 2001b;422:127–135. doi: 10.1016/s0014-2999(01)01051-2. [DOI] [PubMed] [Google Scholar]
- KENNY B.A., CHALMERS D.H., PHILPOTT P.C., NAYLOR A.M. Characterization of an α1D-AR mediating the contractile response of rat aorta to NA. Br. J. Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNEPPER S.M., BUCKNER S.A., BRUNE M.E., DEBERNARDIS J.F., MEYER M.D., HANCOCK A.A. A-61603, a potent α1-adrenergic receptor agonist, selective for the α1A-receptor subtype. J. Pharmacol. Exp. Ther. 1995;274:97–103. [PubMed] [Google Scholar]
- KOHNO Y., SAITO H., TAKITA M., KIGOSKI S., MURAMATSU I. Heterogeneity of α1-adrenoceptor subtypes involved in adrenergic contractions of dog blood vessels. Br. J. Pharmacol. 1994;112:1167–1173. doi: 10.1111/j.1476-5381.1994.tb13206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ L., CARMONA L., VILLALOBOS-MOLINA R. Vascular α1D-adrenoceptor function is maintained during congestive heart failure after myocardial infarction in the rat. Arch. Med. Res. 1999;30:290–297. doi: 10.1016/s0188-0128(99)00033-0. [DOI] [PubMed] [Google Scholar]
- MINNEMAN K.P., THEROUX T.L., HOLLINGER S., HAN C., ESBENSHADE T.A. Selectivity of agonists for cloned α1-adrenergic receptor subtypes. Mol. Pharmacol. 1994;46:929–936. [PubMed] [Google Scholar]
- MURAMATSU I., KIGOSHI S., OHMURA T. Subtypes of α1-adrenoceptor involved in noradrenaline-induced contractions of rat thoracic aorta and dog carotid artery. Jpn. J. Pharmacol. 1991;57:535–544. doi: 10.1254/jjp.57.535. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., OHMURA T., HASHIMOTO S., OSHITA M. Functional subclassification of vascular α1-adrenoceptors. Pharmacol. Commun. 1995;6:23–28. [Google Scholar]
- PIASCIK M.T., GUARINO R.D., SMITH M.S., SOLTIS E.E., SAUSSY JR D.L., PEREZ D.M. The specific contribution of the novel α1D-AR to the contraction of vascular smooth muscle. J. Pharmacol. Exp. Ther. 1995;275:1583–1589. [PubMed] [Google Scholar]
- PIASCIK M.T., HROMETZ S.L., EDELMANN S.E., GUARINO R.D., HADLEY RW BROWN R.D. Immunocytochemical localization of the α1B-AR and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J. Pharmacol. Exp. Ther. 1997;283:854–868. [PubMed] [Google Scholar]
- ROKOSH G.D., SIMPSON P.C. Knockout of the α1A/C-AR subtype: the α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci U.S.A. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAUSSY D.L., JR, GOETZ A.S., KING H.K., TRUE T.A. BMY7378 is a selective antagonist of α1D-ARs: evidence that rat vascular α1-ARs are of the α1D-subtype. Can. J. Physiol. Pharmacol. 1994;72 Suppl. 1:323. [Google Scholar]
- SCHWINN D.A., JOHNSTON G.I., PAGE S.O., MOSLEY M.J., WILSON K.H., WORMAN N.P., CAMPBELL S., FIDOCK M.D., FURNESS M., PARRY-SMITH D.J., PETER B., BAILEY S. Cloning and pharmacological characterization of human α1-ARs: sequence corrections and direct comparison with other species homologues. J. Pharmacol. Exp. Ther. 1995;272:134–142. [PubMed] [Google Scholar]
- SCOFIELD M.A., LIU F., ABEL P.W., JEFFRIES W.B. Quantification of steady state expression of mRNA for α1-adrenergic receptor subtypes using reverse transcription and a competitive polymerase chain reaction. J. Pharmacol. Exp. Ther. 1995;275:1035–1042. [PubMed] [Google Scholar]
- STANASILA L., PEREZ J.B., VOGEL H., COTECCHIA S. Oligomerization of the α1a- and α1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J. Biol. Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- STASSEN F.R., MAAS R.G., SCHIFFERS P.M., JANSSEN G.M., DE MEY JG. A positive and reversible relationship between adrenergic nerves and alpha-1A adrenoceptors in rat arteries. J. Pharmacol. Exp. Ther. 1998;284:399–405. [PubMed] [Google Scholar]
- TANOUE A., NASA Y., KOSHIMIZU T., SHINOURA H., OSHIKAWA S., KAWAI T., SUNADA S., TAKEO S., TSUJIMOTO G. The α1D-AR directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESTA R., DESTEFANI C., GUARNERI L., POGGESI E., SIMONAZZI I., TADDEI C., LEONARDI A. The α1D-adrenoceptor subtype is involved in the noradrenaline-induced contractions of rat aorta. Life Sci. 1995a;57:PL159–PL163. doi: 10.1016/0024-3205(95)02079-x. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., POGGESI E., SIMONAZZI I., TADDEI C., LEONARDI A. Mediation of noradrenaline-induced contractions of rat aorta by the α1B-adrenoceptor subtype. Br. J. Pharmacol. 1995b;114:745–750. doi: 10.1111/j.1476-5381.1995.tb13267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UBERTI M.A., HALL R.A., MINNEMAN K.P. Subtype-specific dimerization of α1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol. Pharmacol. 2003;64:1379–1390. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- VILLALOBOS-MOLINA R., IBARRA M. α1-Adrenoceptors mediating contraction in arteries of normotensive and spontaneously hypertensive rats are of the α1D or α1A subtypes. Eur. J. Pharmacol. 1996;298:257–263. doi: 10.1016/0014-2999(95)00781-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., BLUE D.R., DANIELS D.V., DAVIS B., ELWORTHY T., GEVER J.R., KAVA M.S., MORGANS D., PADILLA F., TASSA S., VIMONT R.L., CHAPPLE C.R., CHESS-WILLIAMS R., EGLEN R.M., CLARKE D.E., FORD A.P.D.W. In vitroα1-AR pharmacology of Ro 70-0004 and RS100329, novel α1A-AR selective antagonists. Br. J. Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG M., VERFURTH F., BUSCHER R., MICHEL M.C. Is α1D-adrenoceptor protein detectable in rat tissues. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:438–446. doi: 10.1007/pl00004966. [DOI] [PubMed] [Google Scholar]