Abstract
To gain further insight into the mechanisms underlying the antihyperalgesic and antiallodynic actions of gabapentin, a chronic pain model was prepared by partially ligating the sciatic nerve in mice. The mice then received systemic or local injections of gabapentin combined with either central noradrenaline (NA) depletion by 6-hydroxydopamine (6-OHDA) or α-adrenergic receptor blockade.
Intraperitoneally (i.p.) administered gabapentin produced antihyperalgesic and antiallodynic effects that were manifested by elevation of the withdrawal threshold to a thermal (plantar test) or mechanical (von Frey test) stimulus, respectively.
Similar effects were obtained in both the plantar and von Frey tests when gabapentin was injected intracerebroventricularly (i.c.v.) or intrathecally (i.t.), suggesting that it acts at both supraspinal and spinal loci. This novel supraspinal analgesic action of gabapentin was only obtained in ligated neuropathic mice, and gabapentin (i.p. and i.c.v.) did not affect acute thermal and mechanical nociception.
In mice in which central NA levels were depleted by 6-OHDA, the antihyperalgesic and antiallodynic effects of i.p. and i.c.v. gabapentin were strongly suppressed.
The antihyperalgesic and antiallodynic effects of systemic gabapentin were reduced by both systemic and i.t. administration of yohimbine, an α2-adrenergic receptor antagonist. By contrast, prazosin (i.p. or i.t.), an α1-adrenergic receptor antagonist, did not alter the effects of gabapentin.
It was concluded that the antihyperalgesic and antiallodynic effects of gabapentin are mediated substantially by the descending noradrenergic system, resulting in the activation of spinal α2-adrenergic receptors.
Keywords: Gabapentin; hyperalgesia; neuropathic pain; descending noradrenergic system, α2-adrenergic receptors
Introduction
Gabapentin (Neurontin) is an antiepileptic agent with established clinical efficacy in patients with refractory partial seizures and secondarily generalized tonic–clonic seizures (Goa & Sorkin, 1993). More recent studies have demonstrated that gabapentin also exhibits analgesic effects in patients with several neuropathic conditions, including diabetic neuropathy and postherpetic neuralgia (Segal & Rordorf, 1996; Backonja et al., 1998). Its clinical efficacy in the regulation of chronic pain is supported by animal studies employing various models of hyperalgesia and tactile allodynia (Singh et al., 1996; Field et al., 1997; Pan et al., 1999b; Luo et al., 2001).
Gabapentin was originally designed and synthesized as a structural analogue of γ-aminobutyric acid (GABA) that would be able to penetrate the blood–brain barrier. However, it exhibits negligible affinity for a number of widely studied neurotransmitter-binding sites, including GABAA and GABAB receptors (Taylor et al., 1998; but see Ng et al. (2001) and Bertrand et al., 2001). Nevertheless, the finding that it specifically binds to the α2δ-1 auxiliary subunit of the voltage-sensitive Ca2+ channel (Gee et al., 1996) may provide a molecular explanation for the inhibition of high-threshold Ca2+ channels (Sutton et al., 2002), the modulation of synaptic transmission (Patel et al., 2000; Shimoyama et al., 2000; Bayer et al., 2004), and the antiallodynic effects (Luo et al., 2001, 2002) observed with gabapentin. The studies by Luo et al. (2001, 2002) revealed that upregulation of the α2δ-1 subunits of voltage-sensitive Ca2+ channels in the spinal cord and dorsal root ganglia correlates with the development of allodynia following spinal nerve injury, and that gabapentin is effective only in animal models of neuropathic pain accompanied by upregulation of the α2δ-1 subunit. While these lines of experimental evidence have contributed considerably to our knowledge of the actions of gabapentin, much more information will be required to reach a full and detailed understanding of the mechanisms responsible for its anticonvulsant/analgesic effects.
Based on the importance of spinal plasticity and sensitization following peripheral nerve injury (Sandkühler & Liu, 1998; Yaksh et al., 1999; Miletic & Miletic, 2000; Sandkühler, 2000), attention has been focused on the spinal cord as the primary site of analgesic action of gabapentin, and indeed intrathecal (i.t.) administration of gabapentin has been shown to produce analgesic effects in chronic pain (Hwang & Yaksh, 1997; Cheng et al., 2000; Kaneko et al., 2000; Luo et al., 2001). In the present paper, we provide evidence that gabapentin also has a supraspinal site of action, in which central noradrenaline (NA), an essential neurotransmitter in the endogenous pain-inhibitory system, is involved.
Methods
All of the experimental protocols used here were approved by the Animal Care and Use Committee of Nagoya City University, and were carried out according to the guidelines of the National Institutes of Health and the Japanese Pharmacological Society.
Preparations of the animal model
The surgical procedure was based on that described by Seltzer et al. (1990). In brief, 4-week-old, male ddY mice were anesthetized by intraperitoneal (i.p.) administration of pentobarbital sodium (60 mg kg−1). One-third to one-half of the dorsal aspect of the right sciatic nerve was ligated just distal to its branch to the posterior biceps and semitendinosus muscles. Thermal hyperalgesia and tactile allodynia were assessed 7 days after ligation.
Assessment of thermal responsiveness
Thermal hyperalgesia was assessed using the plantar test (Ugo Basile, Comerio, Italy), following a modification of the method of Hargreaves et al. (1988). Mice were placed in a clear plastic chamber with a glass floor and allowed to acclimate to their environment before testing. During this time, the mice initially demonstrated exploratory behavior, but subsequently stopped exploring and stood quietly with occasional bouts of grooming. A mobile radiant heat source, which was located under the glass floor, was focused onto the plantar surface of the right hindpaw, and paw withdrawal latencies (PWLs) were recorded. PWLs were measured in duplicate for the right hindpaw of each animal, and the mean of the two values was used for analysis. A cutoff latency of 15 s was imposed to avoid tissue damage.
Assessment of tactile allodynia
Mice were placed in individual transparent Perspex cubicles with a wire mesh bottom, and a series of calibrated von Frey filaments (Semmes-Weinstein monofilaments, Stoelting, Wood Dale, IL, U.S.A.) was used to determine the 50% likelihood of a paw withdrawal response (50% threshold) using the up–down method of Dixon (1980). Eight von Frey filaments, with approximately equal logarithmic incremental bending forces, were chosen (von Frey number: 2.36, 2.44, 2.83, 3.22, 3.61, 3.84, 4.08, 4.17; equivalent to 0.02, 0.03, 0.07, 0.17, 0.41, 0.69, 1.20, and 1.48 g force, respectively). Testing was initiated with the 0.17 g hair, and each hair was applied perpendicularly to the plantar surface of the right hindpaw with sufficient force to bend the filaments, for 3–4 s. Lifting of the paw indicated a positive response and promoted the use of the next weaker (i.e., lighter) filament. Absence of a paw withdrawal response prompted the use of the next stronger (i.e., heavier) filament. This paradigm was continued until four measurements had been obtained after an initial change in behavior, or until four consecutive negative (score of 0.01 g) or five positive scores (score of 1.5 g) had been obtained. The resulting scores were used to calculate the 50% threshold (Chaplan et al., 1994).
In the study presented here, mice that exhibited a PWL of less than 5 s in the plantar test and a 50% threshold of 0.1 g in the von Frey test 7 days after partial ligation of the sciatic nerve were considered to be developing thermal hyperalgesia and tactile allodynia.
Locomotor activities
Locomotor activities during exploratory behavior in an open arena (18 cm × 28 cm floor with 13-cm-high walls) were determined by using an automated behavioral experimental apparatus (Animex IIIA, Shimazu, Kyoto, Japan). In this equipment, the movement detector operates by counting the number of times an animal elicits a capacitance change. Mice developing thermal hyperalgesia and tactile allodynia were injected intracerebroventricularly (i.c.v.) with either saline (as a control) or gabapentin. Assessment of locomotor activities was carried out for 1 h postinjection, locomotion was measured in 5-min windows during that time.
Effects on acute nociception
The degree of antinociception was determined using the plantar test (see above) and the paw pressure test in normal (nonligated) mice.
In the plantar test, an intensity of radiant heat higher than that used in ligated animals was applied. Otherwise at the weaker intensity as was used in ligated animals (including before ligation), mice before ligation occasionally exhibited the PWL values above 10 s, which is close to the cutoff latency of 15 s, which hampered us to make a proper evaluation of the analgesics on acute nociception.
Following the plantar test, mice were subjected to the paw pressure test (Pressure Analgesy-Meter, Muromachi Kikai, Tokyo, Japan) to assess their threshold for acute mechanical nociception. In brief, while the experimenter gently held the body of each mouse, the right hindpaw was exposed to increasing mechanical pressure. The pressure level was increased at a rate of 10 mmHg s−1, and the pressure (mmHg) required to elicit a response was determined for each mouse; that pressure was defined as the nociceptive threshold. Paw pressure measurements were made in duplicate, and the mean of the two values was used for calculations. The cutoff pressure was 200 mmHg.
Depletion of NA
Immediately before sciatic nerve ligation, under anesthesia with pentobarbital sodium (60 mg kg−1, i.p.), some groups of mice were injected intracisternally with 50 μg of the catecholaminergic neurotoxin 6-hydroxydopamine hydrobromide (6-OHDA), which was dissolved in 5 μl of 0.9% saline containing ascorbic acid (100 μg ml−1). Control animals were injected with the vehicle alone.
After the assessment of thermal hyperalgesia and tactile allodynia, the mice were killed by excessive inhalation of ether. The brain stem and spinal cord were dissected out, weighed, and then frozen on dry ice. Each brain stem or spinal cord was homogenized in 450 μl of 0.1 M perchloric acid containing the synthetic monoamine dihydroxybenzylamine (0.01 μg ml−1) as an internal standard. The contents of NA, serotonin, and dopamine (DA) were measured using reverse-phase high-performance liquid chromatography with electrochemical detection.
Drugs
Gabapentin, yohimbine HCl, prazosin HCl, and 6-OHDA were purchased from Sigma Chemical (St Louis, MO, U.S.A.). Gabapentin and yohimbine HCl were dissolved in 0.9% saline, and prazosin HCl in distilled water. When given i.p., the drugs were administered in a volume of 0.1 ml (10 g body weight)−1. For i.t. injection, the drugs were administered in a volume of 5 μl via a disposable 27-gauge needle, which was inserted into the subarachnoid space through the intervertebral foramen between L5 and L6 according to the method described by Hylden & Wilcox (1980). For i.c.v. injection, the drugs were also administered in a volume of 5 μl via a disposable 27-gauge needle, which was inserted into the lateral ventricle (Haley & McCormick, 1957). The α-adrenergic receptor antagonists were administered 15 min before gabapentin injection.
Statistical analysis
All data are expressed as the mean±s.e.m. The effects of gabapentin on the nociceptive threshold in both the plantar and von Frey tests were evaluated with respect to time; the time of administration of gabapentin was defined as time zero. Two-tailed nonparametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (Glantz, 1992) were used for multiple comparisons between the control and treated groups. The Mann–Whitney U-test was used for direct comparisons between two groups. Differences with P<0.05 (two-tailed) were considered significant.
Results
Gabapentin exhibits antihyperalgesic and antiallodynic effects in the Seltzer model
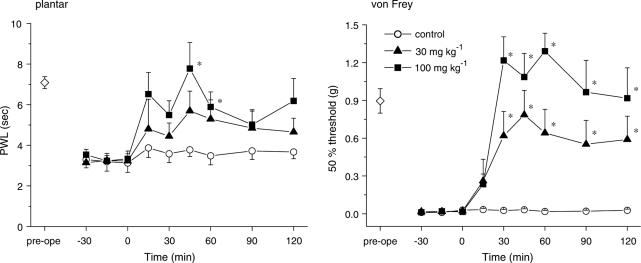
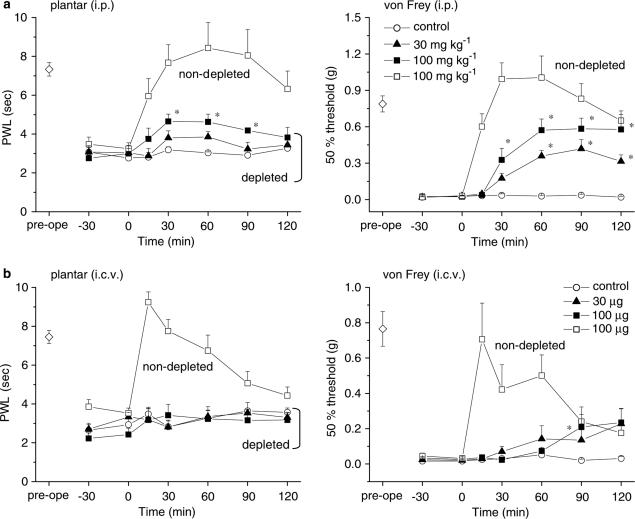
We first confirmed that gabapentin suppressed the thermal hyperalgesia and tactile allodynia that developed in mice with partially ligated sciatic nerves (the Seltzer model). As shown in Figure 1, i.p. gabapentin (30 and 100 mg kg−1) significantly elevated the PWL in the plantar test (P<0.05 at 100 mg kg−1) and markedly elevated the 50% threshold in the von Frey test (P<0.05 at 30 and 100 mg kg−1).
Figure 1.
Gabapentin exhibits antihyperalgesic and antiallodynic effects. Thermal hyperalgesia and tactile allodynia were assessed using the plantar and von Frey tests, respectively. Gabapentin (30 and 100 mg kg−1) was administered i.p. at time zero. Each point represents the mean±s.e.m. of seven separate experiments. Ordinates: mean PWLs (plantar test; left) and 50% thresholds (von Frey test; right). Abscissae: 7 days before (pre-ope) and time in minutes after gabapentin application. Open diamond in each graph shows the mean of pooled PWLs (left) or 50% thresholds (right) obtained before ligation in the three groups of mice. The asterisks indicate data points for which a significant difference between the control (open circle) and gabapentin-treated groups (closed triangle and square) was observed, as determined by two-tailed nonparametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05).
Gabapentin has both supraspinal and spinal actions
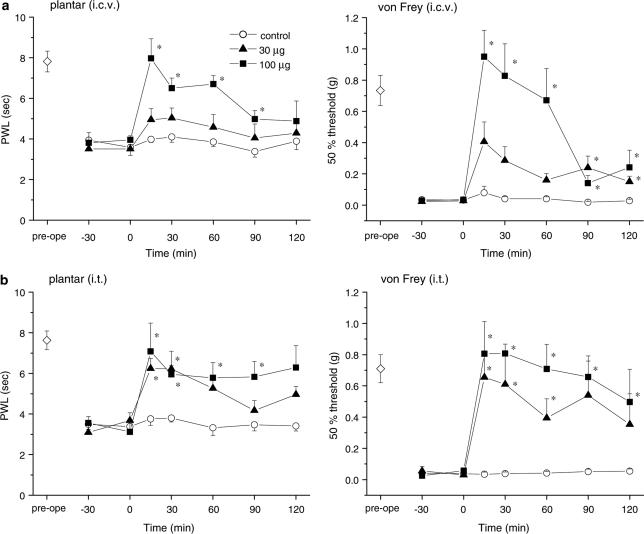
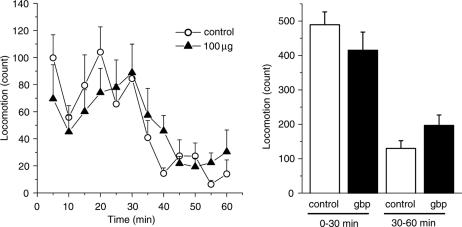
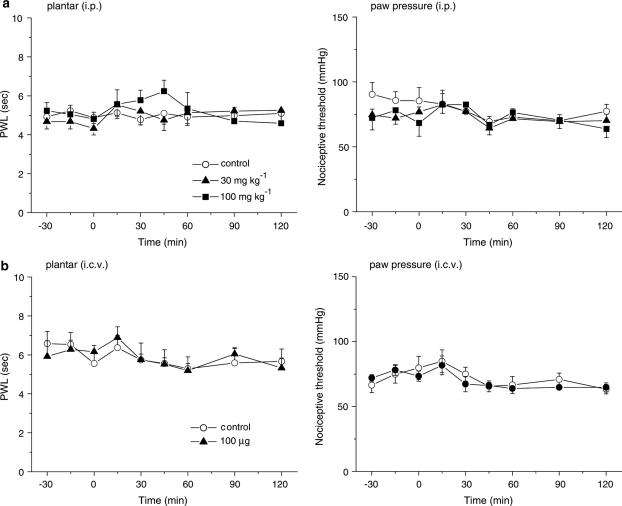
To explore the sites at which it exerts its antihyperalgesic and antiallodynic effects, gabapentin (30 and 100 μg) was injected via the i.c.v. and i.t. routes. Figure 2 shows the dose-dependent elevations of PWL in the plantar test and the 50% threshold in the von Frey test obtained in response to both i.c.v. and i.t. gabapentin (Figure 2a and b, respectively). Thus, both supraspinal and spinal sites contribute to the antihyperalgesic and antiallodynic effects produced by systemically administered gabapentin. Gabapentin (100 μg, i.c.v.) did not affect locomotor activities (Figure 3), indicating that the novel supraspinal analgesic action of gabapentin was not elicited secondarily via impaired motor activities. Moreover, the analgesic action of gabapentin mediated by supraspinal as well as systemic administration was observed exclusively in the neuropathic pain model (Seltzer) mice, since gabapentin (100 μg, i.c.v. or 100 mg kg−1, i.p.) did not exert any antinociceptive effects against acute thermal and mechanical nociception, as assessed by the plantar and paw pressure tests, respectively, in nonligated mice (Figure 4).
Figure 2.
Effects of a local injection of gabapentin. Both (a, i.c.v.) and (b, i.t.) administration of gabapentin (30 and 100 μg) ameliorated the symptoms of thermal hyperalgesia and tactile allodynia, indicating that gabapentin has both supraspinal and spinal actions. Gabapentin was injected at time zero. Each point represents the mean±s.e.m. of six or seven separate experiments. Ordinates: mean PWLs (plantar test; left) and 50% thresholds (von Frey test; right). Abscissae: 7 days before (pre-ope) and time in minutes after gabapentin injection. Open diamond in each graph shows the mean of pooled PWLs (left in a and b) or 50% thresholds (right in (a) and (b)) obtained before ligation in the three groups of mice. The asterisks indicate data points for which a significant difference between the control (open circle) and gabapentin-treated groups (closed triangle and square) was observed, as determined by two-tailed nonparametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05).
Figure 3.
Gabapentin administered i.c.v. does not affect locomotor activity. Mice developing thermal hyperalgesia and tactile allodynia were injected i.c.v. with either saline (vehicle control) or gabapentin (gbp, 100 μg), and the assessment of locomotor activities was carried out for 1 h postinjection (n=9–10). Ordinates: locomotion measured in 5-min periods (left) and between 0–30 and 30–60 min after injection (right). Gabapentin was administered at time zero.
Figure 4.
Gabapentin does not produce antinociceptive effects against acute thermal and mechanical nociception in nonligated mice. Acute thermal and mechanical nociception were assessed by the plantar and paw pressure tests, respectively. Note that an intensity of radiant heat higher than that used in ligated animals was applied in the study on acute thermal nociception. Gabapentin (30 and 100 mg kg−1, i.p. in (a) and 100 μg, i.c.v. in (b)) was administered at time zero. Each point represents the mean±s.e.m. of six or seven separate experiments. Ordinates: mean PWLs (plantar test; left) and nociceptive threshold (paw pressure; right). Abscissae: time in minutes after gabapentin injection.
Depletion of central NA decreases the effects of gabapentin
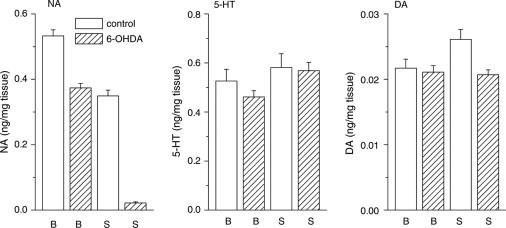
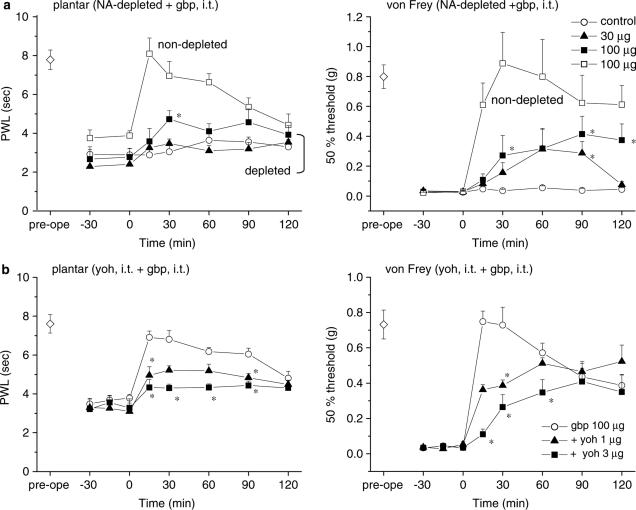
The endogenous pain-inhibitory system, which descends to the lumbar spinal cord from supraspinal structures, regulates spinal nociceptive transmission (Sagen & Proudfit, 1984; Jones, 1991). NA originating from the locus coeruleus is an essential neurotransmitter in the endogenous pain-inhibitory system. Based on our current observation that gabapentin has supraspinal sites of action, we expected central NA to play a role in the generation of its antihyperalgesic and antiallodynic effects. Experiments were therefore performed using mice in which central NA levels had been depleted by intracisternal injection of 6-OHDA immediately before ligation of the sciatic nerve. This treatment with 6-OHDA reduced the NA contents of the brain stem and spinal cord to 70.1 and 6.2%, respectively, of that in control animals treated with vehicle (ascorbic acid) alone (data obtained from 17 control and 52–53 6-OHDA-treated animals), as quantified after the assessment of thermal hyperalgesia and tactile allodynia (Figure 5). Simultaneously measured serotonin and DA contents in both the brain stem and spinal cord were not prominently changed by intracisternal injection of 6-OHDA (Figure 5). Furthermore, thermal hyperalgesia was more severe in the mice that had been pretreated with 6-OHDA. In mice undergoing sciatic ligation without vs with 6-OHDA pretreatment, PWL values in the plantar test were 3.6±0.2 vs 2.8±0.1 s (n=17 and 54, respectively; P<0.01, two-tailed Mann–Whitney U-test), while the 50% thresholds in the von Frey test were 0.031±0.004 vs 0.025±0.003 g (n=17 and 54, respectively; not significant by two-tailed Mann–Whitney U-test). In mice pretreated with 6-OHDA, i.p. gabapentin (30 and 100 mg kg−1) elicited a weak elevation of the PWL in the plantar test, and the elevation of the 50% threshold in the von Frey test was substantially suppressed (Figure 6a).
Figure 5.
Depletion of descending NA. 6-OHDA was injected intracisternally immediately before ligation of the sciatic nerve. After the assessment of thermal hyperalgesia and tactile allodynia (see Figures 6 and 9), the contents of NA, 5-HT, and DA in the brain stem (B) and spinal cord (S) obtained from vehicle control (open columns, n=17) and 6-OHDA-treated mice (hatched columns, n=49–53) were measured using reverse-phase high-performance liquid chromatography with electrochemical detection.
Figure 6.
Depletion of descending NA levels strongly reduces the antihyperalgesic and antiallodynic effects of systemically and i.c.v. administered gabapentin. 6-OHDA was injected intracisternally immediately before ligation of the sciatic nerve. Gabapentin (30 and 100 mg kg−1, i.p. in (a), and 30 and 100 μg, i.c.v. in (b)) was administered at time zero. Each point represents the mean±s.e.m. of six separate experiments. Ordinates: mean PWLs (plantar test; left) and 50% thresholds (von Frey test; right). Abscissae: 7 days before (pre-ope) and time in minutes after gabapentin administration. Open diamond in each graph shows the mean of pooled PWLs (left in (a) and (b)) or 50% thresholds (right in (a) and (b)) obtained before ligation in the four groups of mice. The asterisks indicate data points for which a significant difference between the control (open circle) and gabapentin-treated groups (closed triangle and closed square) was observed, as determined by two-tailed nonparametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05). Pretreatment with vehicle (ascorbic acid) alone did not affect the antihyperalgesic and antiallodynic actions of gabapentin (100 mg kg−1, i.p. in (a) and 100 μg, i.c.v. in (b)), as shown superimposed in the graphs (n=5–6, open square).
We then evaluated the effect of i.c.v. gabapentin (30 and 100 μg) in mice that had been pretreated with 6-OHDA. Again, gabapentin was unable to increase the PWL in the plantar test and failed to raise the 50% threshold in the von Frey test (Figure 6b).
In control mice that had been pretreated with ascorbic acid, we confirmed the antihyperalgesic and antiallodynic actions of gabapentin (100 mg kg−1, i.p., 100 μg, i.c.v., or 100 μg, i.t.), indicating that ascorbic acid alone does not impair the analgesic action of gabapentin (shown in Figures 6 and 9a).
Figure 9.
Depletion of descending NA levels or blockade of spinal α2-adrenergic receptors partly reduces the antihyperalgesic and antiallodynic effects of i.t. administered gabapentin. 6-OHDA was injected intracisternally immediately before ligation of the sciatic nerve. Gabapentin (gbp, 30 and 100 μg, i.t. in (a) and (b)) was administered at time zero. Yohimbine HCl (yoh, 1 and 3 μg, i.t. in (b)) was administered 15 min before the administration of gabapentin. Each point represents the mean±s.e.m. of seven separate experiments. Ordinates: mean PWLs (plantar test; left) and 50% thresholds (von Frey test; right). Abscissae: 7 days before (pre-ope) and time in minutes after gabapentin administration. Open diamond in each graph shows the mean of pooled PWLs (left in (a) and (b)) or 50% thresholds (right in (a) and (b)) obtained before ligation in the four (a) and three (b) groups of mice. In (a), pretreatment with vehicle (ascorbic acid) alone did not affect the antihyperalgesic and antiallodynic actions of gabapentin (100 μg, i.t.), as shown superimposed in the graphs (n=6, open square). In (a) and (b), the asterisks indicate data points for which a significant difference between (a) the control (open circle) and gabapentin-treated groups (closed triangle and closed square) or (b) the gabapentin-only (open circle) and yohimbine-treated groups (closed triangle and square) was observed, as determined by two-tailed nonparametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05).
Taken together, these results indicate that the actions of systemically administered gabapentin are mediated largely by central NA originating from the brain stem, which presumably descends to the spinal cord to inhibit pain transmission. To investigate further the involvement of the descending noradrenergic system in the supraspinal action of gabapentin, we next performed pharmacological experiments with specific antagonists of α-adrenergic receptors.
Spinal α2-adrenergic receptors mediate the supraspinal actions of gabapentin
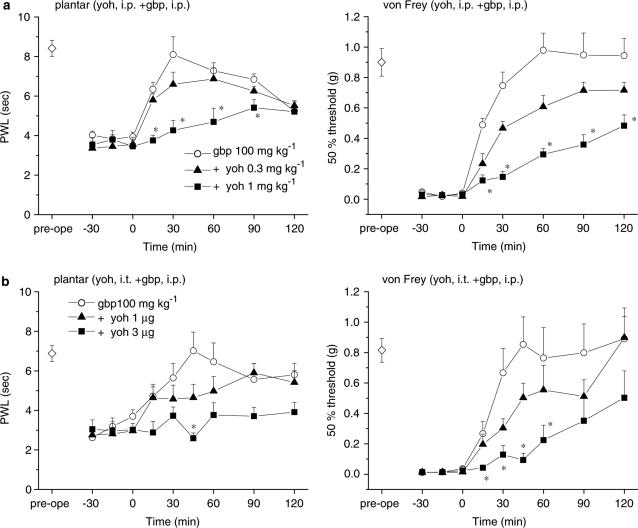
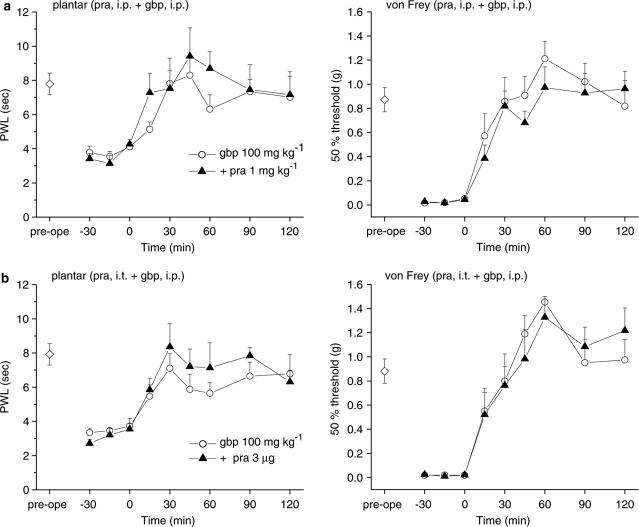
Activation of the noradrenergic endogenous pain-inhibitory system stimulates α2-adrenergic receptors in the lumbar spinal cord, resulting in analgesic effects (Sagen & Proudfit, 1984; Jones, 1991; Jasmin et al., 2002). In our mice with partially ligated sciatic nerves, systemic administration of the α2-adrenergic receptor antagonist yohimbine HCl, at doses of 0.3 and 1 mg kg−1 (i.p.), did not alter the nociceptive thresholds measured using either the plantar or von Frey tests. Subsequent application of gabapentin (100 mg kg−1, i.p., 15 min after yohimbine) elicited only slight elevations of the PWL in the plantar test and the 50% threshold in the von Frey test (Figure 7a). Moreover, i.t. yohimbine HCl (1 and 3 μg) reduced the analgesic effects of systemically administered gabapentin (100 mg kg−1, i.p., Figure 7b) against thermal and mechanical stimuli in a dose-dependent manner. By contrast, blockade of spinal α1-adrenergic receptors by systemic or i.t. injection of prazosin HCl (1 mg kg−1, i.p. or 3 μg, i.t.) did not alter the analgesic effects of systemic gabapentin (Figure 8).
Figure 7.
The α2-adrenergic receptor antagonist yohimbine reduces the antihyperalgesic and antiallodynic effects of gabapentin. Yohimbine HCl (yoh) was administered either i.p. ((a); 0.3 and 1 mg kg−1) or i.t. ((b); 1 and 3 μg) 15 min before the administration of gabapentin (gbp, 100 mg kg−1, i.p., administered at time zero). Each point represents the mean±s.e.m. of six or seven separate experiments. Ordinates: mean PWLs (plantar test; left) and 50% thresholds (von Frey test; right). Abscissae: 7 days before (pre-ope) and time in minutes after gabapentin application. Open diamond in each graph shows the mean of pooled PWLs (left in (a) and (b)) or 50% thresholds (right in (a) and (b)) obtained before ligation in the three groups of mice. The asterisks indicate data points for which a significant difference between the gabapentin-only (open circle) and yohimbine-treated groups (closed triangle and square) was observed, as determined by two-tailed nonparametric Bonferroni-type multiple comparisons following the Kruskal–Wallis test (two comparisons in three groups, *P<0.05).
Figure 8.
The α1-adrenergic receptor antagonist prazosin does not affect the antihyperalgesic and antiallodynic effects of gabapentin. Prazosin HCl (pra) was administered either i.p. ((a); 1 mg kg−1) or i.t. ((b); 3 μg) 15 min before the administration of gabapentin (gbp, 100 mg kg−1, i.p., administered at time zero). Each point represents the mean±s.e.m. of six or seven separate experiments (open circle; gabapentin-only, closed triangle; prazosin-treated). Ordinates: mean PWLs (plantar test; left) and 50% thresholds (von Frey test; right). Abscissae: 7 days before (pre-ope) and time in minutes after gabapentin application. Open diamond in each graph shows the mean of pooled PWLs (left in (a) and (b)) or 50% thresholds (right in (a) and (b)) obtained before ligation in the two groups of mice.
Taken together, our results indicate that the descending noradrenergic system, coupled with spinal α2-adrenergic receptors, mediates the supraspinal actions of gabapentin.
The descending noradrenergic system also influences the spinal action of gabapentin
Finally, we assessed whether the spinal action of gabapentin was dependent on the descending noradrenergic system, and found that i.t. gabapentin was less effective in mice with depleted NA or after blockade of spinal α2-adrenergic receptors (Figure 9). These results suggest that the spinal action of gabapentin is partially dependent on the descending noradrenergic system. We did not investigate further the relationship between the spinal action of gabapentin and the spinal levels of NA.
Discussion
Our results indicate that gabapentin acts on supraspinal structures to activate the descending noradrenergic system. This terminates in the lumbar spinal cord, where NA interacts with α2-adrenergic receptors to reduce the transmission of nociceptive information. Considered together with the well-established effects of gabapentin on the spinal cord (Hwang & Yaksh, 1997; Kaneko et al., 2000; Patel et al., 2000; Luo et al., 2001, 2002), this novel, supraspinally mediated effect provides a substantial explanation of the analgesia produced by systemically administered gabapentin in mice that develop thermal hyperalgesia and tactile allodynia following partial ligation of the sciatic nerve.
In the present experiments, we confirmed that gabapentin has no antinociceptive effects against acute pain, a finding that is consistent with observations from previous studies (Field et al., 1997; Laughlin et al., 2002). Previous findings also support the notion that gabapentin is likely to activate the NA-mediated endogenous pain inhibitory system only in chronic hyperalgesic conditions. The hyperalgesia-dependent inhibition of excitatory synaptic transmission by gabapentin has been demonstrated in the dorsal horn of spinal cord slices prepared from hyperalgesic rats with streptozotocin-induced diabetic neuropathy (Patel et al., 2000; but see Shimoyama et al., 2000). Moreover, it has been shown that gabapentin reduces the release of substance P and calcitonin gene-related peptide from spinal tissues only after inflammation-induced sensitization (Fehrenbacher et al., 2003). The molecular basis for the supraspinal action of gabapentin remains unclear; however, there is evidence that the α2δ-1 subunit of the voltage-dependent calcium channel, the only known specific binding site of gabapentin (Gee et al., 1996), is upregulated in the dorsal root ganglion and spinal cord in association with the development of gabapentin-sensitive allodynia (Luo et al., 2001, 2002). Similar upregulation of this subunit, or specific expression of as yet unidentified gabapentin-binding sites, could take place in the supraspinal structures of mice with thermal hyperalgesia and tactile allodynia induced by partial ligation of the sciatic nerve. In addition to antihyperalgesic and antiallodynic effects, gabapentin sometimes generated the analgesic effects beyond nociceptive withdrawal levels obtained before ligation in ligated animals, which may be also explained by such plastic changes in the spinal and supraspinal structures.
It has been suggested that the reduction in the analgesic effects of opiates observed in some chronic pain patients (Arner & Meyerson, 1988) is partly attributable to noradrenergic dysfunction. However, the neuropathic pain model employed in this study (the Seltzer model) is likely to involve little or no noradrenergic dysfunction. The model mice that developed thermal hyperalgesia and tactile allodynia in this study exhibited the same sensitivity to morphine as normal mice in the plantar test (data not shown). Moreover, when sciatic nerve ligation was combined with 6-OHDA-induced NA depletion, we observed further decreases in the withdrawal threshold against radiant heat (plantar test), supporting the results of Jasmin et al. (2002). Thus, the mechanism involved in the development of thermal hyperalgesia and tactile allodynia following partial ligation of the sciatic nerve is independent of the central noradrenergic system, and animals with chronic pain induced by such methods seem to have functional descending noradrenergic influences on the lumbar spinal cord, via which supraspinal gabapentin exerts its analgesic action. After disabling the noradrenergic inhibitory system by pretreatment with 6-OHDA, supraspinally injected gabapentin no longer produced antihyperalgesic and antiallodynic effects. Thus, the ability of the supraspinal action of gabapentin to contribute to its systemic effects appears to depend on the existence of a functional descending noradrenergic system.
Our pharmacological experiments revealed that systemically or i.t. administered yohimbine (a specific antagonist of α2-adrenergic receptors), but not systemically administered prazosin (a specific antagonist of α1-adrenergic receptors), produced a marked reduction in the effects of gabapentin on thermal hyperalgesia and tactile allodynia. This is in good agreement with the evidence presented here that gabapentin acts on supraspinal structures to activate the descending noradrenergic system, which terminates in the spinal cord. Hence, the supraspinal action of gabapentin would induce increased release of NA, with consequential activation of the α2-adrenergic receptors in the dorsal horn of the spinal cord. Experimental and clinical evidence shows that α2-adrenergic agonists such as clonidine produce the analgesic effects at the spinal cord not only in acute (Reddy et al., 1980; Takano & Yaksh, 1992; Hunter et al., 1997; Stone et al., 1997) and chronic (Puke & Wiesenfeld-Hallin, 1993; Yaksh et al., 1995; Pan et al., 1999a; Kawamata et al., 2003) experimental pain animal models, but also in human patients suffering from acute (Eisenach et al., 1989; Mendez et al., 1990) and chronic (Glynn & O'Sullivan, 1996; Siddall et al., 2000) pain. In agreement with previous studies (Hwang & Yaksh, 1997; Cheng et al., 2000; Kaneko et al., 2000; Luo et al., 2001), i.t. gabapentin generated antihyperalgesic and antiallodynic effects, which undoubtedly contributed to the analgesic effects of systemically applied gabapentin. However, considering that the systemic and supraspinal effects were markedly suppressed by NA depletion, an action on supraspinal structures, leading in turn to the activation of spinal α2-adrenergic receptors, is likely to explain most of the systemic effect of gabapentin. Recent studies have provided evidence indicating that activation of spinal α2-adrenergic receptors is followed by the release of spinal acetylcholine and nitric oxide, which are thought to play an important role in generating the analgesic action of clonidine in the spinal cord (Xu et al., 1997; Pan et al., 1998, 1999a). Whether a similar mechanism mediates the analgesic action of gabapentin on thermal hyperalgesia and tactile allodynia after activation of the descending noradrenergic system remains to be studied.
Unlike the supraspinal action of gabapentin that leads to activation of the noradrenergic endogenous pain inhibitory system, we expected that its spinal actions, which may include effects on the spinal neurons and/or the primary sensory afferent terminals, would be solely independent of the descending noradrenergic system. However, the results show that the spinal action of gabapentin requires the functional noradrenergic system coupled with activation of α2-adrenergic receptors, which may support the finding that clonidine and gabapentin, when administered i.t., interact synergistically against tactile allodynia (Cheng et al., 2000). The systemic effects of gabapentin may include a synergistic interaction between gabapentin and NA at the spinal cord, where NA is released as a result of the supraspinal action of gabapentin.
Further studies focusing on the alterations in neuronal activity in supraspinal structures elicited by spinal sensitization may provide some explanation as to how gabapentin influences the descending noradrenergic system. The present results show the importance of supraspinal structures in the pharmacological regulation of thermal hyperalgesia and tactile allodynia.
Abbreviations
- DA
dopamine
- GABA
γ-aminobutyric acid
- 5-HT
5-hydroxytriptamine (serotonin)
- NA
noradrenaline
- 6-OHDA
6-hydroxydopamine hydrobromide
- PWL
paw withdrawal latency
References
- ARNER S., MEYERSON B.A. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- BACKONJA M., BEYDOUN A., EDWARDS K.R., SCHWARTZ S.L., FONSECA V., HES M., LAMOREAUX L., GAROFALO E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. J. Am. Med. Assoc. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- BAYER K., AHMADI S., ZEILHOFER H.U. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology. 2004;46:743–749. doi: 10.1016/j.neuropharm.2003.11.010. [DOI] [PubMed] [Google Scholar]
- BERTRAND S., NG G.Y.K., PURISAI M.G., WOLFE S.E., SEVERIDT M.W., NOUEL D., ROBITAILLE R., LOW M.J., O'NEILL G.P., METTERS K., LACAILLE J.C., CHRONWALL B.M., MORRIS S.J. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain γ-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channels. J. Pharmacol. Exp. Ther. 2001;298:15–24. [PubMed] [Google Scholar]
- CHAPLAN S.R., BACH F.W., POGREL J.W., CHUNG J.M., YAKSH T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- CHENG J.K., PAN H.L., EISENACH J.C. Antiallodynic effect of intrathecal gabapentin and its interaction with clonidine in a rat model of postoperative pain. Anesthesiology. 2000;92:1126–1131. doi: 10.1097/00000542-200004000-00031. [DOI] [PubMed] [Google Scholar]
- DIXON W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- EISENACH J.C., LYSAK S.Z., VISCOMI C.M. Epidural clonidine analgesia following surgery: phase I. Anesthesiology. 1989;71:640–646. doi: 10.1097/00000542-198911000-00003. [DOI] [PubMed] [Google Scholar]
- FEHRENBACHER J.C., TAYLOR C.P., VASKO M.R. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- FIELD M.J., OLES R.J., LEWIS A.S., MCCLEARY S., HUGHES J., SINGH L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br. J. Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEE N.S., BROWN J.P., DISSANAYAKE V.U.K., OFFORD J., THURLOW R., WOODRUFF G.N. The novel anticonvulsant drug, gabapentin (neurontin), binds to the α2δ subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- GLANTZ S.A.Alternatives to analysis of variance and the t test based on ranks Primer of Biostatistics 1992New York: McGraw-Hill; 320–371.3rd edn. ed. Jeffers, J.D. & Englis, M.R. pp [Google Scholar]
- GLYNN C., O'SULLIVAN K. A double-blind randomized comparison of the effects of epidural clonidine, lignocaine and the combination of clonidine and lignocaine in patients with chronic pain. Pain. 1996;64:337–343. doi: 10.1016/0304-3959(95)00119-0. [DOI] [PubMed] [Google Scholar]
- GOA K.L., SORKIN E.M. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46:409–427. doi: 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- HALEY T.J, MCCORMICK W.G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br. J. Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARGREAVES K., DUBNER R., BROWN F., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HUNTER J.C., FONTANA D.J., HEDLEY L.R., JASPER J.R., LEWIS R., LINK R.E., SECCHI R., SUTTON J., EGLEN R.M. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br. J. Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG J.H., YAKSH T.L. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg. Anesth. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L., WILCOX G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- JASMIN L., TIEN D., WEINSHENKER D., PALMITER R.D., GREEN P.G., JANNI G., OHARA P.T. The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1029–1034. doi: 10.1073/pnas.012598599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES S.L. Descending noradrenergic influences on pain. Prog. Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- KANEKO M., MESTRE C., SÁNCHEZ E.H., HAMMOND D.L. Intrathecally administered gabapentin inhibits formalin-evoked nociception and the expression of Fos-like immunoreactivity in the spinal cord of the rat. J. Pharmacol. Exp. Ther. 2000;292:743–751. [PubMed] [Google Scholar]
- KAWAMATA T., OMOTE K., YAMAMOTO H., TORIYABE M., WADA K., NAMIKI A. Antihyperalgesic and side effects of intrathecal clonidine and tizanidine in a rat model of neuropathic pain. Anesthesiology. 2003;98:1480–1483. doi: 10.1097/00000542-200306000-00027. [DOI] [PubMed] [Google Scholar]
- LAUGHLIN T.M., TRAM K.V., WILCOX G.L., BIRNBAUM A.K. Comparison of antiepileptic drugs tiagabine, lamotrigine, and gabapentin in mouse models of acute, prolonged, and chronic nociception. J. Pharmacol. Exp. Ther. 2002;302:1168–1175. doi: 10.1124/jpet.302.3.1168. [DOI] [PubMed] [Google Scholar]
- LUO Z.D., CALCUTT N.A., HIGUERA E.S., VALDER C.R., SONG Y.-H., SVENSSON C.I., MYERS R.R. Injury type-specific calcium channel α2δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- LUO Z.D., CHAPLAN S.R., HIGUERA E.S., SORKIN L.S., STAUDERMAN K.A., WILLIAMS M.E., YAKSH T.L. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDEZ R., EISENACH J.C., KASHTAN K. Epidural clonidine analgesia after cesarean section. Anesthesiology. 1990;73:848–852. doi: 10.1097/00000542-199011000-00009. [DOI] [PubMed] [Google Scholar]
- MILETIC G., MILETIC V. Long-term changes in sciatic-evoked A-fiber dorsal horn field potentials accompany loose ligation of the sciatic nerve in rats. Pain. 2000;84:353–359. doi: 10.1016/s0304-3959(99)00227-4. [DOI] [PubMed] [Google Scholar]
- NG G.Y.K., BERTRAND S., SULLIVAN R., ETHIER N., WANG J., YERGEY J., BELLEY M., TRIMBLE L., BATEMAN K., ALDER L., SMITH A., MCKERNAN R., METTERS K., O'NEILL G.P., LACAILLE J.C., HÉBERT T.E. γ-Aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin action. Mol. Pharmacol. 2001;59:144–152. [PubMed] [Google Scholar]
- PAN H.L., CHEN S.R., EISENACH J.C. Role of spinal NO in antiallodynic effect of intrathecal clonidine in neuropathic rats. Anesthesiology. 1998;89:1518–1523. doi: 10.1097/00000542-199812000-00031. [DOI] [PubMed] [Google Scholar]
- PAN H.L., CHEN S.R., EISENACH J.C. Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999a;90:509–514. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- PAN H.L., EISENACH J.C., CHEN S.R. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J. Pharmacol. Exp. Ther. 1999b;288:1026–1030. [PubMed] [Google Scholar]
- PATEL M.K., GONZALEZ M.I., BRAMWELL S., PINNOCK R.D., LEE K. Gabapentin inhibits excitatory synaptic transmission in the hyperalgesic spinal cord. Br. J. Pharmacol. 2000;130:1731–1734. doi: 10.1038/sj.bjp.0703530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUKE M.J., WIESENFELD-HALLIN Z. The differential effects of morphine and the alpha 2-adrenoceptor agonists clonidine and dexmedetomidine on the prevention and treatment of experimental neuropathic pain. Anesthesia Analgesia. 1993;77:104–109. doi: 10.1213/00000539-199307000-00021. [DOI] [PubMed] [Google Scholar]
- REDDY S.V., MADERDRUT J.L., YAKSH T.L. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J. Pharmacol. Exp. Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- SAGEN J., PROUDFIT H.K. Effect of intrathecally administered noradrenergic antagonists on nociception in the rat. Brain Res. 1984;310:295–301. doi: 10.1016/0006-8993(84)90152-5. [DOI] [PubMed] [Google Scholar]
- SANDKÜHLER J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- SANDKÜHLER J., LIU X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur. J. Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- SEGAL A.Z., RORDORF G. Gabapentin as a novel treatment for postherpetic neuralgia. Neurology. 1996;46:1175–1176. doi: 10.1212/wnl.46.4.1175. [DOI] [PubMed] [Google Scholar]
- SELTZER Z., DUBNER R, SHIR Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- SHIMOYAMA M., SHIMOYAMA N., HORI Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405–414. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- SIDDALL P.J., MOLLOY A.R., WALKER S., MATHER L.E., RUTKOWSKI S.B., COUSINS M.J. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesthesia Analgesia. 2000;91:1493–1498. doi: 10.1097/00000539-200012000-00037. [DOI] [PubMed] [Google Scholar]
- SINGH L., FIELD M.J., FERRIS P., HUNTER J.C., OLES R.J., WILLIAMS R.G., WOODRUFF G.N. The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-serine. Psychopharmacology. 1996;127:1–9. doi: 10.1007/BF02805968. [DOI] [PubMed] [Google Scholar]
- STONE L.S., MACMILLAN L.B., KITTO K.F., LIMBIRD L.E., WILCOX G.L. The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J. Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON K.G., MARTIN D.J., PINNOCK R.D., LEE K., SCOTT R.H. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br. J. Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO Y., YAKSH T.L. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J. Pharmacol. Exp. Ther. 1992;261:764–772. [PubMed] [Google Scholar]
- TAYLOR C.P., GEE N.S., SU T.-Z., KOCSIS J.D., WELTY D.F., BROWN J.P., DOOLEY D.J., BODEN P., SINGH L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- XU Z., TONG C., PAN H.L., CERDA S.E., EISENACH J.C. Intravenous morphine increases release of nitric oxide from spinal cord by an α-adrenergic and cholinergic mechanism. J. Neurophysiol. 1997;78:2072–2078. doi: 10.1152/jn.1997.78.4.2072. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., HUA X.Y., KALCHEVA I., NOZAKI-TAGUCHI N., MARSALA M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAKSH T.L., POGREL J.W., LEE Y.W., CHAPLAN S.R. Reversal of nerve ligation-induced allodynia by spinal alpha-2 adrenoceptor agonists. J. Pharmacol. Exp. Ther. 1995;272:207–214. [PubMed] [Google Scholar]