Abstract
Rheumatoid arthritis is a serious, inflammatory disease of the distal joints that has a possible neurogenic component underlying its pathology.
Substance P (SP), an endogenous neuropeptide that acts upon the neurokinin 1 (NK1) receptor, is released from sensory nerves and is involved in neurogenic inflammation.
In this study, we have developed novel techniques to determine the contribution of SP to microvascular responses in a model of complete Freund's adjuvant (CFA)-induced arthritis in NK1 knockout mice.
Detailed analysis in normal mice revealed that CFA (20 μg i.art.)-induced plasma extravasation was raised from 18 to 72 h, when compared with intravascular volume. By comparison, knee swelling was sustained for 3 weeks. Neutrophil accumulation mirrored plasma extravasation. SP (10 pmol i.art.) caused significant acute plasma extravasation, but not other parameters, in wild type (WT), but not NK1 knockout mice. CFA (10 μg i.art.) induced a significantly decreased intravascular volume, presumably due to decreased blood flow, at early time points (5 and 7 h) in WT but not NK1 knockouts. Otherwise, similar responses in WT and NK1 knockout mice were observed. However, injection of SP into CFA-pretreated joints caused a significant enhancement of plasma extravasation and knee swelling in the WT but not NK1 knockouts.
In conclusion, the present study has used novel techniques in WT and NK1 knockout mice to show that SP can modulate vascular tone and permeability in the inflamed joint via activation of the NK1 receptor and that SP-induced responses are more pronounced where pre-existing inflammation is present.
Keywords: Substance P, rheumatoid arthritis, neurokinin 1 receptor, plasma extravasation, intravascular volume, neutrophil accumulation, knee swelling
Introduction
Rheumatoid arthritis in humans is a chronic, debilitating, inflammatory disease that affects connective tissue in distal joints and is characterized by spontaneous remissions and unpredictable exacerbations of the condition (Ignatavicius, 2001). Inflammatory processes involved in joint disease include plasma extravasation leading to joint swelling, vascular remodeling and cellular infiltration of the synovia. The precise microvascular mechanisms underlying these events are not fully understood, but it is possible that a neurogenic component is involved in joint disease. For example, in experimental models of joint disease, transection of sensory nerves has been shown to reduce hyperalgesia, swelling and joint destruction (Levine et al., 1986). Furthermore, there is a strong tendency for symmetrical joint inflammation in patients with rheumatoid arthritis suggesting a possible neural influence on the progression of the disease (Niissalo et al., 2002). Indeed, a neurogenic influence of contralateral responses has been demonstrated in a rat model of joint disease (Kidd et al., 1995).
Neurogenic inflammation is defined as the oedema formation, increased blood flow and inflammatory cell involvement observed after stimulation of sensory nerve fibres (usually C and Aδ fibres) and release of neuropeptides, such as substance P (SP), calcitonin gene-related peptide and neuropeptide Y. SP, acting on the neurokinin 1 (NK1) receptor, exerts a variety of proinflammatory effects. For example, SP is a potent mediator of increased microvascular permeability in species that include the mouse through its action on postcapillary endothelial cells (Cao et al., 1999) and SP has a potent effect on the cellular components of inflammation, such as neutrophil accumulation (Cao et al., 2000).
The involvement of SP in joint inflammation has been implicated in arthritis, both in human and experimental animal studies. In human rheumatoid synovia, a strong hybridization signal of the NK1 receptor has been detected and, moreover, the signal intensity of NK1 receptor mRNA positively correlated with the severity of joint disease (Sakai et al., 1998). In animal studies, antigen-induced mono-arthritis leads to a pronounced bilateral upregulation of expression of NK1 receptors in the rat dorsal root ganglion (Segond von Banchet et al., 2000). Furthermore, adjuvant-induced joint inflammation has been shown to cause a very rapid transcription of the SP precursor, β-preprotachykinin, in the innervating dorsal root ganglia of the rat (Bulling et al., 2001).
The availability of NK1 receptor knockout mice now enables the investigation of neurogenic mechanisms in arthritis in this species. However, to date, published methods for investigating joint disease in mice do not permit a detailed analysis of the early stages of microvascular responses in which neuropeptides are known to have their most potent effects. With this in mind, we have developed techniques whereby both the early and late microvascular responses in the mouse knee joint can be determined. In particular, a quantitative radiometric assay for measuring plasma extravasation or intravascular volume has been developed and used in conjunction with a traditional technique for measuring knee swelling. Furthermore, a method has been developed for collecting a synovial lavage from mouse knee joints. To our knowledge, this is the first time that a technique that permits the analysis of the cellular and soluble contents of mouse joint synovial fluid has been published. Using the developed techniques, we have elucidated the time course of microvascular responses to intra-articular (i.art.) injection of complete Freund's adjuvant (CFA), determined the relationship between plasma extravasation and knee swelling and investigated the contribution of SP to joint inflammation.
Methods
Preparation of animals
Female CD1 mice (25–30 g) were purchased from Harlan, U.K. Female Sv129+C57BL/6 mice (25–30 g), either genetically unaltered or lacking the gene for the NK1 receptor, were bred in house from mice donated by N. Gerard (Boston, U.S.A.). Mice were kept in a climatically controlled environment and had access to food and water ad libitum. Experiments were carried out in accordance with the U.K. Home Office Animals (Scientific Procedures) Act, 1986. All recovery procedures were performed under isoflurane anaesthesia (2% vol isoflurane, 2% vol O2) and all non-recovery procedures were carried out under urethane anaesthesia (2.5 mg g−1 i.p.). 30 G needles were used for all i.art. injections (BD Micro-Fine insulin syringes, 0.3 ml).
Joint inflammation
Adjuvant-induced joint inflammation was induced via i.art. injections of CFA into the ipsilateral hind knee joint and an equivalent volume of saline (0.9% sodium chloride, pyrogen free) injected into the contralateral hind knee joint for control and inflammation was allowed to develop for 1 h–3 weeks. In total, 20 μg of CFA in 20 μl was injected into the joints of CD1 mice. The dose of CFA was reduced to 10 μg in 10 μl for Sv129+C57BL/6 mice due to their significantly smaller knee diameters when compared with CD1 mice (3.54±0.03 vs 3.93±0.03 mm, respectively; n=20, P<0.001). The inflammatory effects of SP (10–1000 pmol, 10 μl ipsilateral joint, Sigma, U.K.) and saline (10 μl, contralateral joint) were examined over 30 min for plasma extravasation and intravascular volume and over 5 h for neutrophil accumulation. The effect of SP on adjuvant-induced joint inflammation was examined as follows: NK1 receptor wild type (WT) and knockout mice were given CFA (10 μg, 10 μl i.art.) into both joints and inflammation was allowed to develop for 4 h 30 min. After this time, the mice were given SP (100 pmol, 10 μl, i.art.) or saline (10 μl, i.art. contralateral joint) and inflammation was allowed to develop for a further 30 min.
Measurement of plasma extravasation and intravascular volume
Plasma extravasation was determined by the extravascular accumulation of intravenously injected (i.v.) 125I-albumin (36 kBq in 0.1 ml) administered 1 h prior to the end of the experimental period for CFA experiments, or 30 min prior to the end for experiments involving SP. Mice were killed by cervical dislocation at the end of the accumulation period and the skin overlying the knee was excised. 125I-albumin accumulation in the joint was then determined using a collimated gamma probe (Europrobe, Bright Technologies, U.K.). The head of the probe was held against the joint region and the counts/min detected in the joint were recorded. Four readings were taken for each joint.
Alternatively, for an assessment of intravascular volume, 125I-albumin was injected 2 min prior to the end of the experimental period. Thus, the 125I-albumin was distributed throughout the cardiovascular system for 2 min but with minimal time for plasma extravasation to take place. The measurements were taken as for plasma extravasation.
Collection of synovial fluid and assessment of neutrophil accumulation
The assay of myeloperoxidase (MPO) activity in synovial fluid was determined as a measurement of neutrophil accumulation. In order to collect a synovial lavage, mice were killed and the skin overlying the knee was excised. The patellar ligament was then carefully dissected to expose the synovial membrane. A 30 G needle (BD Micro-Fine insulin syringe, 0.3 ml) was inserted through the membrane and the synovial cavity was washed by injecting and immediately aspirating 25 μl of heparinized saline (5 U ml−1) to obtain the synovial lavage. This was repeated once (total of 50 μl of synovial lavage). The collected fluid was snap frozen in liquid nitrogen and stored at −20°C prior to assay. A spectrophotometric assay was used to measure MPO activity, as described previously (Cao et al., 2000). The quantity of MPO in mouse joint synovial lavage is expressed in U ml−1.
Measurement of knee swelling
The skin around the knee joints was shaved and the distance between the medial and lateral aspects of each knee joint was measured using calipers (Mitutoyo), at the level of the patellar ligament.
Histological analysis
Whole mouse joints were dissected and immersed in a paraformaldehyde solution (2.5% in PBS, pH 7.4). Joints were decalcified in buffered formic acid for 2 weeks and then embedded in paraffin. Sections were cut on a microtome at 3 μm and mounted on glass slides. They were then stained with haematoxylin and eosin (H&E), fixed to the slides using DPX and analysed using an Olympus BX62 microscope.
Data analysis
The reproducibility of radioactivity measurements in the joint was determined by taking four replicate measurements at each of 10 different joints. The mean was taken as 100% for each joint and the s.e.m. calculated to give 100±3.5%, n=10. The probe was validated by measuring counts in serial dilutions of radioactivity. Plasma extravasation was expressed as a ratio of counts/min detected in the agent-treated joint compared to the saline-treated joint, to allow for unavoidable differences in the quantity of radioactivity injected i.v. A ratio of 1 indicates that the same quantity of 125I-albumin is present in both joints and, thus, no plasma extravasation. A ratio of >1 shows that more 125I-albumin is present in the test joint compared to the control joint. The larger the ratio, the greater the amount of plasma extravasation. To determine whether 125I-accumulation in the test joint was significantly greater than that in the control joint, ratios were compared to a value of 1.0 using one-sampled Student's t-tests. All other statistical analysis throughout the study was carried out using two-sampled paired or unpaired Student's t-tests, as appropriate. The ability to detect a change in blood flow was determined through measure of intravascular volume 30 min after i.art. endothelin 1. A 30 pmol dose of endothelin-1 induced a significant (P<0.05, one-sampled t-test) reduction in blood flow measured as a decrease in the ratio of counts compared with contralateral saline joint to 0.71±0.06, n=4.
Drugs
The anaesthetics, urethane and isoflurane, were purchased from Sigma-Aldrich, U.K., and Abbott Laboratories, U.K., respectively. Urethane was dissolved in 0.9% saline (Baxter Healthcare, U.K.) at a concentration of 0.25 g ml−1. SP (Sigma-Aldrich, U.K.) was first dissolved in 0.9% saline containing 0.01% BSA (Sigma-Aldrich, U.K.) at a concentration of 10 nmol ml−1 and further diluted in saline for injection. 125I-albumin (ICN, U.K.) was dissolved in saline at a concentration of 18 MBq ml−1 and further diluted 1 : 4 with saline prior to injection. MPO (Sigma-Aldrich, U.K.) was dissolved in saline at a concentration of 1 U ml−1 and further diluted in saline to produce a standard curve. H&E and DPX were purchased from VWR International, U.K.
Results
Time course of adjuvant-induced joint inflammation
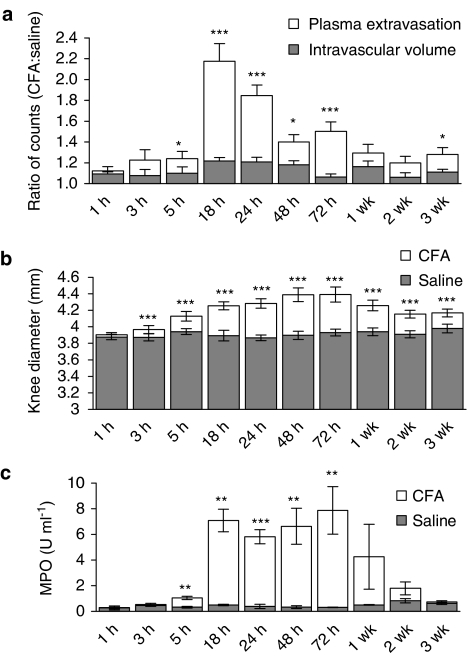
The initial results in this study show the time course of plasma extravasation, intravascular volume, knee swelling and neutrophil accumulation over 3 weeks, following i.art. injection of CFA in the mouse knee joint. The effect of CFA on plasma extravasation and intravascular volume is shown in Figure 1a. Plasma extravasation reached a maximum at 18 h after injection of CFA and then decreased thereafter to low levels that remained for the duration of the experiment. 125I-albumin accumulation due to plasma extravasation was significantly higher than that due to increased intravascular volume at 18–72 h after injection of CFA (P<0.001; unpaired Student's t-tests). No discernable pattern to the time course of intravascular volume was observed, although intravascular volume was significantly greater in CFA-treated compared to saline-treated joints at several time points.
Figure 1.
Time course of CFA-induced inflammation in the mouse knee joint. Effect of intra-articular injection of CFA (20 μg, 20 μl) and saline (contralateral joint, 20 μl) on (a) plasma extravasation (n=5–7) and intravascular volume (n=4–6), (b) knee swelling (n=9–12) and (c) neutrophil accumulation as synovial MPO activity (n=3–7) over a 3-week time course in CD1 mice. All results are expressed as mean±s.e.m. (a) Results are expressed as a ratio of counts detected in the CFA-treated compared to the saline-treated joint. Statistical analysis was performed to determine whether 125I-albumin accumulation due to plasma extravasation was significantly greater than that due to increased intravascular volume using unpaired t-tests. *P<0.05; ***P<0.001 vs intravascular volume data. (b) Statistical significance was shown by paired t-tests. ***P<0.001 vs saline-treated joints. (c) Statistical significance was shown by unpaired t-tests. **P<0.01; ***P<0.001 vs saline-treated joints.
Results showing changes in knee swelling, as measured by calipers, over the 3-week time course are shown in Figure 1b. The knee diameters of CFA-treated joints were significantly greater than those of saline-treated joints from 3 h onwards (P<0.001; paired Student's t-tests), reaching a maximum between 48 and 72 h and remained significantly higher than saline-treated joints throughout the time course. No significant increase in knee diameter was detected in the saline-treated joints.
Figure 1c shows a significant increase in the MPO activity of synovial fluid (used as a marker of neutrophil accumulation) from CFA-treated knee joints compared to saline-treated joints (P<0.001; paired Student's t-tests). MPO activity was maximal between 18 and 72 h after injection of CFA and started to decrease at 1 week. The MPO content of the joint synovial fluid returned to baseline levels by 3 weeks. No significant increase in MPO activity was observed in saline-treated joints at any point over the 3-week time course.
SP-induced joint inflammation
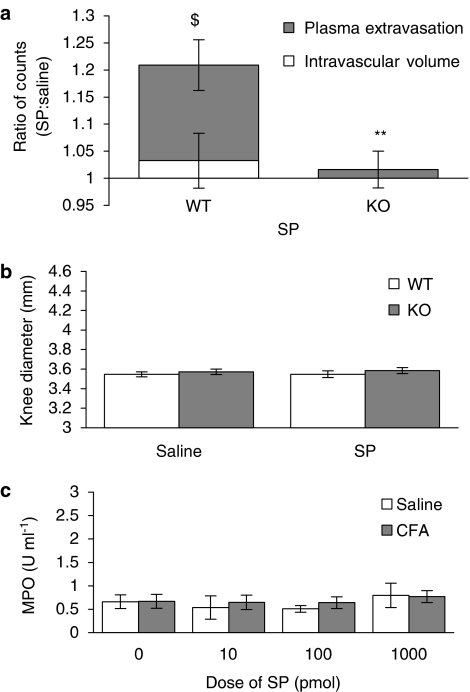
SP (i.art.) caused a significant increase in plasma extravasation 30 min after i.art. injection into the WT mouse knee joint (P<0.01; Figure 2a), when compared with the measure of intravascular volume. An essential role of the NK1 receptor was determined through the observation of a lack of plasma extravasation in NK1 receptor knockout mice (Figure 2a). Despite SP causing plasma extravasation in WT mice, no significant increase in knee diameter was observed and, as expected, SP also had no effect on knee diameter in NK1 receptor knockout mice (Figure 2b).
Figure 2.
Effect of exogenous SP on plasma extravasation and knee swelling in the naïve joint. Effect of SP (100 pmol, 10 μl) and saline (contralateral joint, 10 μl) on (a) intravascular volume and plasma extravasation (n=4–6) and (b) knee swelling (n=6–7) after a 30-min time course in wild type (WT) and NK1 receptor knockout (KO) mice. (c) Effect of SP (1–1000 pmol, 10 μl) on neutrophil accumulation, assessed as MPO activity after a 5-h time course in WT mice. Results are mean±s.e.m. Statistical significance was evaluated using an unpaired t-test to compare WT vs NK1 receptor KO mice. **P<0.01 vs WT mice; $P<0.01 vs intravascular volume data.
Subsequent experiments were performed to determine the effect of SP on neutrophil accumulation. SP was injected into the knee joint and inflammation was allowed to develop over a 5 h time course to give sufficient time for neutrophil accumulation to take place in these mice (see Cao et al., 2000). A range of doses of SP was used (10–1000 pmol), but no increase in the MPO activity of synovial fluid of SP-treated joints compared to saline-treated joints was detected at any of the doses tested (Figure 2c). Thus, i.art. injection of SP alone caused plasma extravasation, but neither neutrophil accumulation nor knee swelling in the naïve mouse knee joint.
CFA-induced joint inflammation in NK1 receptor knockout mice
The previous experiments to determine the time course of CFA-induced inflammation showed that the onset of physical signs of the inflammation began after approximately 3 h and were maximal at approximately 18 h. Thus, in subsequent experiments the role of SP in the response to CFA was investigated in NK1 receptor WT and knockout mice at 3–18 h after i.art. injection of CFA in order to determine the role of SP in the onset of joint inflammation and in the peak inflammatory response.
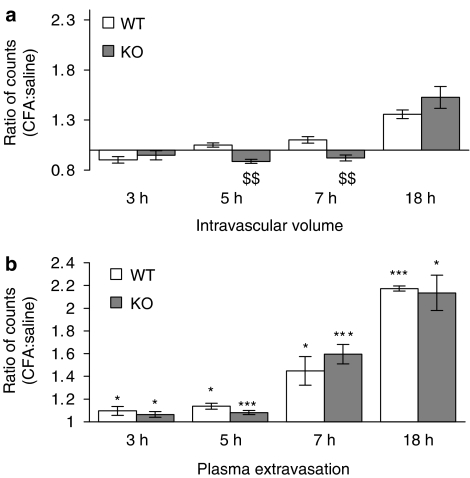
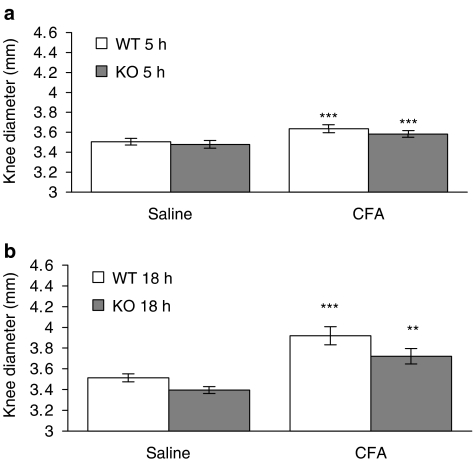
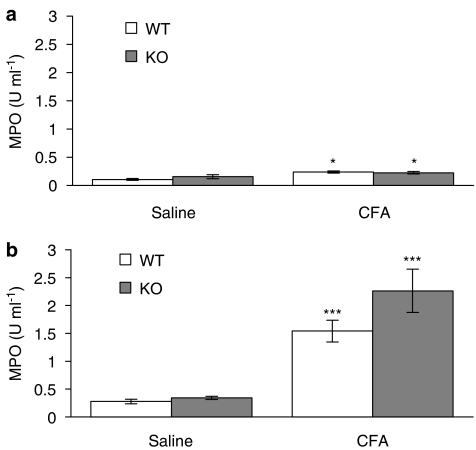
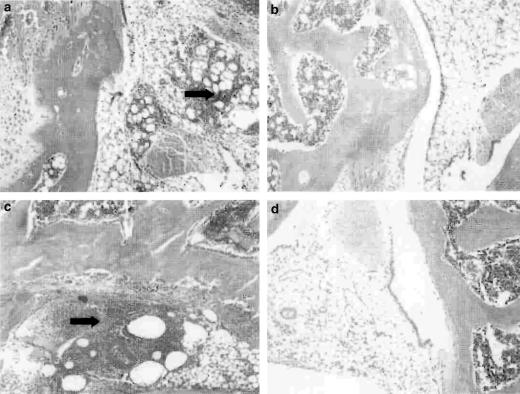
Interestingly, at the 5 and 7 h time points, a significant decrease (P<0.01) in intravascular volume was found in the CFA-treated joints of NK1 receptor knockout mice (Figure 3), indicating a decreased blood flow in these mice. The intravascular volume of the CFA-treated joints was significantly less than that of the saline-treated joints in the knockout mice and the CFA-treated joints in WT controls. By 18 h after injection of CFA, the intravascular volume of CFA-treated joints in NK1 receptor knockout mice had substantially increased compared to the 5 h time point. Meanwhile, 125I-albumin accumulation due to plasma extravasation was significantly greater than that due to increased intravascular volume in all mice at all of the time points investigated, but no significant differences between WT and knockout mice were detected for plasma extravasation. Similarly, knee swelling (Figure 4) and synovial MPO activity were significantly increased in the CFA-treated compared to the saline-treated joints of all mice but no significant differences were observed between WTs and knockouts (Figure 5). The significant increase in neutrophils in CFA-treated joints compared to saline-treated joints of both WT and NK1 knockout joints at 18 h, demonstrated by the MPO assay, was confirmed by H&E staining of whole joint sections. Neutrophils, as opposed to mononuclear cells, were identified in the synovium of CFA-treated joints in both WT (Figure 6a) and knockout mice (Figure 6c). No significant presence of neutrophils was observed in the contralateral, saline-treated joints (Figure 6b & d).
Figure 3.
Role of NK1 receptors in CFA-induced plasma extravasation and intravascular volume changes. The effect of intra-articular injection of CFA (10 μg, 10 μl) and saline (contralateral joint, 10 μl) on (a) intravascular volume and (b) plasma extravasation after a 3 h (n=4–5), 5 h (n=5–7), 7 h (n=5) or 18 h (n=3–5) time course in wild type (WT) and NK1 receptor knockout (KO) mice. Results are expressed as mean±s.e.m. Statistical significance was evaluated using unpaired t-tests. *P<0.05; ***P<0.001 vs intravascular volume data at the equivalent time point; $$P<0.01 vs WT mice.
Figure 4.
Role of NK1 receptors in CFA-induced knee swelling. Effect of intra-articular injection of CFA (10 μg, 10 μl) and saline (contralateral joint, 10 μl) on knee swelling after (a) a 5-h time course (n=13) and (b) an 18-h time course (n=7) in wild type (WT) and NK1 receptor knockout (KO) mice. Results are expressed as mean±s.e.m. Statistical significance was evaluated using unpaired t-tests to compare WT vs NK1 receptor KO mice and CFA-treated joints vs saline-treated joints. **P<0.01; ***P<0.001 vs saline-treated joints.
Figure 5.
Role of NK1 receptors in CFA-induced neutrophil infiltration. Effect of intra-articular injection of CFA (10 μg, 10 μl) and saline (contralateral joint, 10 μl) on synovial MPO activity after (a) a 5-h time course (n=4–5) and (b) an 18-h time course (n=5) in wild type (WT) and NK1 receptor knockout (KO) mice. Results are expressed as mean±s.e.m. Statistical significance was evaluated using unpaired t-tests to compare WT vs NK1 receptor KO mice and CFA-treated joints vs saline-treated joints. *P<0.05; ***P<0.001 vs saline-treated joints.
Figure 6.
Histology of joint sections. Haemotoxylin and eosin staining of joint sections to show the effect of intra-articular injection of CFA (10 μg, 10 μl) on synovial neutrophil infiltration in (a) WT and (c) KO mice. Staining for neutrophils in the saline-treated joints of (b) WT and (d) KO mice is also shown. Arrows indicate areas of neutrophil accumulation.
Effect of SP on adjuvant-induced joint inflammation
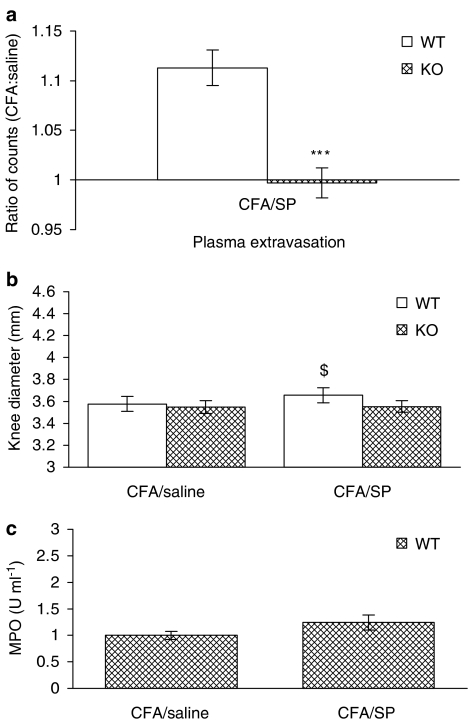
The potent plasma extravasation activity of SP, but lack of difference between plasma extravasation in WT and NK1 knockout mice, led us to investigate the effect of exogenous SP in CFA-induced inflammation at 18 h. SP caused a significant increase in plasma extravasation in the CFA-pretreated joints of WT mice, similar to the effect of SP in the naïve joint (Figure 7a; open bar) that was not observed in NK1 receptor knockout mice as expected. SP had no effect on knee diameter in naïve joints (Figure 2b); however, a slight but significantly enhanced response was observed (Figure 7b). This effect on knee swelling was not observed in NK1 receptor knockout mice (Figure 7b). By comparison, neutrophil accumulation was not further increased in CFA-treated WT mice after treatment with SP and was therefore not investigated in NK1 receptor knockout mice (Figure 7c).
Figure 7.
Effect of exogenous SP on knee swelling, plasma extravasation and neutrophil infiltration in the CFA-pretreated joint. Effect of SP (100 pmol, 10 μl) and saline (contralateral joint, 10 μl) on (a) plasma extravasation (n=5–6), (b) knee swelling (n=6) and (c) synovial MPO activity (n=6–7) in CFA-pretreated joints of wild type (WT) and NK1 receptor knockout (KO) mice. Results are mean±s.e.m. Statistical significance was evaluated using an unpaired t-test to compare WT vs NK1 receptor KO mice. ***P<0.001 vs WT mice; $P<0.05 vs saline-treated joint.
Discussion
The results of the present study demonstrate that CFA causes significant, time-dependent changes in neutrophil accumulation, plasma extravasation, and knee swelling in the mouse knee joint. Furthermore, they suggest that endogenous SP plays a significant role in maintaining intravascular volume in the CFA-treated joint and that exogenous SP can potentiate the plasma extravasation and swelling effects of CFA. These results have been obtained using novel techniques that have been developed in our laboratory for investigating microvascular mechanisms in the mouse knee joint.
A major objective was to determine whether the time course of plasma extravasation in the joint correlates with that of knee swelling over the 3-week experimental period of CFA-induced joint inflammation. Plasma extravasation is used as a measure of oedema formation and occurs as a direct consequence of increased microvascular permeability (Plante et al., 1996). The associated measure of intravascular volume in this study allowed a clear quantification of plasma extravasation per se. It is generally assumed that plasma extravasation leads to an increase in knee diameter (Ahlqvist, 2000). The results of the detailed time course analysis reveal that plasma extravasation, measured by the extravascular accumulation (for 60 min after i.v. 125I-albumin) as compared with an assessment of intravascular accumulation (for 2 min after i.v. 125I-albumin), was maximal 18 h after injection of CFA and decreased thereafter. Knee swelling was maximal at 48 h and remained significantly higher until the end of the experiment. These experiments were unable to account for the draining of the inflammatory exudate through the lymphatic system, thus one possibility is that as plasma extravasation decreased, the residual plasma extravasation was acting to maintain knee swelling rather than further increase it. The measurement of the MPO content in synovial fluid allowed assessment of neutrophil accumulation. This reached significance in the CFA-treated joint within 5 h of injection and was maximal between 18 and 72 h. Interestingly, the time course of onset of neutrophil accumulation was similar to that of plasma extravasation and knee swelling, suggesting a close relationship between these inflammatory processes in the joint.
The i.art. administration of SP allowed its acute activity as a mediator of plasma extravasation to be determined. The discreet measure of plasma extravasation in the joint was revealed under these acute conditions, as no significant effect of SP on knee swelling was observed. Importantly, the use of mice that lacked the major vasoactive NK1 receptor for SP confirmed the importance of this receptor in mediating plasma extravasation as previously observed in the joint of other species (e.g. cat, Ferrell & Russell, 1986) and skin of this colony of NK1 knockout mice (Cao et al., 1999). This supports studies involving other NK1 knockout mice and mice lacking the gene for SP, preprotachykinin knockouts (Cao et al., 1998; De Felipe et al., 1998). SP can both influence the recruitment of neutrophils at inflammatory sites (Von essen et al., 1992; Cao et al., 2000) and modulate their activity (Hafstrom et al., 1989; Böckmann et al., 2001). Previous authors have demonstrated that SP causes the upregulation of neutrophil adhesion molecules on microvascular endothelial cells (Quinlan et al., 1999), thus SP might have been expected to cause neutrophil accumulation. However, when SP was injected into the naïve mouse knee joint, neutrophil accumulation was not detected. The study by Quinlan et al. (1999) was performed in vitro in the absence of some enzymes that degrade SP such as neutral endopeptidase (Skidgel et al., 1984; Lu et al., 1997), thus possibly allowing exposure to SP for several hours, which may be critical for gene expression to be significant. In contrast, SP injected into the joint, in vivo, would be rapidly metabolized. These results are in keeping with previous studies carried out in the skin of these mice that demonstrated that SP was unable to influence neutrophil accumulation into naïve skin (Cao et al., 2000).
The involvement of an SP NK1 receptor component in the response to CFA was then investigated. However, plasma extravasation, knee swelling and synovial neutrophil accumulation were unaltered in NK1 receptor knockout mice compared to WT mice at 5 and 18 h after i.art. injection of CFA. In contrast, the intravascular volume of the CFA-treated joint in NK1 receptor knockout mice was significantly reduced at 5 and 7 h, but not at 3 and 18 h compared to both the contralateral, saline-treated joint and the CFA-treated joints of WT mice. The assumption that the measure of intravascular volume could be used as an assessment of blood flow was established through initial experiments using known vasoconstrictor amounts of endothelin (30 pmol). The significant decrease in intravascular volume of the CFA-treated joint compared to that of the saline-treated joint suggests that the joint tissue is at risk of being hypoxic. Hypoxia leads to tissue injury by causing anoxia and acidosis, which causes the release of hydrolytic enzymes, increased vascular permeability and acceleration of inflammatory processes (Rothschild & Masi, 1982). It is possible that a component of the response to CFA in NK1 receptor knockout mice was hypoxia-induced, which could have masked the absence of a neurogenic component. It is concluded that SP may play an important role in maintaining the normal intravascular volume of the joint, thereby exerting a protective effect in acute joint inflammation. These possibilities are yet to be investigated. This is the first time that a direct study of joint inflammation has been made in NK1 knockout mice. However, previous studies of CFA-induced footpad inflammation in NK1 knockout mice and preprotachykinin-deleted mice have been performed (Cao et al., 1998; De Felipe et al., 1998; Kidd et al., 2003). Interestingly, a lack of effect on foot pad size was observed in all but one set of experiments although no attempt was made to measure blood flow. The exception involved use of ‘a high intensity CFA model' by Kidd et al. (2003), where an inhibition of CFA-induced foot diameter increase was observed in NK1 receptor knockouts. The relevance of this study to the findings presented here is unclear. However, both studies support the concept that CFA-induced inflammation can involve a vasoactive component under certain circumstances. By comparison, it is important to note that an involvement of the NK1 receptor in mechanical hyperalgesia in CFA-induced footpad inflammation appears clear (Kidd et al., 2003).
We next investigated the effect of SP in the presence of CFA in the mouse knee joint. i.art. injection of CFA produces a large inflammatory response and a variety of proinflammatory mediators are present at the site of inflammation, including IL-1β (Szekanecz et al., 2000). As in the naïve joint, exogenous SP caused an increase in plasma extravasation in CFA-pretreated WT mice via activation of the NK1 receptor. Interestingly, in contrast to the situation in the naïve joint, SP exacerbated CFA-induced knee swelling in WT mice via activation of NK1 receptors. These results are in keeping with the findings of Scott et al. (1992) showing that acute carrageenan-induced joint inflammation in the rat enhances SP-mediated responses. As regards joint disease, an analogous situation to this in humans may be in the case of the spontaneous exacerbations of inflammation and joint swelling observed in rheumatoid arthritis patients. If SP is involved in these exacerbations then this makes NK1 receptor antagonists an interesting prospect for the treatment of this aspect of rheumatoid arthritis. In contrast to plasma extravasation and knee swelling, SP caused no significant potentiation of synovial neutrophil accumulation in the CFA-pretreated joint.
To conclude, the present study has used novel techniques to show microvascular responses to CFA and/or SP in the mouse knee joint. Furthermore, this investigation is the first to study joint inflammation in WT and NK1 knockout mice. Significant findings of this study were that knee swelling in response to CFA was not directly proportional to plasma extravasation and, furthermore, that NK1 receptor activation may play an important part in maintaining blood volume during the acute stages of joint disease. Use of NK1 knockout mice also confirmed that responses to exogenous SP caused an acute plasma extravasation, but not knee swelling or neutrophil accumulation, in the naïve mouse knee joint, and, in contrast, that SP causes both plasma extravasation and knee swelling in the CFA-pretreated knee joint. Thus, SP has a greater effect in the presence of other inflammatory mediators, such as those released in response to CFA. Consequently, the findings of this study may be of significance to the understanding of the spontaneous exacerbations of joint disease in rheumatoid arthritis patients with pre-existing inflammation.
Acknowledgments
We thank the Arthritis Research Campaign for support.
Abbreviations
- CFA
complete Freund's adjuvant
- i.art.
intra-articular
- KO
knockout
- NK1
neurokinin 1
- SP
substance P
- WT
wild type
References
- AHLQVIST J. Swelling of synovial joints – an anatomical, physiological and energy metabolical approach. Pathophysiology. 2000;7:1–19. doi: 10.1016/s0928-4680(00)00044-4. [DOI] [PubMed] [Google Scholar]
- BÖCKMANN S., SEEP J., JONES L. Delay of neutrophil apoptosis by the neuropeptide substance P: involvement of caspase cascade. Peptides. 2001;22:661–670. doi: 10.1016/s0196-9781(01)00376-x. [DOI] [PubMed] [Google Scholar]
- BULLING D.G., KELLY D., BOND S., MCQUEEN D.S., SECKL J.R. Adjuvant-induced joint inflammation causes a very rapid transcription of beta-preprotachykinin and alpha-CGRP genes in innervating sensory ganglia. J. Neurochem. 2001;77:372–382. doi: 10.1046/j.1471-4159.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- CAO T., GERARD N.P., BRAIN S.D. Use of NK1 knockout mice to analyze substance P-induced edema formation. Am. J. Physiol. 1999;277:R476–R481. doi: 10.1152/ajpregu.1999.277.2.R476. [DOI] [PubMed] [Google Scholar]
- CAO T., PINTER E., AL-RASHED S., GERARD N., HOULT R., BRAIN S.D. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J. Immunol. 2000;164:5424–5429. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- CAO Y.Q., MANTYH P.W., CARLSON E.J., GILLESPIE A.M., EPSTEIN C.J., BASBAUM A.I. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- DE FELIPE C., HERRERO J.F., O'BRIEN J.A., PALMER J.A., DOYLE C.A., SMITH A.J., LAIRD J.M., BELMONTE C., CERVERO F., HUNT S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- FERRELL W.R., RUSSELL N.J. Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetized cat. J. Physiol. 1986;379:407–416. doi: 10.1113/jphysiol.1986.sp016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAFSTROM I., GYLLENHAMMER H., PALMBLAD J., RINGERTZ B. Substance P activates and modulates neutrophil oxidative metabolism and aggregation. J. Rheumatol. 1989;16:1033–1037. [PubMed] [Google Scholar]
- IGNATAVICIUS D.D. Rheumatoid arthritis and the older adult. Geriatr. Nurs. 2001;22:139–142. doi: 10.1067/mgn.2001.116376. [DOI] [PubMed] [Google Scholar]
- KIDD B.L., CRUWYS S.C., GARRETT N.E., MAPP P.I., JOLLIFFE V.A., BLAKE D.R. Neurogenic influences on contralateral responses during experimental rat monoarthritis. Brain Res. 1995;688:72–76. doi: 10.1016/0006-8993(95)00512-o. [DOI] [PubMed] [Google Scholar]
- KIDD B.L., INGLIS J.J., VETSIKA K., HOOD V.C., DE FELIPE C., BESTER H., HUNT S.P., CRUWYS S.C. Inhibition of inflammation and hyperalgesia in NK-1 receptor knock-out mice. Neuroreport. 2003;14:2189–2192. doi: 10.1097/00001756-200312020-00011. [DOI] [PubMed] [Google Scholar]
- LEVINE J.D., DARDICK S.J., ROIZEN M.F., HELMS C., BASBAUM A.L. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J. Neurosci. 1986;6:3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU B., FIGINI M., EMANUELI C., GEPPETTI P., GRADY E.F., GERARD N.P., ANSELL J., PAYAN D.G., GERARD C., BUNNETT N. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat. Med. 1997;3:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- NIISSALO S., HUKKANEN M., IMAI S., TORNWALL J., KONTTINEN Y.T. Neuropeptides in experimental and degenerative arthritis. Ann. NY. Acad. Sci. 2002;969:384–399. doi: 10.1111/j.1749-6632.2002.tb04239.x. [DOI] [PubMed] [Google Scholar]
- PLANTE G.E., CHAKIR M., ETTAOUIL M., LEHOUX S., SIROIS P. Consequences of alteration in capillary permeability. Can. J. Physiol. Pharmacol. 1996;74:824–833. doi: 10.1139/cjpp-74-7-824. [DOI] [PubMed] [Google Scholar]
- QUINLAN K.L., SONG I.S., NAIK S.M., LETRAN E.L., OLREUD J.E., BUNNETT N.W., ARMSTRONG C.A., CAUGHMAN S.W., ANSEL J.C. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically upregulated by substance P. J. Immunol. 1999;16:1656–1661. [PubMed] [Google Scholar]
- ROTHSCHILD B.M., MASI A.T. Pathogenesis of rheumatoid arthritis: a vascular hypothesis. Semin. Arthritis Rheum. 1982;12:11–31. doi: 10.1016/0049-0172(82)90020-8. [DOI] [PubMed] [Google Scholar]
- SAKAI K., MATSUNO H., TSUJI H., TOHYAMA M. Substance P receptor gene expression in synovial fluid in rheumatoid arthritis and osteoarthritis. Scand. J. Rheumatol. 1998;27:135–141. doi: 10.1080/030097498441010. [DOI] [PubMed] [Google Scholar]
- SCOTT D.T., LAM F.Y., FERRELL W.R. Acute inflammation enhances substance P-induced plasma protein extravasation in the rat knee joint. Regul. Peptides. 1992;39:227–235. doi: 10.1016/0167-0115(92)90543-4. [DOI] [PubMed] [Google Scholar]
- SEGOND VON BANCHET G., PETROW P.K., BRAUER R., SCHAIBLE H.G. Monoarticular antigen-induced arthritis leads to a pronounced bilateral upregulation of the expression of neurokinin 1 and bradykinin 2 receptors in dorsal root ganglion neurons of rats. Arthritis Res. 2000;2:424–427. doi: 10.1186/ar121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKIDGEL R.A., ENGELBRECHT S., JOHNSON A.R., ERDOS E.G. Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- SZEKANECZ Z., HALLORAN M.M., VOLIN M.V., WOODS J.M., STRIETER R.M., HAINES G., KUNKEL S.L., BURDICK M.D., KOCH A.E. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:1266–1277. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- VON ESSEN S.G., RENNARD S.I., O'NEILL D., ERTL R.F., ROBBINS R.A., KOYAMA S., RUBENSTEIN I. Bronchial epithelial cells release neutrophil chemotactic activity in response to tachykinins. Am. J. Physiol. 1992;263:L226–L231. doi: 10.1152/ajplung.1992.263.2.L226. [DOI] [PubMed] [Google Scholar]