Abstract
The amino acid, D-aspartate, exists in the mammalian brain and is an agonist at the N-methyl-D-aspartate (NMDA) subtype of ionotropic glutamate receptors. Here, for the first time, we studied the actions of D-aspartate on α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptors (AMPARs) in acutely isolated rat hippocampal neurons.
In the presence of the NMDA receptor channel blocker, MK801, D-aspartate inhibited kainate-induced AMPAR current in hippocampal neurons. The inhibitory action of D-aspartate on kainate-induced AMPAR current was concentration-dependent and was voltage-independent in the tested voltage range (−80 to +60 mV).
The estimated EC50 of the L-glutamate-induced AMPAR current was increased in the presence of D-aspartate, while the estimated maximum L-glutamate-induced AMPAR current was not changed. D-aspartate concentration-dependently shifted the dose–response curve of kainate to the right. Schild plot analysis indicated that D-aspartate acts competitively to block AMPARs. The Kb for D-aspartate was estimated to be 0.93 mM.
D-Aspartate also blocked L-glutamate-induced current in Xenopus laevis oocytes that expressed recombinant homomeric AMPARs.
NMDA possessed similar inhibitory action on AMPARs. However, L-aspartate had little inhibitory action on AMPARs.
D-Aspartate, but not L-aspartate, was found to reduce the amplitude of miniature excitatory postsynaptic current in cultured hippocampal neurons.
Our data are consistent with a model in which D-aspartate directly competes with kainate and L-glutamate in binding to the agonist binding site of AMPARs. The prevalence of D-aspartate in the brain suggests a possible role of D-aspartate in modulating AMPAR-mediated fast excitatory synaptic transmission.
Keywords: D-aspartate, NMDA, AMPA receptor, D-amino acid, hippocampus, patch clamp
Introduction
Fast excitatory synaptic transmission in the mammalian brain is mediated primarily through α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptors (AMPARs) and N-methyl-D-aspartate receptors (NMDARs), two major subtypes of ionotropic glutamate receptors, each named after its selective agonist. AMPARs are the major mediator of synaptic transmission and NMDARs modulate synaptic plasticity (Dingledine et al., 1999). These receptors are ubiquitous in the brain and play important roles in virtually every central process including sensory, motor control and higher functions such as learning and memory (Bliss & Collingridge, 1993).
It has been documented that L-glutamate is the predominant native neurotransmitter for both AMPARs and NMDARs. L-aspartate and glycine are important native ligands participating in the activation of NMDARs (Fleck et al., 1993). In addition, several studies indicate that D-amino acids, notably D-serine and D-aspartate, exist in the mammalian brain (Dunlop et al., 1986; Hashimoto et al., 1993, 1995; Kera et al., 1995; Hamase et al., 1997).
Mounting evidence has been accumulated to support the notion that there is a possible role of D-aspartate in native synaptic transmission. Firstly, free D-aspartate exists in the mammalian brain in low micromolar to submillimolar concentrations (Dunlop et al., 1986; Hashimoto et al., 1993, 1995). A high concentration of D-aspartate is present during neonatal development and also in adult neuroendocrine nuclei (Neidle & Dunlop, 1990; Hashimoto et al., 1993, 1995; Fisher et al., 1994; D'Aniello et al., 1996; Schell et al., 1997). Secondly, although it is not clear how endogenous D-aspartate is transported, stored and released in vivo, it has been characterized that D-aspartate can be taken up into cells through high-affinity L-glutamate and L-aspartate transporters (Davies & Johnston, 1975; Albus & Habermann, 1983; Palmer & Reiter, 1994) and accumulated in astrocytes and neurons, including synaptosomes (Gundersen et al., 1995; Fleck et al., 2001; Waagepetersen et al., 2001). The preloaded exogenous D-aspartate can be released during cell depolarization induced by a high concentration of potassium in a calcium-dependent manner (Albus & Habermann, 1983; Palmer & Reiter, 1994; Savage et al., 2001; Waagepetersen et al., 2001) and this release can also be seen from synaptosomes (Martire et al., 2000). The possible mechanisms of D-aspartate release include the reverse operation of glutamate transporters (Waagepetersen et al., 2001) and exocytosis of vesicles (Nakatsuka et al., 2001; Savage et al., 2001). Thirdly, functional studies have demonstrated that D-aspartate is able to activate NMDARs (Watkins & Evans, 1981; Mayer & Westbrook, 1985; Kiskin et al., 1990). Finally, the enzyme responsible for the degradation of D-aspartate, D-aspartate oxidase, is present in the brain (Yusko & Neims, 1973; Davies & Johnston, 1975) and is enriched in several brain areas including the olfactory bulb, hippocampus and cerebral cortex (Schell et al., 1997; Zaar et al., 2002). The localization of D-aspartate oxidase appears to be reciprocal to the concentration of D-aspartate, suggesting that this enzyme may play a role in degradation of endogenous D-aspartate (Schell et al., 1997).
Apart from the well-documented agonist action on NMDARs, little is known of the functions of D-aspartate in the brain. Several studies suggest an inhibitory action of NMDA, a synthetic structural analog of D-aspartate, on AMPAR function. In retinal ganglion cells, kainate-induced current was inhibited by coapplication of NMDA with kainate (Karschin et al., 1988). Similar inhibitory actions of NMDA on the recombinant AMPARs expressed in Xenopus laevis oocytes have been reported (Lerma et al., 1989b; Brackley & Usherwood, 1993; Ruppersberg et al., 1993). Interestingly, in addition to NMDA, a number of NMDAR agonists were also found to partially block recombinant AMPARs, including D-aspartate (Magazanik & Usherwood, 1996). It is not clear whether the AMPARs in hippocampus can be inhibited by D-aspartate. Here we found that D-aspartate is able to inhibit AMPAR-mediated current in hippocampal neurons by competing at the agonist binding site of AMPARs. However, the enantiomer of D-aspartate, L-aspartate, is virtually without inhibitory action. The prevalence of D-aspartate in several brain areas suggests a potential role for D-aspartate in modulating AMPAR-mediated synaptic transmission. Some preliminary data of this work have been reported in abstract form (Gong & Bai, 2003).
Methods
All animal experiments were conducted under the guidelines of Animal Care and Veterinary Services at The University of Western Ontario and The Danish University of Pharmaceutical Sciences.
Acute isolation of hippocampal neurons and patch clamp recording
Enzymatic digestion was used to isolate CA1 pyramidal neurons from 2 to 5 week postnatal rat hippocampus (Bai et al., 2002). Briefly, Sprague–Dawley rats were anesthetized with halothane and decapitated. The brain was rapidly removed and rinsed in cold extracellular fluid (ECF). The hippocampus was surgically isolated and cut into 750-μm thick transverse slices with a tissue chopper. For digestion, the slices were incubated at room temperature (22–24°C) in ECF containing 2–4 mg ml−1 papain (derived from papaya latex, Sigma) for 30 min. The CA1 region was separated from the rest of the slice and fine surgical forceps were used to tease out single CA1 pyramidal cells. Pyramid shaped cells were selected for recording. The ECF contained (in mM): 140 NaCl, 1.3 CaCl2, 5.0 KCl, 25 HEPES and 33 glucose. The pH was adjusted to 7.3 with NaOH and the osmolarity of the solution was adjusted to 325 mOsmol l−1. The pH was measured and readjusted where necessary for all the agonist- and antagonist-containing solutions to avoid pH-dependent modulation of AMPARs (Lei et al., 2001). The whole-cell patch electrode had a resistance of 3–5 MΩ and was filled with a solution that contained (in mM): 140 CsF, 2 tetraethylammonium (TEA), 30 HEPES, 11 EGTA, 2 MgATP. The pH was adjusted to 7.2 using CsOH and the osmolarity was 295 mOsmol l−1. A triple barrel perfusion system (SF-77B Perfusion Fast-Step, Warner Instruments Corp., Hamden, CT, U.S.A.) was employed to rapidly switch from normal ECF to solutions that contained glutamate or other agonists (Figure 1). The optimized open pipette solution exchange time is measured to be 1–3 ms (10–90% rise time). The time interval between agonist applications was 20–30 s. Patches that displayed a ‘rundown' greater than 2% per minute were discarded. All recordings were performed at room temperature (22–24°C). Recording electrodes were prepared with a puller (Narishige, PP83, Tokyo, Japan). Voltage clamp for whole-cell recordings was carried out with an Axopatch 200B (Axon Instruments Inc., Union City, CA, U.S.A.). Series resistance was compensated by 85–90%. Current signals were filtered at 2 kHz and digitized at 5–10 kHz using a DigiData 1200 interface and pClamp6 software.
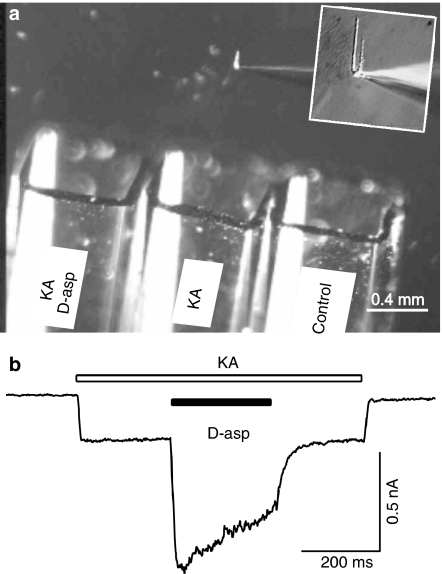
Figure 1.
Experimental setup and kainate and D-aspartate-induced current in isolated hippocampal neurons. (a) The photograph illustrates the triple barrel perfusion system. An isolated hippocampal neuron was lifted from the dish and was constantly perfused by one of the three barrels. An enlarged view on the patch pipette and the attached hippocampal neuron is shown in the inset. The barrel positions were controlled by a step-motor under the command of pClamp software via Digidata 1200 interface. Three different solutions (indicated on the barrel) were constantly perfused. (b) Kainate (KA, 200 μM, open bar) induced an inward current in this hippocampal neuron (VH=−60 mV) and a mixture of D-aspartate (D-asp, 1 mM, filled bar) and kainate (200 μM) induced a larger inward current.
Miniature excitatory postsynaptic current recording
Miniature excitatory postsynaptic currents (mEPSCs) were recorded in cultured embryonic rat hippocampal neurons in the presence of the sodium channel blocker tetrodotoxin (1 μM) and the GABAA receptor antagonist bicuculline (30 μM). MK801 (10 μM) was included in the bath medium to isolate the AMPAR-mediated mEPSCs (mEPSCsAMPA). Nonstop recording of the mEPSCsAMPA into a PC computer was made by pClamp (Axon Instrument). The amplitude and frequency of mEPSCsAMPA were analyzed using Mini Analysis program (Synaptosoft Inc., Decatur, GA, U.S.A.). To estimate the actions of D- and L-aspartate on the amplitude of mEPSCsAMPA, we averaged the top 30–70 events in a 2-min period during the drug application. The averaged amplitude of top mEPSCsAMPA was normalized to that of the identical number of top events during a 2-min control period. However, all super threshold mEPSCsAMPA were analyzed to determine the event frequency.
cRNA preparation
The rat AMPAR clones GluR1o, GluR2(Q)i, GluR3i and GluR3o were inserted into the vector pGEMHE (Liman et al., 1992) for preparation of high-expression cRNA transcripts. cDNAs were grown in XL1 Blue bacteria (Stratagene, La Jolla, CA, U.S.A.) and prepared using column purification (Qiagen, Chatsworth, CA, U.S.A.). cRNAs were synthesized from these cDNAs using the mMessage mMachine T7 mRNA-capping transcription kit (Ambion Inc., Austin, TX, U.S.A.).
Oocyte two-electrode voltage clamp electrophysiology
Mature female X. laevis (African Reptile Park, Tokai, South Africa) were anesthetized using 0.1% ethyl 3-aminobenzoate methanesulfonate (tricaine) and ovaries were surgically removed. The ovarian tissue was dissected and treated with 2 mg ml−1 collagenase in nominally Ca2+-free Barth's medium for 2 h at room temperature and subsequently defolliculated using fine forceps. On the second day, oocytes were injected with 50 nl of (∼1 μg μl−1) cRNA and incubated in Barth's medium (in mM: 88 NaCl, 1 KCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 2.4 NaHCO3, 10 HEPES, pH 7.4) with gentamicin (0.10 mg ml−1) at 17°C. Oocytes were used for recordings from 3–10 days postinjection and were voltage-clamped by a two-electrode voltage clamp amplifier (GeneClamp 500B, Axon Instruments, Union City, CA, U.S.A.) with both microelectrodes filled with 3 M KCl. Recordings were made at room temperature at holding potentials in the range of −80 to −30 mV while the oocytes were continuously superfused with Ca2+-free frog Ringer's solution (in mM: 115 NaCl, 2 KCl, 1.8 BaCl2, 5 HEPES, pH 7.0). Drugs were dissolved in Ca2+-free frog Ringer's solution and added by bath application.
Chemicals
Kainic acid (Ocean Produce International, Shelburne, Nova Scotia, Canada) was dissolved in 0.05 MNaOH to make a stock solution (50 mM). The NMDAR antagonists (+)-MK801, 3-(2-carboxypiperazin-4-yl) propanephosphonic acid (CPP) and D(−)-2-amino-5-phosphonopentanoic acid (D-AP5) were obtained from Tocris Cookson Inc. (Ellisville, MO, U.S.A.). DL-TBOA (DL-threo-β-benzyloxyaspartic acid) was generously donated by Dr Keiko Shimamoto at Suntory Institute for Bioorganic Research, Japan. All the other chemicals are products of Sigma (St Louis, MO, U.S.A.).
Data analysis
All the data are expressed as means±s.e.m. Paired-Student's t test, unpaired t test or two-way ANOVA were used to test statistical significance (*P<0.05 or **P<0.01) between paired, unpaired and groups of data respectively. Concentration–response relationships and estimation of EC50/IC50 were determined using Prism software (GraphPad Software Inc., San Diego, CA, U.S.A.).
Results
D-aspartate, but not L-aspartate, inhibits AMPARs in hippocampal neurons
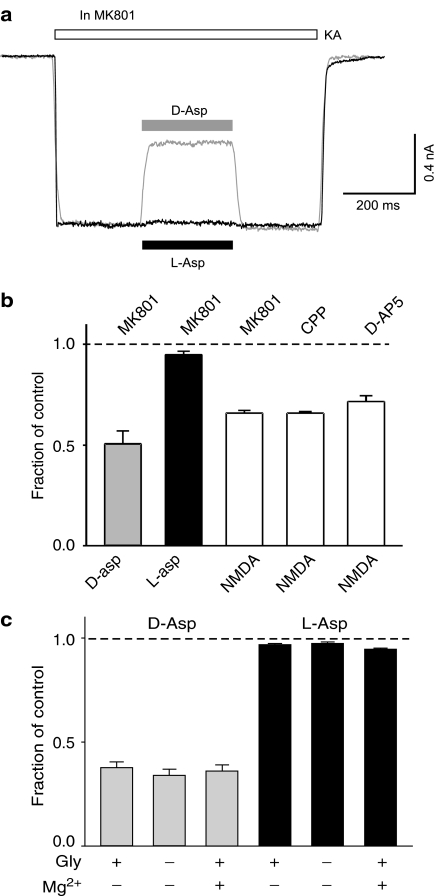
In acutely isolated hippocampal neurons (VH=−60 mV), kainate (200 μM) induced an inward current that was almost entirely mediated through AMPARs due to the sensitivity (97% block) to the AMPAR-selective antagonist GYKI 53655 (Bai et al., 2002). Addition of D-aspartate (1 mM) in the kainate solution induced a larger inward current (Figure 1b). Application of the selective NMDAR channel blocker, MK801 (5 μM), gradually and completely blocked NMDAR-mediated current. Surprisingly, after a complete block of D-aspartate-induced current by MK801, kainate (200 μM)-induced current was consistently inhibited by D-aspartate (49±7% blockade, n=5, P=0.002 with paired Student's t-test, Figure 2a, b). This inhibition was totally eliminated when D-aspartate was removed from the bath. Interestingly, L-aspartate (1 mM) only produced a marginal inhibition (5±2% blockade, n=5, P=0.04). The synthetic analog of D-aspartate, NMDA, also significantly inhibited kainate-induced current (Figure 2b).
Figure 2.
D-aspartate and NMDA, but not L-aspartate, block kainate-induced current in hippocampal neurons. (a) Superimposed current records to show the actions of D-aspartate (grey trace, 1 mM) and L-aspartate (black trace, 1 mM) on kainate (200 μM, KA)-induced current. Kainate-induced current was partially inhibited by D-aspartate and little inhibition was observed with L-aspartate. MK801 (5 μM) was added in all solutions to block NMDAR-mediated current. (b) Bar graph illustrates the inhibitory action of D-aspartate (D-asp, 1 mM, grey bar), L-aspartate (L-asp, 1 mM, black bar) and NMDA (1 mM, open bars) on kainate (200 μM)-induced current. The antagonist for NMDARs used for each experiment was indicated on the top of each bar. Concentrations of the antagonists used are: MK801, 5 μM; CPP, 50 μM and D-AP5, 100 μM. (c) Bar graph summarizing the blocking action D-aspartate (1 mM, grey bars) and L-aspartate (1 mM, black bars) on kainate (100 μM)-induced current in the presence and/or absence of glycine (Gly, 10 μM) and magnesium (Mg2+, 2 mM). Minus represents no added glycine/magnesium.
MK801 is a noncompetitive blocker on NMDARs by binding to the channel pore region and does not affect NMDA binding to the NMDAR and subsequent receptor activation (Huettner & Bean, 1988; MacDonald et al., 1991). It is possible that the inhibitory action of D-aspartate and NMDA on kainate-induced current was dependent on NMDAR activation, even though there was no current passing through the NMDAR channel. If this were the case, then prevention of the activation of NMDAR by a competitive antagonist would eliminate the inhibitory action of D-aspartate and NMDA on kainate-induced current. This idea was tested with the competitive NMDAR antagonists, CPP and D-AP5. Replacing MK801 with either CPP (50 μM) or D-AP5 (100 μM) did not significantly alter the degree of blockade of NMDA on kainate-induced current (from 34.1±1.3 to 34.2±0.9 and 28.4±2.9% blockade, respectively, Figure 2b). Removal of the NMDAR coagonist, glycine, or adding Mg2+ in the bath will also substantially inhibit NMDAR-mediated current. However, none of these manipulations was able to alter the inhibitory action of D-aspartate on kainate (100 μM)-induced current (Figure 2c). L-aspartate produced little inhibition on kainate-induced current in any of these conditions (Figure 2c). These data suggest that the inhibitory action of D-aspartate and NMDA on kainate-induced current is independent of NMDAR activation. The lack of inhibitory action of L-aspartate on kainate-induced current was not due to a higher affinity of L-aspartate to the glutamate transporters than that of D-aspartate. The addition of the potent glutamate transporter inhibitor DL-TBOA (100 μM) failed to modify the inhibitory action of D-aspartate and the lack of inhibition of L-aspartate on AMPARs (data not shown).
D-aspartate dose-dependently inhibits kainate-induced current
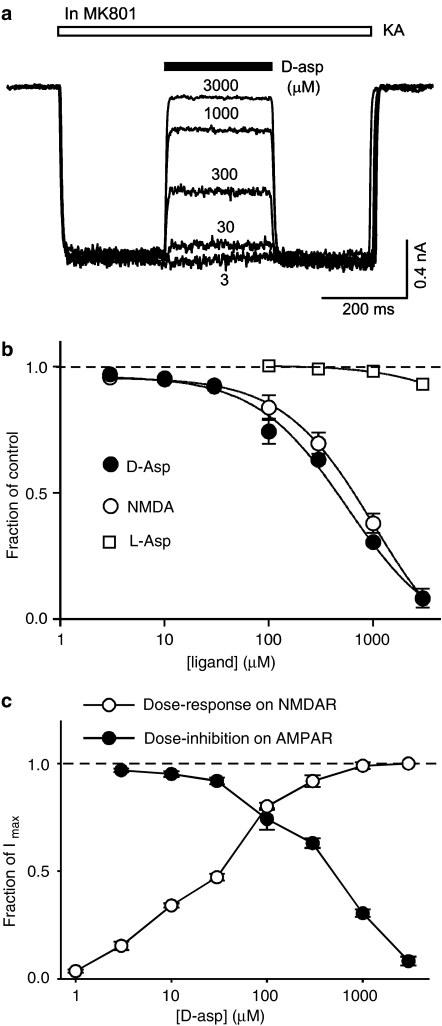
An alternative hypothesis is that D-aspartate is able to inhibit kainate-induced current by competing with kainate on the agonist-binding site of AMPARs. This hypothesis predicts that increasing concentrations of D-aspartate will increase the inhibitory action of D-aspartate on kainate-induced current. To test this we studied the actions of different concentrations of D-aspartate (3–3000 μM) on kainate (100 μM)-induced current (Figure 3a, b). The results support the prediction and demonstrate that D-aspartate concentration-dependently inhibits kainate-induced current (Figure 3a, b). The estimated IC50 for D-aspartate was 0.38±0.08 mM. NMDA produced similar dose-dependent inhibition on kainate-induced current with a lower potency (IC50=0.68±0.13 mM, n=3, P=0.03 with paired Student's t-test). We also tested L-aspartate (100 μM–3 mM) on kainate-induced current. A significant block on kainate (100 μM)-induced current was observed with 3 mM L-aspartate (7±1% block, n=7, P=0.006).
Figure 3.
D-aspartate concentration-dependently inhibits kainate-induced current. (a) Superimposed current recordings are from the same isolated hippocampal neuron in the presence of MK801. Kainate (100 μM, KA, open bar)-induced current was concentration-dependently inhibited by coapplication of D-aspartate (black bar). The concentration of D-aspartate is indicated (in μM) for each current recording. (b) Concentration–inhibition curve of D-aspartate, NMDA and L-aspartate on kainate (100 μM)-induced current. The estimated IC50 for D-aspartate was 0.38 mM and for NMDA was 0.68 mM. (c) Superimposed concentration–inhibition curve (filled circles) of D-aspartate on kainate-induced current in the presence of MK801 (5 μM) and concentration–response curve (open circles) for D-aspartate-induced current in hippocampal neurons. A concentration of 10 μM glycine was included in the extracellular solution. The estimated EC50 for the agonist action of D-aspartate was 28±3 μM (n=5).
To directly compare the agonist action on NMDARs and the blocking action on kainate-induced current of D-aspartate under the same experimental conditions we constructed concentration–response curves for D-aspartate-induced NMDAR-mediated current in the presence of a saturating concentration of the coagonist glycine (10 μM). As illustrated in Figure 3c the agonist action of D-aspartate on NMDARs was more potent than the antagonist action on AMPARs. The estimated EC50 (28±3 μM) for the agonist action was approximately an order of magnitude lower than the estimated IC50 (380±80 μM) for the blockade of kainate-induced current.
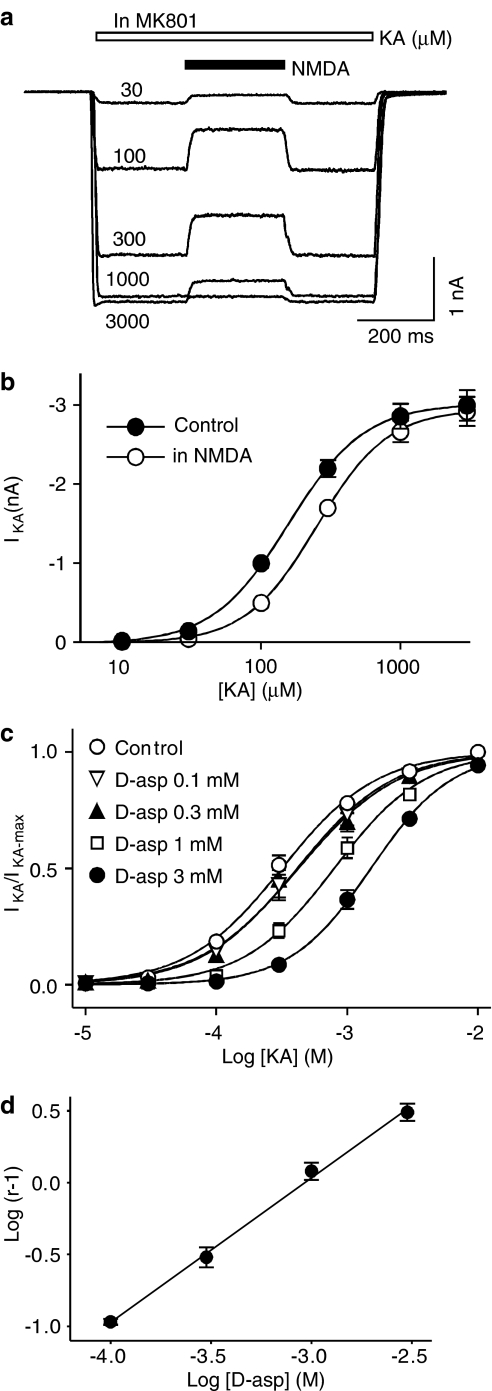
The second prediction of the hypothesis was that D-aspartate should be able to reduce the apparent affinity of kainate at AMPARs, that is, in the presence of D-aspartate or NMDA the concentration–response curve for kainate-induced current should be shifted to the right without a decrease in the maximum current. This was indeed the case (Figure 4a, b). The estimated EC50 for the kainate concentration–response curve was increased from 169±22 to 260±10 μM (n=7; P<0.001, Figure 4a, b) in the presence of NMDA (1 mM). However, there was no significant difference between the maximum current under control conditions (2.8±0.2 nA) and that in the presence of NMDA (2.8±0.2 nA, n=7, P=0.35). We have also studied the blocking action of four different concentrations of D-aspartate on the kainate concentration–response curve. D-aspartate dose-dependently shifted the kainate concentration–response curve to the right without changing the maximum current amplitude (Figure 4c). The estimated EC50 was significantly increased at all tested D-aspartate concentrations from the control kainate concentration–response curve of the same cell (1.11±0.004 fold of control for 0.1 mM D-aspartate, P=0.001; 1.32±0.06 fold for 0.3 mM D-aspartate, P=0.003; 2.28±0.17 fold for 1 mM D-aspartate, P<0.001; 4.30±0.53 fold for 3 mM D-aspartate, P<0.001). As illustrated in Figure 4d, the Schild plot was linear (r2=0.997) with a slope (1.01±0.04) of unity, indicating that the antagonist action of D-aspartate on AMPARs was competitive. The Kb of D-aspartate was calculated to be 0.93 mM.
Figure 4.
D-aspartate and NMDA shift the kainate concentration–response curve to the right. (a) Superimposed current recordings obtained from the same isolated hippocampal neurons in response to different concentrations of kainate (KA, 30, 100, 300, 1000 and 3000 μM, open bar). In the presence of MK801, NMDA (1 mM) partially blocked kainate-induced current. (b) Kainate concentration–response curve in the control conditions (filled circles) and in the presence of NMDA (1 μM, open circles). The estimated EC50 was significantly increased (control 0.17±0.02 mM vs in the presence of NMDA 0.26±0.01 mM, n=7, P<0.001). The estimated maximum currents were not changed. (c) Kainate concentration–response curves in the control conditions and in the presence of different concentrations of D-aspartate. Data were normalized to the maximum control kainate-induced current. The estimated maximum kainate-induced currents in the presence of different concentrations of D-aspartate were not different from those obtained from their corresponding maximum control kainate current in control conditions. (d) A Schild plot was constructed to show the antagonist action of D-aspartate on kainate-induced current was concentration dependent. Linear regression line (r2=0.997) was shown with a slope of 1.01±0.04 and an X-intercept at −3.03, giving Kb=0.93 mM for D-aspartate. Dose ratio r=(EC50 in D-asp)/(EC50 control).
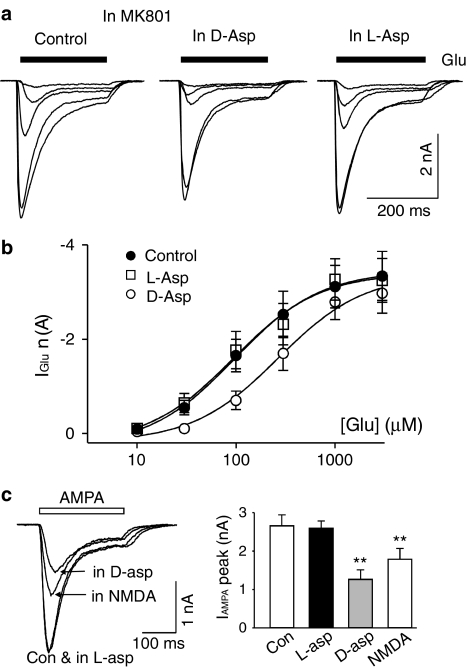
D-aspartate inhibits L-glutamate-induced AMPAR current
L-glutamate is the native and full agonist for AMPARs. It was next tested if the L-glutamate-induced AMPAR current was also subject to the inhibition by D-aspartate. MK801 was used to block D-aspartate- and L-glutamate-induced NMDA current. Under these conditions, L-glutamate induced an inward AMPAR current with a transient peak followed by various degrees of apparent desensitization. L-glutamate concentration–response curves measured from the peak current were constructed in the control conditions and in the presence of D-aspartate (1 mM). As illustrated in Figure 5, D-aspartate (1 mM) shifted the L-glutamate concentration–response curve to the right similar to that observed for kainate-induced current. The estimated EC50 for the L-glutamate concentration–response curve increased from 192±73 to 295±63 μM in D-aspartate (n=7, P=0.04), while the maximum L-glutamate-induced current did not change. Our data are consistent with a model that D-aspartate is able to inhibit L-glutamate-induced AMPAR current by competing with glutamate at its binding site on AMPARs. A significant increase in the EC50 of L-glutamate-induced current was also observed in the presence of NMDA (1 mM, data not shown). However, L-aspartate (1 mM) failed to modify the L-glutamate concentration–response curve (Figure 5a, b).
Figure 5.
D-aspartate shifts the L-glutamate concentration–response curve to the right. (a) Current records obtained from the same isolated hippocampal neuron in response to different concentrations of L-glutamate (30, 100, 300, 1000 and 3000 μM) in the control conditions (left panel), in the presence of D-aspartate (1 mM, middle panel) and in the presence of L-aspartate (1 mM, right panel). (b) L-glutamate concentration–response curve in the control conditions (filled circles), in the presence of D-aspartate (open circles) and in the presence of L-aspartate (open squares). (c) Current records obtained from the same isolated hippocampal neuron in response to AMPA (100 μM) under control condition (Con), in the presence of L-aspartate (in L-asp, 1 mM), in the presence of D-aspartate (D-asp, 1 mM) and in the presence of NMDA (1 mM). MK801 (5 μM) was added to all solutions to block NMDAR-mediated current. Right panel illustrates data summary from four different hippocampal neurons to show D-aspartate and NMDA significantly reduced AMPA-induced current (**indicates P<0.01).
AMPA, a selective AMPAR agonist, induced an inward current in hippocampal neurons. AMPA (100 μM)-induced current was characterized with a transient peak followed by a rapid decay to a steady-state current due to the desensitization of AMPARs (Figure 5c). D-aspartate (1 mM) and NMDA (1 mM) were able to significantly reduce the peak current amplitude of AMPA-induced current (Figure 5c). L-aspartate (1 mM) failed to reduce the amplitude of AMPA-induced current.
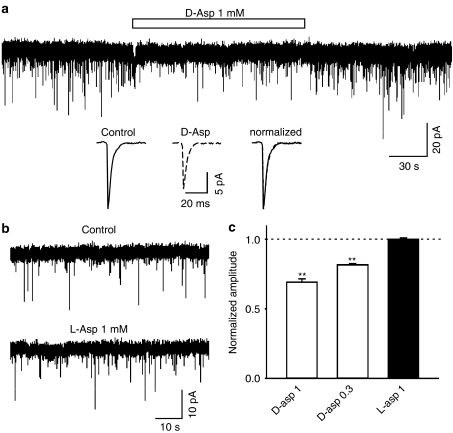
D-aspartate reduces the amplitude of AMPAR-mediated miniature excitatory postsynaptic current (mEPSCAMPA)
To further investigate if D-aspartate is able to modulate fast excitatory synaptic transmission in hippocampal neurons, we studied the mEPSCsAMPA in cultured hippocampal neurons. Whole-cell recording was made at a holding potential of −60 mV and in the presence of TTX (1 μM), bicuculline (30 μM) and MK801 (10 μM). The mEPSCsAMPA were stable in frequency and amplitude during the control conditions. Application of D-aspartate (0.3 and 1 mM) significantly and reversibly reduced the amplitude of mEPSCs (Figure 6a, c, at 1 mM, 69±2% of control, n=5, P<0.001; at 0.3 mM, 82±0.9% of control, n=3, P<0.001) without substantial change in the kinetic properties (Figure 6a inset). D-aspartate also significantly reduced the frequency of mEPSCs (at 1 mM, 66±10% of control, P=0.005; at 0.3 mM, 86±5% of control, P=0.043). However, the reduction in mEPSCAMPA frequency might be, at least in part, due to the fact that the small amplitude mEPSCAMPA became undetected during D-aspartate application. In contrast, L-aspartate (1 mM) exhibited no effect on the amplitude or frequency of mEPSCs (Figure 6b, c).
Figure 6.
D-aspartate, but not L-aspartate, reduced the amplitude of mEPSCsAMPA in cultured hippocampal neurons. (a) An example current trace shows mEPSCsAMPA before, during and after the application of D-aspartate (open bar, 1 mM). Averaged individual mEPSCsAMPA before and during D-aspartate (dotted line, 1 mM), and the superimposed normalized traces are illustrated as insets. (b) Current recordings are mEPSCsAMPA before and during application of L-aspartate (1 mM). (c) Bar graph exhibits that the amplitude of mEPSCAMPA is significantly reduced by D-aspartate (open bars, P<0.01 for 1 and 0.3 mM). L-aspartate has no effect on the amplitude of mEPSCAMPA. Each bar represents averaged data obtained from 3–5 neurons.
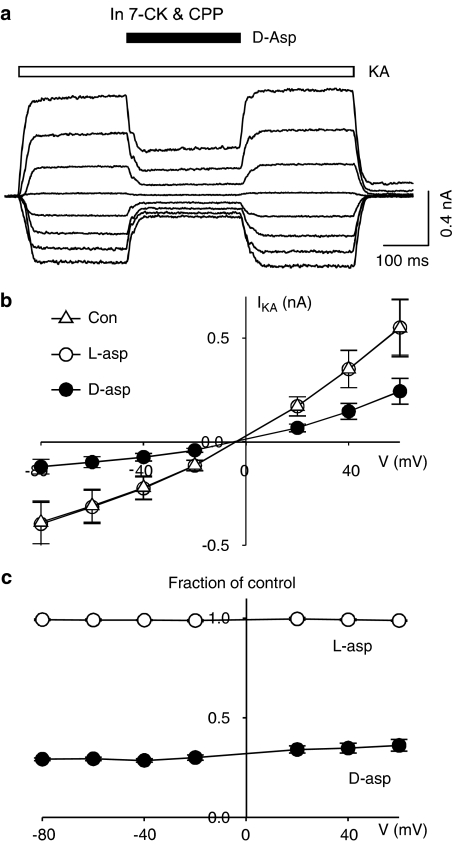
D-aspartate voltage-independently inhibits kainate-induced current
We demonstrated that D-aspartate was able to inhibit AMPARs at a holding potential close to resting membrane potential (−60 mV). We subsequently investigated whether the inhibitory action varied with different holding potentials in isolated hippocampal neurons. The current–voltage relationship of AMPAR-mediated response was studied by measuring kainate (100 μM)-induced whole-cell current over a range of holding potentials from −80 mV to +60 mV. Addition of CPP (50 μM) and 7-chlorokynurenic acid (7-CK, 20 μM) in nominally glycine-free extracellular medium was necessary to completely block NMDAR-mediated current at all tested holding potentials. Application of D-aspartate in the middle of kainate perfusion resulted in a transient reduction of kainate-induced current consistently at all tested holding potentials (Figure 7), indicating the D-aspartate-mediated inhibition of kainate-induced current was voltage-independent. Minimal blocking action was observed when D-aspartate was replaced with L-aspartate throughout the whole range of holding potentials (Figure 7c open circles).
Figure 7.
The blocking action of D-aspartate on kainate-induced current is observed at all tested holding potentials. (a) Kainate (100 μM, open bar)-induced currents under different holding potentials (−80 to +60 mV, 20 mV increment). Application of D-aspartate (D-asp, filled bar) partially blocked kainate-induced current. (b) Current–voltage relationship for kainate-induced current was plotted in control conditions (Con, open triangles) and in the presence of D-aspartate (D-asp, 1 mM, filled circles) and L-aspartate (L-asp, 1 mM, open circles). (c) Kainate-induced currents in the presence of D-aspartate (filled circles) and L-aspartate (open circles) were calculated as fractions of control kainate currents.
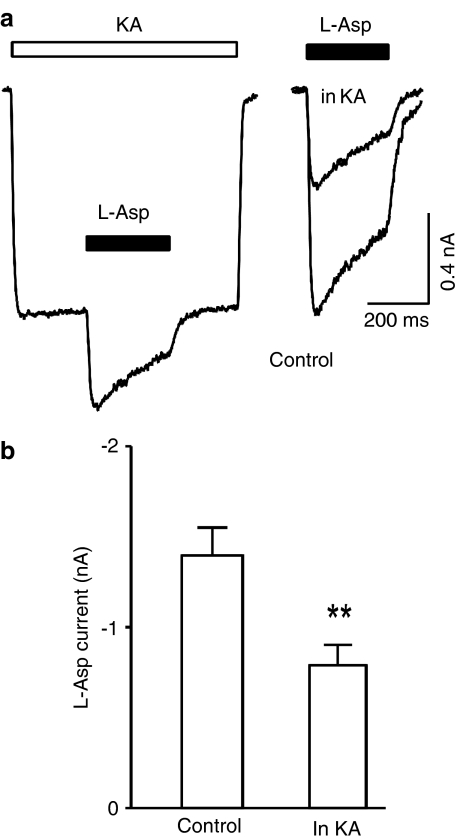
Kainate reduces L-aspartate-induced current in hippocampal neurons
Having observed the blocking action of D-aspartate and NMDA, and the lack of blocking action of L-aspartate, on kainate-induced current, it is necessary to verify if the nonionotropic cross-inhibition between the AMPARs and the NMDARs (Bai et al., 2002) exists when using L-aspartate as an NMDAR agonist. In hippocampal neurons, L-aspartate-induced current in the control conditions (1.4±0.16 nA) was significantly larger than that obtained during kainate-induced current (0.8±0.11 nA, n=7, P=0.003, Figure 8a, b), indicating that the cross-inhibition between the AMPARs and the NMDARs does exist and is similar to that described previously (Bai et al., 2002).
Figure 8.
Kainate reduces L-aspartate-induced current in hippocampal neurons. (a) Current traces obtained from the same hippocampal neuron in response to kainate (200 μM, KA, open bar) and L-aspartate (L-asp, 1 mM, filled bars). L-aspartate alone induced current was superimposed with L-aspartate-induced current in the presence of kainate (right panel). (b) Bar graph illustrates kainate reduced L-aspartate-induced current.
An additional concern for the subadditive actions of NMDA- and kainate-induced current was that kainate could block NMDA-induced current. This was indeed the case on recombinant NMDARs expressed in X. laevis oocytes (Lerma et al., 1989a; Ruppersberg et al., 1993). However, under our experimental conditions, kainate (200 μM) failed to produce any significant block of either L-aspartate (95±3% of control, n=5) or D-aspartate (101±1% of control, n=4)-induced NMDA current in hippocampal neurons.
D-aspartate and NMDA, but not L-aspartate, antagonize recombinant AMPARs
The effects of D-aspartate, NMDA and L-aspartate (1 mM) were evaluated at recombinant rat AMPAR (GluR1o, GluR2(Q)i, GluR3o and GluR3i) expressed in X. laevis oocytes in the absence or presence of 10 μM L-glutamate (Table 1). A concentration of 10 μM L-glutamate was chosen as the control stimulation since this is near the EC50 value for L-glutamate at each receptor subtype (Coquelle et al., 2000). D-aspartate and NMDA have no agonist activity at the recombinant AMPARs but can inhibit the responses to 10 μM L-glutamate, consistent with our observations on hippocampal neurons. L-aspartate is a weak agonist at the recombinant AMPARs and produces additive responses to 10 μM L-glutamate. No responses were detected for any of the compounds in uninjected oocytes (n=20). These data demonstrate that D-aspartate and NMDA directly inhibit these recombinant AMPARs, suggesting this is a fundamental property of AMPARs and not only restricted to the AMPARs in the hippocampus.
Table 1.
NMDA, L- and D-aspartate activities at recombinant AMPAR expressed in Xenopus laevis oocytes
| Stimulation drugs | % of the control current | |||
|---|---|---|---|---|
| GluR1o | GluR2(Q)i | GluR3o | GluR3i | |
| 1 mM NMDA | NR (13)** | ND | ||
| 1 mM D-aspartate | 1.2±0.7 (14)** | ND | ||
| 1 mM L-aspartate | 34±2 (7) | 16±2 (4) | 44±6 (2) | 23±3 (6) |
| NMDA (1 mM)+L-glutamate (10 μM) | 64±6 (21)** | ND | ||
| D-aspartate (1 mM)+L-glutamate (10 μM) | 70±1 (8) | 32±2 (6) | 79±10 (2) | 37±2 (6) |
| L-aspartate (1 mM)+L-glutamate (10 μM) | 111±3 (22)** | ND | ||
Data are expressed as % of control. 100% control is the current response to 10 μM L-glutamate on the recombinant AMPA receptors. ND=not determined; NR=no response. Values are given as means±s.e.m. N value is indicated in parentheses.
These data were pooled from GluR1o, GluR2(Q)i and GluR3o as there was no statistically significant difference between the data to the different subtypes of AMPA receptors by one-way ANOVA.
Discussion
Here we provide functional evidence that D-aspartate inhibits AMPARs in acutely isolated hippocampal neurons. However, L-aspartate is virtually without this blocking action, indicating that the structural requirement for this inhibitory action is stereoselective. Recent findings demonstrate that D-aspartate exists in the brain at concentrations high enough to modify AMPARs. Thus, these novel findings may indicate a possible role of D-aspartate in modulating synaptic transmission in hippocampus. Our observation that D-aspartate was able to reduce the amplitude of mEPSCs provides direct evidence to further support its potential role in modulating excitatory synaptic transmission. Whether the inhibition by D-aspartate on AMPARs plays a role under physiological or pathological conditions remains to be investigated.
Several pieces of evidence support the idea that D-aspartate and NMDA inhibit the function of AMPARs by directly competing with the agonist binding site on AMPARs. First, we have ruled out the possibility that D-aspartate and NMDA inhibit AMPARs by activating NMDARs. We chose to use MK801 to block D-aspartate- and NMDA-induced current due to its high potency on NMDAR. Competitive NMDAR antagonists, CPP and D-AP5, completely prevent the activation of NMDARs. Yet they failed to modify the blocking action of D-aspartate and NMDA on AMPAR current. In addition, removal of the coagonist glycine from the bath or adding Mg2+ to the bathing medium will reduce substantially the NMDAR activities. However, these manipulations again failed to modify the blocking action of D-aspartate on kainate-induced AMPAR current. Second, both kainate and L-glutamate concentration–response curves for AMPARs on hippocampal neurons were shifted to the right by D-aspartate and NMDA without modification of the maximum AMPAR current. The ability to shift kainate concentration–response curves by D-aspartate was concentration-dependent. The Schild plot of D-aspartate blockade of kainate-induced responses was linear with a slope of one and a calculated Kb of 0.93 mM. Third, previous studies have shown that NMDA could inhibit recombinant AMPARs (GluR1, 2, 3 and 4) expressed in X. laevis oocytes (Ruppersberg et al., 1993; Magazanik & Usherwood, 1996). Consistent with these studies we found that both D-aspartate and NMDA blocked L-glutamate-induced current in recombinant homomeric AMPARs, demonstrating that these ligands directly interact with AMPARs. Finally, the block of AMPARs by D-aspartate did not show any voltage-dependence, suggesting that the blocking site of D-aspartate is unlikely to be anywhere within the channel pore area of AMPA channels. Further evidence will be required to verify how D-aspartate fits to the binding pocket of the agonist binding site on AMPARs.
The blocking action of NMDA on kainate-induced current was first described in rat retinal ganglion cells, where coapplication of NMDA and kainate induced a smaller current than that elicited by kainate alone (Karschin et al., 1988). In the presence of NMDAR blockers, AP5 or Mg2+, similar observations were made on receptors expressed in X. laevis oocytes using whole-brain mRNA (Lerma et al., 1989b, Brackley & Usherwood, 1993) and cRNAs encoding combinations of GluR1–4 subunits of AMPARs (Brackley & Usherwood, 1993; Ruppersberg et al., 1993; Magazanik & Usherwood, 1996). In the present study, NMDA and D-aspartate are both acting as antagonists at all recombinant homomeric AMPARs tested. D-aspartate displays significantly different degrees of blockade at different homomeric AMPARs. L-aspartate was observed to be a weak agonist in all tested recombinant AMPARs. However we did not see any agonist action of L-aspartate (1 mM) at hippocampal AMPARs (see also Patneau & Mayer, 1990).
Our present data demonstrate that NMDA concentration-dependently blocks kainate-induced current in hippocampal neurons. This blocking action is not only on kainate-induced current but also on AMPA- and L-glutamate-induced AMPAR current. A recent report suggests that NMDA exists in endocrine nuclei in the brain (D'Aniello et al., 2000). However, the concentration was estimated to be in micromolar range and is probably too low to produce a substantial block on AMPARs in synapses. On the other hand, D-aspartate was found to be enriched in the brain and D-aspartate mimics the blocking action of NMDA on AMPAR-mediated current. Due to its existence in the brain at concentrations that are possibly high enough to modify AMPAR function and due to its higher potency in blocking AMPARs compared to that of NMDA, D-aspartate antagonism offers a novel mechanism in modulating AMPAR activation. D-aspartate is also a potent NMDAR agonist and could participate in activation of NMDARs in synapses. Both activation of NMDARs and inhibition of AMPARs make D-aspartate a unique ligand as it is able to regulate the balance between AMPAR and NMDAR activities, the two key ionotropic glutamate receptors.
We observed that D-aspartate was able to concentration-dependently reduce mEPSCAMPA amplitude at the tested doses, indicating an antagonist action of D-aspartate on postsynaptic AMPA receptors. In addition, a significant reduction in mEPSCAMPA frequency by these doses of D-aspartate was also observed. The reduction in mEPSCAMPA frequency can be simply interpreted as that D-aspartate has an additional presynaptic action to reduce synaptic release of glutamate. However, we cannot rule out a possibility that the frequency change was, in part, due to the reduction in the amplitude of mEPSCAMPA, as the amplitude of small events may be dropped below the mEPSC detection threshold and is similar to the baseline noise level. The distribution of the amplitude of mEPSCAMPA in our hippocampal culture is skewed with more small amplitude events than the large amplitude ones, which made the frequency of mEPSCAMPA likely to reduce by a small decrease in the amplitude. Further experiments are necessary to fully clarify if D-aspartate possesses an action at the presynaptic site.
D-aspartate and NMDA are commonly considered to be specific agonists for NMDARs. Our current findings reveal novel additional actions of these compounds, that is, to competitively block AMPARs. Therefore, caution should be taken in the interpretation of the effects of NMDA and D-aspartate when used pharmacologically in the brain or neuronal cultures. Future experiments are required to further explore the physiological role of D-aspartate in regulating synaptic transmission in the brain.
Acknowledgments
We thank Drs John MacDonald, Stephen Sims and Dan Belliveau for reading an earlier version of the manuscript and Dr James Hammond for helpful discussions. This work was supported by Canadian Institute of Health Research (D.B. and W.Y.L.), Canada Research Chair in Cellular Communication (D.B.) and The Novo Nordisk Foundation (D.S.P.).
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate
- 7-CK
7-chlorokynurenic acid
- CPP
3-(2-carboxypiperazin-4-yl) propanephosphonic acid
- D-AP5
D(−)-2-amino-5-phosphonopentanoic acid
- D-asp
D-aspartate
- DL-TBOA
DL-threo-β-benzyloxyaspartic acid
- ECF
extracellular fluid
- KA
kainate
- L-asp
L-aspartate
- MK801
dizocilpine or (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- NMDA
N-methyl-D-aspartate
References
- ALBUS U., HABERMANN E. Tetanus toxin inhibits the evoked outflow of an inhibitory (GABA) and an excitatory (D-aspartate) amino acid from particulate brain cortex. Toxicon. 1983;21:97–110. doi: 10.1016/0041-0101(83)90053-3. [DOI] [PubMed] [Google Scholar]
- BAI D., MULLER R.U., RODER J.C. Non-ionotropic cross-talk between AMPA and NMDA receptors in rodent hippocampal neurones. J. Physiol. 2002;543:23–33. doi: 10.1113/jphysiol.2002.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLISS T.V., COLLINGRIDGE G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- BRACKLEY P.T., USHERWOOD P.N. Are chimeric kainate/N-methyl-D-aspartate receptors expressed in Xenopus laevis oocytes from mammalian and amphibian RNA. J. Pharmacol. Exp. Ther. 1993;265:910–919. [PubMed] [Google Scholar]
- COQUELLE T., CHRISTENSEN J.K., BANKE T.G., MADSEN U., SCHOUSBOE A., PICKERING D.S. Agonist discrimination between AMPA receptor subtypes. Neuroreport. 2000;11:2643–2648. doi: 10.1097/00001756-200008210-00008. [DOI] [PubMed] [Google Scholar]
- D'ANIELLO A., DI COSMO A., DI CRISTO C., ANNUNZIATO L., PETRUCELLI L., FISHER G. Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996;59:97–104. doi: 10.1016/0024-3205(96)00266-4. [DOI] [PubMed] [Google Scholar]
- D'ANIELLO A., DI FIORE M.M., FISHER G.H., MILONE A., SELENI A., D'ANIELLO S., PERNA A.F., INGROSSO D. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J. 2000;14:699–714. doi: 10.1096/fasebj.14.5.699. [DOI] [PubMed] [Google Scholar]
- DAVIES L.P., JOHNSTON G.A. D-aspartate oxidase activity in extracts of mammalian central nervous tissue. J. Neurochem. 1975;25:299–304. doi: 10.1111/j.1471-4159.1975.tb06970.x. [DOI] [PubMed] [Google Scholar]
- DINGLEDINE R., BORGES K., BOWIE D., TRAYNELIS S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- DUNLOP D.S., NEIDLE A., MCHALE D., DUNLOP D.M., LAJTHA A. The presence of free D-aspartic acid in rodents and man. Biochem. Biophys. Res. Commun. 1986;141:27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- FISHER G.H., PETRUCELLI L., GARDNER C., EMORY C., FREY W.H., AMADUCCI L., SORBI S., SORRENTINO G., BORGHI M., D'ANIELLO A. Free D-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Mol. Chem. Neuropathol. 1994;23:115–124. doi: 10.1007/BF02815405. [DOI] [PubMed] [Google Scholar]
- FLECK M.W., BARRIONUEVO G., PALMER A.M. Synaptosomal and vesicular accumulation of L-glutamate, L-aspartate and D-aspartate. Neurochem. Int. 2001;39:217–225. doi: 10.1016/s0197-0186(01)00018-3. [DOI] [PubMed] [Google Scholar]
- FLECK M.W., HENZE D.A., BARRIONUEVO G., PALMER A.M. Aspartate and glutamate mediate excitatory synaptic transmission in area CA1 of the hippocampus. J. Neurosci. 1993;13:3944–3955. doi: 10.1523/JNEUROSCI.13-09-03944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONG X.Q., BAI D. D-aspartate, but not L-aspartate, blocks AMPA receptors in hippocampal neurons. Soc. Neurosci. Abstr. 2003;29:895.8. [Google Scholar]
- GUNDERSEN V., SHUPLIAKOV O., BRODIN L., OTTERSEN O.P., STORM-MATHISEN J. Quantification of excitatory amino acid uptake at intact glutamatergic synapses by immunocytochemistry of exogenous D-aspartate. J. Neurosci. 1995;15:4417–4428. doi: 10.1523/JNEUROSCI.15-06-04417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMASE K., HOMMA H., TAKIGAWA Y., FUKUSHIMA T., SANTA T., IMAI K. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochim. Biophys. Acta. 1997;1334:214–222. doi: 10.1016/s0304-4165(96)00095-5. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO A., KUMASHIRO S., NISHIKAWA T., OKA T., TAKAHASHI K., MITO T., TAKASHIMA S., DOI N., MIZUTANI Y., YAMAZAKI T. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J. Neurochem. 1993;61:348–351. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO A., OKA T., NISHIKAWA T. Anatomical distribution and postnatal changes in endogenous free D-aspartate and D-serine in rat brain and periphery. Eur. J. Neurosci. 1995;7:1657–1663. doi: 10.1111/j.1460-9568.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- HUETTNER J.E., BEAN B.P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARSCHIN A., AIZENMAN E., LIPTON S.A. The interaction of agonists and noncompetitive antagonists at the excitatory amino acid receptors in rat retinal ganglion cells in vitro. J. Neurosci. 1988;8:2895–2906. doi: 10.1523/JNEUROSCI.08-08-02895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERA Y., AOYAMA H., MATSUMURA H., HASEGAWA A., NAGASAKI H., YAMADA R. Presence of free D-glutamate and D-aspartate in rat tissues. Biochim. Biophys. Acta. 1995;1243:283–286. [PubMed] [Google Scholar]
- KISKIN N.I., KRISHTAL O.A., TSYNDRENKO A.Y. Cross-desensitization reveals pharmacological specificity of excitatory amino acid receptors in isolated hippocampal neurons. Eur. J. Neurosci. 1990;2:461–470. doi: 10.1111/j.1460-9568.1990.tb00437.x. [DOI] [PubMed] [Google Scholar]
- LEI S., ORSER B.A., THATCHER G.R., REYNOLDS J.N., MACDONALD J.F. Positive allosteric modulators of AMPA receptors reduce proton-induced receptor desensitization in rat hippocampal neurons. J. Neurophysiol. 2001;85:2030–2038. doi: 10.1152/jn.2001.85.5.2030. [DOI] [PubMed] [Google Scholar]
- LERMA J., KUSHNER L., SPRAY D.C., BENNETT M.V., ZUKIN R.S. mRNA from NCB-20 cells encodes the N-methyl-D-aspartate/phencyclidine receptor: a Xenopus laevis oocyte expression study. Proc. Natl. Acad. Sci. U.S.A. 1989a;86:1708–1711. doi: 10.1073/pnas.86.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMA J., KUSHNER L., ZUKIN R.S., BENNETT M.V. N-methyl-D-aspartate activates different channels than do kainate and quisqualate. Proc. Natl. Acad. Sci. U.S.A. 1989b;86:2083–2087. doi: 10.1073/pnas.86.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIMAN E.R., TYTGAT J., HESS P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- MACDONALD J.F., BARTLETT M.C., MODY I., PAHAPILL P., REYNOLDS J.N., SALTER M.W., SCHNEIDERMAN J.H., PENNEFATHER P.S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J. Physiol. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGAZANIK L.G., USHERWOOD P.N. Competitive antagonism of recombinant AMPA/kainate receptors by N-methyl-D-aspartate and analogues. Neuroreport. 1996;8:257–259. doi: 10.1097/00001756-199612200-00051. [DOI] [PubMed] [Google Scholar]
- MARTIRE M., ALTOBELLI D., MAURIZI S., PREZIOSI P., FUXE K. K(+)-evoked [(3)H]D-aspartate release in rat spinal cord synaptosomes: modulation by neuropeptide Y and calcium channel antagonists. J. Neurosci. Res. 2000;62:722–729. doi: 10.1002/1097-4547(20001201)62:5<722::AID-JNR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J. Physiol. 1985;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATSUKA S., HAYASHI M., MUROYAMA A., OTSUKA M., KOZAKI S., YAMADA H., MORIYAMA Y. D-Aspartate is stored in secretory granules and released through a Ca(2+)-dependent pathway in a subset of rat pheochromocytoma PC12 cells. J. Biol. Chem. 2001;276:26589–26596. doi: 10.1074/jbc.M011754200. [DOI] [PubMed] [Google Scholar]
- NEIDLE A., DUNLOP D.S. Developmental changes in free D-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci. 1990;46:1517–1522. doi: 10.1016/0024-3205(90)90424-p. [DOI] [PubMed] [Google Scholar]
- PALMER A.M., REITER C.T. Comparison of the superfused efflux of preaccumulated D-[3H]aspartate and endogenous L-aspartate and L-glutamate from rat cerebrocortical minislices. Neurochem. Int. 1994;25:441–450. doi: 10.1016/0197-0186(94)90020-5. [DOI] [PubMed] [Google Scholar]
- PATNEAU D.K., MAYER M.L. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J. Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPPERSBERG J.P., MOSBACHER J., GUNTHER W., SCHOEPFER R., FAKLER B. Studying block in cloned N-methyl-D-aspartate (NMDA) receptors. Biochem. Pharmacol. 1993;46:1877–1885. doi: 10.1016/0006-2952(93)90627-9. [DOI] [PubMed] [Google Scholar]
- SAVAGE D.D., GALINDO R., QUEEN S.A., PAXTON L.L., ALLAN A.M. Characterization of electrically evoked [3H]-D-aspartate release from hippocampal slices. Neurochem. Int. 2001;38:255–267. doi: 10.1016/s0197-0186(00)00077-2. [DOI] [PubMed] [Google Scholar]
- SCHELL M.J., COOPER O.B., SNYDER S.H. D-aspartate localizations imply neuronal and neuroendocrine roles. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAAGEPETERSEN H.S., SHIMAMOTO K., SCHOUSBOE A. Comparison of effects of DL-threo-beta-benzyloxyaspartate (DL-TBOA) and L-trans-pyrrolidine-2,4-dicarboxylate (t-2,4-PDC) on uptake and release of [3h]D-aspartate in astrocytes and glutamatergic neurons. Neurochem. Res. 2001;26:661–666. doi: 10.1023/a:1010939304104. [DOI] [PubMed] [Google Scholar]
- WATKINS J.C., EVANS R.H. Excitatory amino acid transmitters. Annu. Rev. Pharmacol. Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- YUSKO S.C., NEIMS A.H. D-aspartate oxidase in mammalian brain and choroid plexus. J. Neurochem. 1973;21:1037–1039. doi: 10.1111/j.1471-4159.1973.tb07555.x. [DOI] [PubMed] [Google Scholar]
- ZAAR K., KOST H.P., SCHAD A., VOLKL A., BAUMGART E., FAHIMI H.D. Cellular and subcellular distribution of D-aspartate oxidase in human and rat brain. J. Comp. Neurol. 2002;450:272–282. doi: 10.1002/cne.10320. [DOI] [PubMed] [Google Scholar]