Abstract
We examined the contribution of each α1-adrenoceptor (AR) subtype in noradrenaline (NAd)-evoked contraction in the thoracic aortas and mesenteric arteries of mice. Compared with the concentration–response curves (CRCs) for NAd in the thoracic aortas of wild-type (WT) mice, the CRCs of mutant mice showed a significantly lower sensitivity. The pD2 value in rank order is as follows: WT mice (8.21)>α1B-adrenoceptor knockout (α1B-KO) (7.77)>α1D-AR knockout (α1D-KO) (6.44)>α1B- and α1D-AR double knockout (α1BD-KO) (5.15). In the mesenteric artery, CRCs for NAd did not differ significantly between either WT (6.52) and α1B-KO mice (7.12) or α1D-KO (6.19) and α1BD-KO (6.29) mice. However, the CRC maximum responses to NAd in α1D- and α1BD-KO mice were significantly lower than those in WT and α1B-KO mice.
Except in the thoracic aortas of α1BD-KO mice, the competitive antagonist prazosin inhibited the contraction response to NAd with high affinity. However, prazosin produced shallow Schild slopes in the vessels of mice lacking the α1D-AR gene. In the thoracic aorta, pA2 values in WT mice for KMD-3213 and BMY7378 were 8.25 and 8.46, respectively, and in α1B-KO mice they were 8.49 and 9.13, respectively. In the mesenteric artery, pA2 values in WT mice for KMD-3213 and BMY7378 were 8.34 and 7.47, respectively, and in α1B-KO mice they were 8.11 and 7.82, respectively. These pharmacological findings were in fairly good agreement with findings from comparison of CRCs, with the exception of the mesenteric arteries of WT and α1B-KO mice, which showed low affinities to BMY7378.
We performed a quantitative analysis of the mRNA expression of each α1-AR subtype in these vessels in order to examine the correlation between mRNA expression level and the predominance of each α1-AR subtype in mediating vascular contraction.
The rank order of each α1-AR subtype in terms of its vasoconstrictor role was in fairly good agreement with the level of expression of mRNA of each subtype, that is, α1D-AR>α1B-AR>α1A-AR in the thoracic aorta and α1D-AR>α1A-AR>α1B-AR in the mesenteric artery. No dramatic compensatory change of α1-AR subtype in mutant mice was observed in pharmacological or quantitative mRNA expression analysis.
Keywords: α1-Adrenoceptor subtype, noradrenaline, mouse thoracic aorta, mouse mesenteric artery, α1D-knockout mouse, α1B-knockout mouse, α1BD-double knockout mouse, vasoconstriction, real-time PCR, mRNA expression
Introduction
The sympathetic nervous system plays an important role in vasoconstriction and blood pressure regulation. Catecholamines cause vascular smooth muscle contraction, primarily by activating α1-adrenoceptor (α1-ARs) (Hoffman, 2001). Currently, α1-AR can be characterized as three subtypes, α1A-, α1B-, and α1D-AR, by molecular cloning (Cotecchia et al., 1988; Schwinn et al., 1990; Perez et al., 1991; Hirasawa et al., 1993; Esbenshade et al., 1995; Hieble et al., 1995b) and by pharmacological analysis (McGrath, 1982; Han et al., 1987; Foglar et al., 1995; Guarino et al., 1996). The functional roles of each α1-AR subtype, in particular in relation to vasoconstriction and blood pressure regulation, have been elucidated through the recent development of α1-AR subtype-selective agonists and antagonists (Goetz et al., 1995; Hieble et al., 1995a, 1995b; Ford et al., 1996; Saussy et al., 1996; Yamamoto & Koike, 2001a, 2001b). The thoracic aorta and mesenteric artery, vessels with great potential as experimental models, have often been used to analyze the vasoconstrictor role of each α1-AR subtype in noradrenaline (NAd)-evoked vasoconstriction (Shi et al., 1989; Han et al., 1990; Kenny et al., 1995; Hussain & Marshall, 2000; Yamamoto & Koike, 2001a, 2001b). For example, α1D-AR-mediated vasoconstriction has been demonstrated to predominate in the rat thoracic aorta (Ford et al., 1996) and mouse thoracic aorta (Yamamoto & Koike, 2001b). In contrast, in the mouse mesenteric artery, it is still uncertain whether α1D-AR-mediated vasoconstriction predominates (Yamamoto & Koike, 2001a), although α1D-AR has been shown pharmacologically to be present in this artery (Goetz et al., 1995; Saussy et al., 1996). In addition, the gene knockout approach is increasingly being used to elucidate the functional roles of individual α1-AR subtypes in vasoconstriction and blood pressure regulation (Cavalli et al., 1997; Rokosh & Simpson, 2001; Daly et al., 2002; Tanoue et al., 2002). This gene knockout approach has revealed that all three α1-AR subtypes might be involved in vasoconstriction and blood pressure regulation (Cavalli et al., 1997; Rokosh & Simpson, 2001; Daly et al., 2002; Tanoue et al., 2002). However, we could not simply compare these results, because of the different genetic backgrounds and potential compensatory effects. Hence, the exact roles of the α1-AR subtypes in vasoconstriction need further investigation, both from a pharmacological and a gene expression viewpoint. To date, we have obtained limited information on the mRNA expression profiles of each α1-AR subtype in the cardiovascular system (Guarino et al., 1996; Miller et al., 1996). Real-time PCR techniques (Heid et al., 1996; Harrison et al., 2000; Medhurst et al., 2000) have enabled us to analyze the mRNA expression of each α1-AR subtype quantitatively in a variety of tissues (Volgin et al., 2001; Tanoue et al., 2002; Nomiya & Yamaguchi, 2003). Here, we performed a pharmacological characterization of the thoracic aorta and mesenteric arteries of wild-type (WT) and mutant mice with the same genetic background, in order to compare the expression of each α1-AR subtype in these vessels, as monitored by real-time PCR assay.
Methods
Generation of mice lacking both the α1B-AR and α1D-AR subtypes
α1B-KO and α1D-KO mice had already been generated and their viability confirmed (Cavalli et al., 1997; Tanoue et al., 2002). α1B-KO mice with the genetic background of 129Sv and a mixture of C57Black/6J strains (Cavalli et al., 1997) were mated with α1D-KO mice with the genetic background of 129Sv and a mixture of C57Black/6J strains (Tanoue et al., 2002) to produce F1 mice heterozygous for both traits. F1 heterozygous mice were mated to produce F2 WT and α1B- and α1D-AR double knockout (α1BD-KO) mice. Breeding pairs from these two lines produced offspring, which were used in our experiments. Thus, the genetic backgrounds of the WT, α1B-KO, α1D-KO, and α1BD-KO mice were the same. The genotypes of each α1-AR subtype were determined from DNA isolated from the tail (Cavalli et al., 1997; Tanoue et al., 2002). Four groups of male mice, WT, α1B-KO, α1D-KO, and α1BD-KO, with body weights of about 20–30 g, were used. All mice were housed in animal quarters with a 12-h light–dark cycle and were given food and distilled water ad libitum.
Mechanical responses
All experiments were conducted in accordance with the guidelines for the care and use of animals, as approved by the ethical committee of the National Research Institute for Child Health and Development. Each mouse was killed by a blow on the head, and then the thoracic aorta and mesenteric artery were isolated and dissected free of excess fat and connective tissue. The intimal surface of each artery was gently rubbed with a polyethylene tube to remove the endothelium, and functional loss of endothelial cells was confirmed by loss of the relaxation response to acetylcholine (1 μM). Each artery was cut into 4-mm ring segments. Ring segments were suspended in a 20-ml organ bath filled with Ringer–Locke solution (in mM: NaCl 154, KCl 5.6, CaCl2 2.2, MgCl2 2.1, NaHCO3 5.9, and glucose 2.8), kept at 37°C and bubbled with a mixture of 95% O2 and 5% CO2. To prevent oxidation of NAd, ascorbic acid (1 mg ml−1) was added to the solution. The tension was monitored continuously and recorded isometrically by a force displacement transducer. Experiments were conducted in the presence of propranolol (10 μM), yohimbine (0.3 μM), desmethylimipramine (0.1 μM), and normetanephrine (1 μM) to block β-adrenoceptors and α2-adrenoceptors and to inhibit neural and non-neural uptake of NAd, respectively. The strips were allowed to equilibrate for 90 min and then contracted with NAd and allowed to equilibrate for 30 min after washing. This was repeated until two successive contractions of approximately equal size had been obtained. The competitive antagonistic activities were expressed as pA2 values (negative logarithms of the dissociation constant). The concentration–response curves (CRCs) of NAd were obtained cumulatively. A contraction was expressed as grams force of the maximum contraction produced by NAd. After determination of a control CRC, the strips were equilibrated with a competitive antagonist for 30 min. CRCs were then determined in the presence of the antagonist and the procedure repeated with two further concentrations of the antagonist in the same preparation. After determination of the control CRCs, three successive cumulative CRCs for the antagonists were recorded. For each tissue, pD2 values and maximum tensions for the first, second, third, and fourth CRCs for NAd were not significantly different in preliminary experiments. We used the nonselective α1-antagonist prazosin (1–30 × 10−9 M), the α1D-selective antagonist 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]-ethyl]-8-azaspiro[4,5]decane-7,9-dione dihydrochloride (BMY7378) (10–3000 × 10−9 M), and the α1A-selective antagonist (−)-1-(3-hydroxypropyl)-5-((2R)-2-{[2-({2-[(2,2,2-trifluoroethyl) propyl)-2,3-dihydro-1H-indole-7-carboxamide (KMD-3213) (10–100 × 10−9 M) to determine the pA2 values. The pA2 values were calculated according to the method of Tallarida et al. (1979), which was originally reported by Arunlakshana & Schild (1959). Antagonist pA2 values were obtained from the X-intercept of the plot of log(agonist DR−1) against the log antagonist concentration using regression analysis. pA2 provides an estimate of the pKB, when the antagonism has been shown to meet all the criteria of competition. In cases where prazosin produced shallow Schild slopes, which were significantly different from unity, further pharmacological analyses using KMD-3213 or BMY7378 were not carried out.
RNA isolation and cDNA synthesis
The thoracic aorta and mesenteric artery were isolated and dissected free of excess fat and connective tissue. These materials were then immediately pooled in RNAlater RNA stabilization solution (Ambion Inc., Austin, TX, U.S.A.) for 1 day at room temperature to preserve as much RNA as possible. After that, total RNA was extracted from each sample using Isogen (Nippon Gene Co., Ltd, Tokyo, Japan). Total RNA (<5 μg) was treated with RNase-free DNase (Takara Shuzo Co., Tokyo, Japan) and reverse-transcribed using random hexamers (Tanoue et al., 2002). One-tenth of each cDNA sample was amplified by PCR with a receptor-specific primer set and a primer set specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Sabath et al., 1990). Each sample contained the upstream and downstream primers (10 pmol of each), 0.25 mM of each dNTP, 50 mM KCl, 10 mM Tris-HCl (pH 8.6), 1.5 mM MgCl2, and 2.5 U of Taq DNA polymerase (TaKaRa Shuzo Co., Tokyo, Japan). Thermal cycling was performed for 1 min at 94°C, 1 min at 56°C, and 2 min at 72°C for 27 cycles. Control PCR reactions were also performed on non-reverse-transcribed RNA to exclude any contamination by genomic DNA. Amplified DNA was analyzed on a 1.5% agarose gel with 100-bp DNA markers (New England Biolabs Inc., Beverly, MA, U.S.A.).
Real-time PCR assay
For accurate quantification of RT-PCR products, a TaqMan 5′ nuclease fluorogenic quantitative PCR assay (Applied Biosystems Japan Ltd, Tokyo, Japan) was conducted in accordance with the manufacturer's instructions, using total RNA from the thoracic aortas and mesenteric arteries of WT, α1B-KO, α1D-KO, and α1BD-KO mice. cDNAs were synthesized from total RNA (0.5–1.0 μg), as described above. Real-time PCR assays with the ABI Prism 7700 Sequence Detection System (Applied Biosystems Japan Ltd) were then carried out using the following oligonucleotides (5′–3′): 1A reverse primer, TCACACCAATGTATCGGTCGA; 1A probe, 6FAM-CCATCATGGGCCCTGCATCATCT-TAMRA; 1B forward primer, CCTGGTCAT-GTACTGCCGA; 1B reverse primer, GACTCCCGCCTCCAGATTC; 1B probe, 6FAM-TCTACATCGTGGCAAAGAGGACCACC-TAMRA; 1D forward primer, CGCTGTGGTGGGAACCGGCAG; 1D reverse primer, AGTTGGTGACCGTCTGCAAGT; 1D probe, 6FAM-CGGGCAACCT-TCTCGTCATCCTCTC-TAMRA; 1A forward primer, GCGGTGGACGTCTTATGCT. Commercially available TaqMan rodent GAPDH control reagents containing primers and probe for GAPDH were also used (Applied Biosystems Japan Ltd). All primers used for real-time PCR assays were derived from the nucleotide sequences within the first exon of each gene. In this assay, we added 5 pmol of each primer, 10 pmol of the TaqMan probe, 25 μl of Universal Master Mix (Applied Biosystems Japan Ltd), and 1 μl of cDNA in a total reaction volume of 50 μl. After enzyme inactivation for 10 min at 95°C, 50 cycles were performed (15 s at 95°C, 60 s at 60°C). The level of GAPDH expression was measured in all samples for normalization of sample-to-sample differences in RNA input, RNA quality, and reverse transcription efficiency.
Data analysis
Numerical results were expressed as mean±s.e. CRCs to NAd in wild-type (WT) and mutant mice were compared by two-way analysis of variance (ANOVA) for repeated measures. The CRC maximum responses, the pD2 values, pA2 values, and the number of α1-AR subtype copies were compared by a one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. A P-value of <0.05 was considered to denote a significant difference.
Drugs
The following drugs were used: (−)-NAd bitartrate (Wako-Junyaku, Osaka, Japan); BMY7378 (Research Biochemicals, Natick, MA, U.S.A.); prazosin hydrochloride, desmethylimipramine hydrochloride, (±)-propranolol hydrochloride, and yohimbine hydrochloride (Sigma, St Louis, MO, U.S.A.); and KMD-3213 (Kissei Pharmaceutical Co. Ltd, Fukuoka, Japan).
Results
NAd-induced contraction of the thoracic aorta
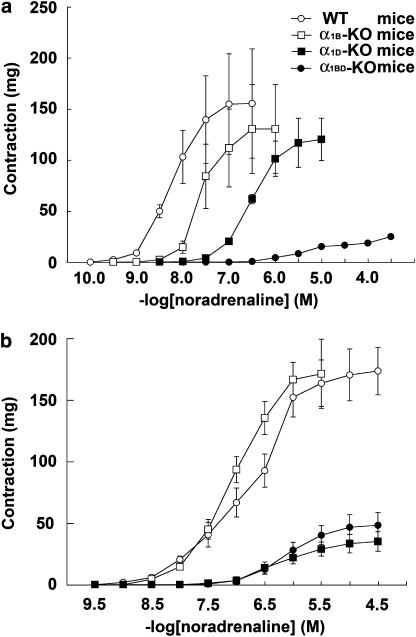
In the thoracic aortas of WT mice, NAd evoked contraction in a concentration-dependent manner (Figure 1a). The maximum responses of the CRCs for NAd in the thoracic aorta were not significantly different between WT (155±53 mg, n=10) and α1B-KO mice (130±43 mg, n=10) (Figure 1a). However, there was a significant difference in sensitivity to NAd between the CRCs for WT and α1B-KO mice (pD2 value in WT was 8.21±0.07 and in α1B-KO was 7.77±0.07, n=10 (P<0.05)) (Figure 1a). The CRCs for NAd in α1D-KO mice were shifted further to the right and were significantly different from those in WT mice (P<0.05). There was a significant difference in sensitivity to NAd (P<0.05) between the CRCs for α1D-KO mice and WT mice (pD2 value in α1D-KO was 6.44±0.05, n=10), without a significant depression in the maximum response (120±21 mg in α1D-KO, n=10) (Figure 1a). The CRC for NAd in α1BD-KO mice was almost completely abolished (maximum response, 25±1 mg; pD2 value, 5.15±0.05; n=10) (Figure 1a).
Figure 1.
Comparison of CRCs for NAd in thoracic aortas (a) and mesenteric arteries (b) taken from each group of mice. Ordinate: contraction, expressed as milligrams force. Abscissa: negative log concentration (M) of NAd. Each value represents the mean±s.e. of 10 experiments.
pA2 values for antagonists in the thoracic aortas of WT mice
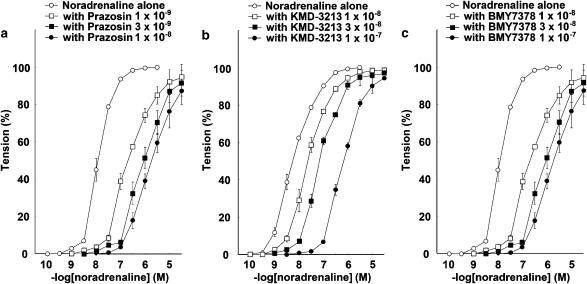
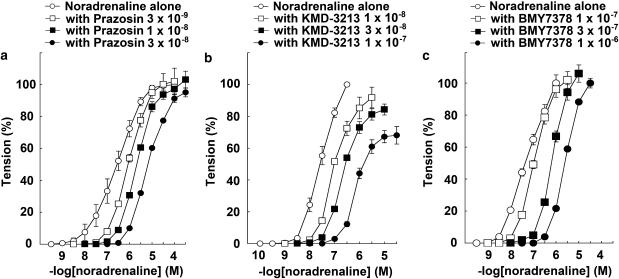
The response to NAd in the thoracic aortas of WT mice was antagonized in the presence of either prazosin, KMD-3213, or BMY7378 in a concentration-dependent manner. The CRCs for NAd were shifted right by prazosin, KMD-3213, or BMY7378. The pA2 values of prazosin, KMD-3213, and BMY7378 were 9.99±0.15 (n=10), 8.25±0.04 (n=7), and 8.46±0.22 (n=5), respectively (Figures 2a–c and Table 1). The slopes of the Schild regression line were not significantly different from unity (Table 1).
Figure 2.
Effects of prazosin, KMD-3213, and BMY7378 on NAd-induced contraction in thoracic aortas from WT mice. Ordinate: contraction, expressed as percentages of the maximum response. Abscissa: negative log concentration (M) of NAd. Each value represents the mean±s.e. of 5–10 experiments. Aortic segments were exposed to vehicle (control) or different concentrations of (a) prazosin, (b) KMD-3213, or (c) BMY7378 before the addition of cumulative concentrations of NAd.
Table 1.
pA2 values for antagonists against NAd in the thoracic aorta of WT and α1B-KO mice
| WT mice | α1B-KO mice | |||
|---|---|---|---|---|
| Antagonist | pA2 value | Slope | pA2 value | Slope |
| Prazosin | 9.99±0.15 | 1.18±0.08 | 10.41±0.04 | 0.92±0.05 |
| KMD-3213 | 8.25±0.04 | 1.06±0.04 | 8.49±0.11 | 1.09±0.06 |
| BMY7378 | 8.46±0.22 | 0.99±0.01 | 9.13±0.10 | 1.06±0.05 |
Each value represents the mean±s.e. of 5–10 experiments.
pA2 values for antagonists in the thoracic aortas of α1B-KO mice
The response to NAd in the thoracic aortas of α1B-KO mice was antagonized in the presence of prazosin, KMD-3213, or BMY7378 in a concentration-dependent manner. The CRCs for NAd were shifted rightwards by prazosin, KMD-3213, or BMY7378. The pA2 values of prazosin, KMD-3213, and BMY7378 were 10.41±0.04 (n=10), 8.49±0.11 (n=9), and 9.13±0.10 (n=7), respectively (Figures 3a–c and Table 1). The slopes of the Schild regression line were not significantly different from unity (Table 1). The mean pA2 value for BMY7378 against NAd-induced contraction in the thoracic aorta of α1B-KO mice tended to be higher than in that of WT mice (P=0.062).
Figure 3.
Effects of prazosin, KMD-3213, and BMY7378 on NAd-induced contraction in thoracic aortas from α1B-KO mice. Ordinate: contraction, expressed as percentages of the maximum response. Abscissa: negative log concentration (M) of NAd. Each value represents the mean±s.e. of 7–10 experiments. Aortic segments were exposed to vehicle (control) or different concentrations of (a) prazosin, (b) KMD-3213, or (c) BMY7378 before the addition of cumulative concentrations of NAd.
pA2 values for antagonists in the thoracic aortas of α1D-KO mice and α1BD-KO mice
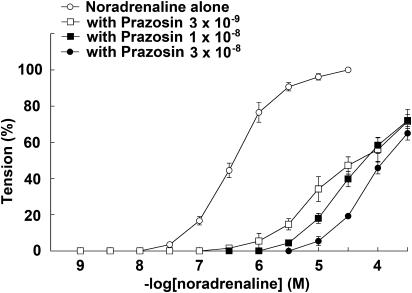
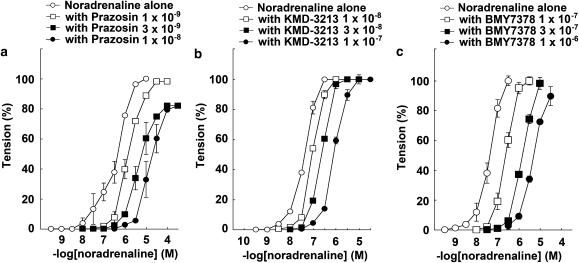
The response to NAd in the thoracic aortas of α1D-KO mice was antagonized in the presence of prazosin in a concentration-dependent manner. The CRCs for NAd were shifted right by prazosin (Figure 4) and the pA2 value was 9.30±0.11 (n=10). However, prazosin produced shallow Schild slopes, which were significantly different from unity (data not shown). There was almost complete abolition of the CRCs for NAd in the thoracic aortas of α1BD-KO mice; therefore, the effects of α1-selective antagonists on the thoracic aortas of α1BD-KO mice could not be examined.
Figure 4.
Effects of prazosin on NAd-induced contraction in thoracic aortas from α1D-KO mice. Ordinate: contraction, expressed as percentages of the maximum response. Abscissa: negative log concentration (M) of NAd. Each value represents the mean±s.e. of 10 experiments. Aortic segments were exposed to vehicle (control) or to different concentrations of prazosin before the addition of cumulative concentrations of NAd.
NAd-induced contraction of the mesenteric artery
In the mesenteric arteries of these groups of mice, NAd evoked contraction in a concentration-dependent manner (Figure 1b). The CRCs for NAd in the mesenteric artery were not significantly different between WT and α1B-KO mice (Figure 1b). Also, CRCs for NAd in the mesenteric artery did not differ significantly between WT and α1B-KO in either the maximum response (173±19 mg in WT, n=10, and 171±28 mg in α1B-KO, n=10) or in sensitivity to NAd (pD2 value, 6.52±0.22 in WT, n=10, and 7.12±0.14 in α1B-KO, n=10) (Figure 1b). Similarly, there was no significant difference between CRCs for NAd in the mesenteric arteries of α1D-KO mice and α1BD-KO mice (Figure 1b). Furthermore, CRCs for NAd in the mesenteric artery did not differ significantly between α1D-KO and α1BD-KO mice in either maximum response (35±8 mg in α1D-KO, n=10, and 48±11 mg in α1BD-KO, n=10) or sensitivity (pD2 value, 6.19±0.07 in α1D-KO, n=10, and 6.29±0.06 in α1BD-KO, n=10) (Figure 1b). The maximum responses of the mesenteric arteries from both α1D-KO and α1BD-KO mice were significantly lower (approximately 70% reduction, P<0.05) than those from either WT or α1B-KO mice (Figure 1b). There was a significant difference in sensitivity between the mesenteric arteries and thoracic aortas of WT mice (P<0.05), but no significant difference in maximum response. Furthermore, NAd evoked contraction in the mesenteric arteries of α1D-KO and α1BD-KO mice with a significantly higher pD2 value (P<0.05) and maximum response than in the thoracic aorta of α1BD-KO mice (Figure 1a and b).
pA2 values for antagonists in the mesenteric arteries of WT mice
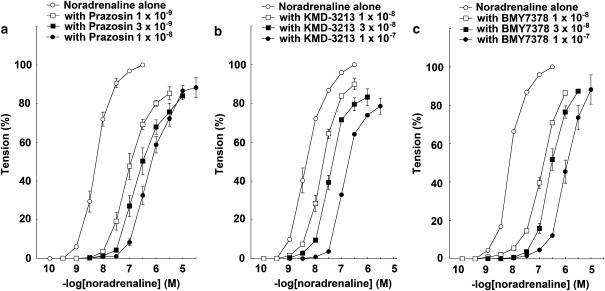
The response to NAd in the mesenteric arteries of WT mice was antagonized by the presence of prazosin, KMD-3213, or BMY7378 in a concentration-dependent manner. CRCs for NAd were shifted rightwards by prazosin, KMD-3213, or BMY7378. The pA2 values of prazosin, KMD-3213, and BMY7378 were 9.92±0.11 (n=10), 8.34±0.40 (n=8), and 7.47±0.18 (n=7), respectively (Figures 5a–c and Table 2). The slopes of the Schild regression lines were not significantly different from unity (Table 1).
Figure 5.
Effects of prazosin, KMD-3213, and BMY7378 on NAd-induced contraction in mesenteric arteries from WT mice. Ordinate: contraction, expressed as percentages of the maximum response. Abscissa: negative log concentration (M) of NAd. Each value represents the mean±s.e. of 7–10 experiments. Mesenteric arterial segments were exposed to vehicle (control) or to different concentrations of (a) prazosin, (b) KMD-3213, or (c) BMY7378 before the addition of cumulative concentrations of NAd.
Table 2.
pA2 values for antagonists against NAd in the mesenteric artery of WT and α1B-KO mice
| WT mice | α1B-KO mice | |||
|---|---|---|---|---|
| Antagonist | pA2 value | Slope | pA2 value | Slope |
| Prazosin | 9.92±0.11 | 0.96±0.07 | 9.83±0.06 | 1.24±0.14 |
| KMD-3213 | 8.34±0.40 | 1.36±0.19 | 8.11±0.05 | 1.06±0.06 |
| BMY7378 | 7.47±0.18 | 1.08±0.12 | 7.82±0.10 | 1.02±0.11 |
Each value represents the mean±s.e. of 6–10 experiments.
pA2 values for antagonists in the mesenteric arteries of α1B-KO mice
The response to NAd in the mesenteric arteries of WT mice was antagonized by the presence of prazosin, KMD-3213, or BMY7378 in a concentration-dependent manner. Prazosin, KMD-3213, or BMY7378 all shifted the CRCs for NAd to the right. The pA2 values of prazosin, KMD-3213, and BMY7378 were 9.83±0.06 (n=10), 8.11±0.05 (n=7), and 7.82±0.10 (n=6), respectively (Figures 6a–c and Table 2). The slopes of the Schild regression lines were not significantly different from unity (Table 1).
Figure 6.
Effect of prazosin, KMD-3213, and BMY7378 on NAd-induced contraction in mesenteric arteries from α1B-KO mice. Ordinate: contraction, expressed as percentages of the maximum response. Abscissa: negative log concentration (M) of NAd. Each value represents the mean±s.e. of 6–10 experiments. Mesenteric arterial segments were exposed to vehicle (control) or to different concentrations of (a) prazosin, (b) KMD-3213, or (c) BMY7378 before the addition of cumulative concentrations of NAd.
pA2 values for antagonists in the mesenteric arteries of α1D-KO mice and α1BD-KO mice
The responses to NAd in the mesenteric arteries of α1D-KO and α1BD-KO mice were antagonized by the presence of prazosin in a concentration-dependent manner. Prazosin shifted the CRCs for NAd rightwards. The pA2 value for prazosin in the mesenteric arteries of α1D-KO mice was 9.30±0.26 (n=4) and in α1BD-KO mice it was 9.43±0.31 (n=5), and these were not significantly different from each other. However, prazosin produced shallow Schild slopes, which were significantly different from unity (data not shown).
Expression of each α1-AR subtype in the vessels of WT mice
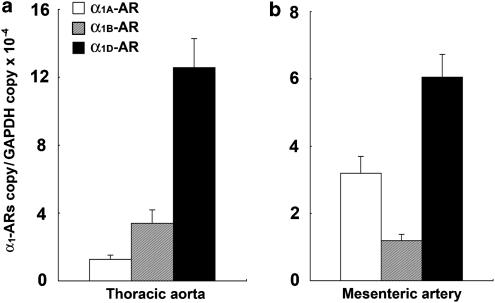
Real-time PCR analysis revealed that the mRNA expression level of α1D-AR was the highest of the α1-AR subtypes in both the thoracic aorta and mesenteric artery (n=5, each group) (Figure 7a and b). The expression level of α1D-AR mRNA in the mesenteric artery was approximately half that in the thoracic aorta. In the thoracic aorta, the level of expression of α1B-AR mRNA was approximately one-quarter that of α1D-AR, and the expression level of α1A-AR was approximately one-tenth that of α1D-AR (Figure 7a). In the mesenteric artery, the level of expression of α1A-AR mRNA was approximately half that of α1D-AR, and expression of α1B-AR was approximately one-sixth that of α1D-AR (Figure 7b). The expression of the mRNA of each α1-AR subtype in order of rank was α1D-AR>α1B-AR>α1A-AR in the mouse thoracic aorta and α1D-AR>α1A-AR>α1B-AR in the mouse mesenteric artery. In addition, the total level of expression of the mRNAs of all the α1-AR subtypes was significantly higher in the thoracic aorta than in the mesenteric artery (P<0.05).
Figure 7.
α1-AR subtype mRNA expression in the thoracic aortas (a) and mesenteric arteries (b) of WT mice. Ordinate: relative level of expression of mRNA of each α1-AR subtype, standardized against the GAPDH level. Abscissa: vessels from which the total RNA was isolated. Values represent means±s.e. of five independent experiments.
Expression of each α1-AR subtype in the thoracic aortas of knockout mice
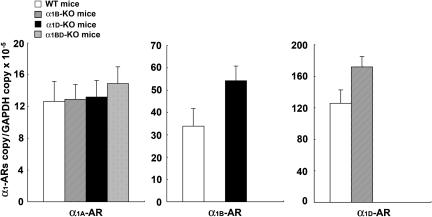
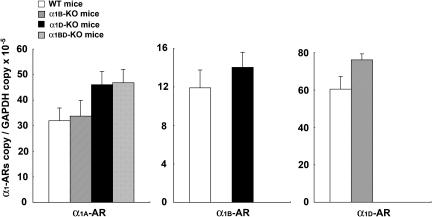
Real-time PCR analysis showed that the level of expression of α1D-AR mRNA in the thoracic aortas of α1B-KO mice tended to be higher (P=0.066) than in WT mice (n=5, each) (Figure 8). However, no other trends in the levels of expression of mRNA of α1-AR subtypes in the thoracic aorta were observed (n=5, each) (Figure 8).
Figure 8.
α1-AR subtype mRNA expression in thoracic aortas of each group of mice. Ordinate: relative level of expression of mRNA of each α1-AR subtype, standardized against the GAPDH level. Abscissa: each α1-AR subtype, as expressed in the thoracic aortas of each group of mice. Values represent means±s.e. of five independent experiments.
Expression of each α1-AR subtype in the mesenteric arteries of knockout mice
Real-time PCR analysis revealed that the expression level of α1A-AR mRNA in the mesenteric arteries of both α1D-KO and α1BD-KO mice tended to be higher than in those of WT mice (P=0.087 in α1D-KO vs WT and P=0.074 in α1BD-KO vs WT, n=5 each) (Figure 9). No higher tendency in the levels of expression of mRNA of different α1-AR subtypes was observed in the mesenteric artery (n=5, each) (Figure 9).
Figure 9.
α1-AR subtype mRNA expression in mesenteric arteries of each group of mice. Ordinate: relative level of expression of mRNA of each α1-AR subtype, standardized against the GAPDH level. Abscissa: each α1-AR subtype, as expressed in the mesenteric arteries of each group of mice. Values represent means±s.e. of five independent experiments.
Discussion
We examined the pharmacological characteristics of the thoracic aorta and mesenteric artery in WT and mutant mice with the same genetic background. The CRC for NAd in the thoracic aorta of α1BD-KO mice was almost completely abolished (Figure 1a), revealing that α1A-AR plays almost no role in α1-AR-mediated contraction of the mouse thoracic aorta. α1D-AR was effectively the only α1-AR expressed in the thoracic aortas of α1B-KO mice and, similarly, α1B-AR was in effect the only α1-AR expressed in the thoracic aortas of α1D-KO mice. Taking into account the pD2 values of the thoracic aortas of α1B-KO and α1D-KO mice (7.77 and 6.44, respectively), contraction mediated through α1D-AR appeared to be approximately 20 times greater than that evoked by α1B-AR. Therefore, the vasoconstrictor role of each α1-AR subtype in the mouse thoracic aorta in rank order of predominance was α1D-AR>α1B-AR≫α1A-AR. In the mouse mesenteric artery, α1B-AR appears to be of little importance in α1-AR-mediated contraction, since no statistically significant difference was observed between CRCs for NAd between WT and α1B-KO mice or between α1D- and α1BD-KO mice. In addition, the CRC for NAd in the mesenteric artery showed that the maximum response in α1D-KO mice was remarkably reduced compared to that in WT mice (173±19 mg in WT and 35±8 mg in α1D-KO). These results indicate that the majority of α1-ARs were absent from the mesenteric arteries of α1D-KO mice, suggesting that α1D-AR could play a major vasoconstrictor role and that the vasoconstrictor activity of the other α1-ARs (α1A-AR and α1B-AR) in the mouse mesenteric artery might be minor. Furthermore, CRCs for NAd in the mesenteric arteries of α1BD-KO mice, previously considered to be mediated through only α1A-AR, were not abolished as they were in the thoracic arteries, even though there was lower sensitivity to NAd and a lower maximum response. These findings suggest that α1A-AR might play a minor vasoconstrictor role in the mouse mesenteric artery. Therefore, the rank order of predominance of the vasoconstrictor activity of each α1-AR subtype in the mesenteric artery was α1D-AR>α1A-AR≫α1B-AR. Sensitivity to NAd in α1-AR-mediated contraction is much greater in the thoracic aorta than in the mesenteric artery (approximately 1/50th sensitivity of that in the thoracic aorta) in mice (Figure 1a and b).
We then examined the pharmacological characteristics of these vessels using the nonselective α1-antagonist prazosin. Prazosin effectively antagonized NAd-induced contraction of thoracic aortas from WT, α1B- and α1D-KO mice, and of mesenteric arteries from WT, α1B-, α1D-, and α1BD-KO mice, which showed that the response to NAd was mediated through α1-ARs (Tables 1 and 2, Figures 2, 3, 4, 5 and 6). The pA2 values for prazosin against NAd from the Schild plot were all very similar to each other (9.99 in WT thoracic aorta, 10.41 in α1B-KO thoracic aorta, 9.30 in α1D-KO thoracic aorta, 9.92 in WT mesenteric artery, 9.83 in α1B-KO mesenteric artery, 9.30 in α1D-KO mesenteric artery, and 9.43 in α1BD-KO mesenteric artery), and were in good agreement with those of mice in our previous study (pA2 value for prazosin was 9.71 in mouse thoracic aorta and 9.93 in mouse mesenteric artery; Yamamoto & Koike, 2001a, 2001b). The slopes of the Schild regression lines for the thoracic aorta and mesenteric artery in WT and α1B-KO mice indicated the competitive nature of the antagonism. On the other hand, prazosin produced shallow Schild slopes, which were significantly different from unity, in the thoracic aortas of α1D-KO mice and the mesenteric arteries of α1D- and α1BD-KO mice. These phenomena originally suggested a change in the nature of prazosin from a competitive to a noncompetitive-like antagonist, according to the Schild and Gaddum equations (Arunlakshana & Schild, 1959; Tallarida et al., 1979). However, we have already confirmed that prazosin acts as a competitive antagonist of NAd in the thoracic aorta and mesenteric artery in the mouse (Yamamoto & Koike, 2001a, 2001b) Also, no structural change in the residual α1-ARs in α1-AR subtype knockout mice has yet been reported (Cavalli et al., 1997; Rokosh & Simpson, 2001; Daly et al., 2002; Tanoue et al., 2002). In addition, these phenomena were observed only in the vessels of mice lacking the α1D-AR gene, a major vasoconstrictor of these vessels. These conflicting phenomena suggest a remarkable reduction in the quantity of α1-ARs in these vessels in mice lacking α1D-AR gene compared with WT mice, rather than a change in the nature of prazosin from that of a competitive to a noncompetitive-like antagonist. However, a reduction in receptor number does not, theoretically, change the nature of the antagonism. It is not clear why the reduction in α1D-ARs leads to apparent noncompetitive antagonism, because no similar study using α1D-KO mice has yet been reported.
The pharmacological characterization of these vessels using the α1A-selective antagonist KMD-3213 found that the mean pA2 values of KMD-3213 against NAd-induced contraction in the thoracic aorta (pA2 value for WT mice was 8.25 and for α1B-KO mice was 8.49, Table 1) and in the mesenteric artery (pA2 value for WT mice was 8.34 and for α1B-KO mice was 8.11, Table 2) were in good agreement with the negative logarithm of dissociation constant (pKi value) of human cloned α1d-AR (pKi values were 10.5, 7.5, and 8.5 for human α1a-, α1b-, and α1d-AR, respectively; Shibata et al., 1995) and with the pA2 value of functional α1D-AR (α1A-AR, 10.0 in rat caudal artery; α1B-AR, 7.7 in dog carotid artery; α1D-AR, 8.3 and 8.13 in rat thoracic aorta) (Ford et al., 1996; Yamagishi et al., 1996; Murata et al., 1999, respectively). The slopes of the Schild regression lines in these vessels indicated that the antagonism is competitive. These previous findings strongly show that contraction of these vessels in WT and α1B-KO mice was mediated mainly through α1D-AR.
The mean pA2 value of the α1D-selective antagonist BMY7378 (8.46, Table 1) against NAd-induced contraction in the thoracic aortas of WT mice (Goetz et al., 1995; Saussy et al., 1996) was similar to that reported in our previous study (pA2 value 8.43; Yamamoto & Koike, 2001b), and was in good agreement with the generally accepted value for the rat thoracic aorta (pA2 value, 8.5; Ford et al., 1996). In addition, the mean pA2 value (9.13, Table 1) for BMY7378 against NAd-induced contraction in the thoracic aortas of α1B-KO mice tended to be higher (P=0.062) than that of WT mice and was similar to that reported previously (9.3) in the thoracic aortas of these mice (Daly et al., 2002). The slopes of the Schild regression lines for the thoracic aortas of WT and α1B-KO mice indicated that the antagonism is competitive. On the other hand, in the mouse mesenteric arteries, the mean pA2 values of BMY7378 against NAd-induced contraction in WT (7.47) and α1B-KO (7.82) mice indicated low affinity for BMY7378 (Table 2). The slopes of the Schild regression lines again indicate a competitive antagonism. The effects of BMY7378 on NAd-induced contraction in the mesenteric arteries of WT and α1B-KO mice were in good agreement with those in our previous report (pA2 value for mice mesenteric artery, 7.69; Yamamoto & Koike, 2001a).
We derived pharmacological characterizations for these vessels using these antagonists and found that contraction of the vessels in WT and α1B-KO mice might be mediated primarily through α1D-AR. An exception is in the case of the low affinity for BMY7378 in the mesenteric arteries of WT and α1B-KO mice. Although the pKi value for BMY7378 against α1d-AR of mice was not previously known, other studies have proved that pKi values for BMY7378 against α1d-AR vary from 8.2 (rats) to 9.4 (humans) (Goetz et al., 1995). Moreover, the mouse mesenteric artery is less sensitive to NAd than the thoracic aorta, suggesting that it has lower concentrations of α1-AR subtypes (especially the major vasoconstrictor) and that α1A-AR is a more important vasoconstrictor in the mesenteric artery than in the thoracic aorta. These findings suggest that there is a lower concentration of α1D-AR, and a higher concentration of α1A-AR, in the mesenteric artery than in the thoracic aorta of the mouse. This may cause a lowering of the affinity for BMY7378 in the mouse mesenteric artery. Although the affinity for the α1D-selective antagonist BMY7378 tended to be higher in the thoracic aortas of α1B-KO mice, the patterns of contraction in the thoracic aortas and mesenteric arteries of α1B-KO mice were similar to those in WT mice. This similarity suggests that α1B-AR may play only a minor vasoconstrictor role, or no vasoconstrictor role at all, in contraction of the mouse thoracic aorta and mesenteric artery. The results of our pharmacological analysis using α1-AR antagonists were therefore similar to those found in our comparison of the CRCs for NAd in these vessels.
We investigated the correlation between the vasoconstrictor role of each α1-AR subtype and its mRNA expression in these vessels. There was reasonably close correlation in the rank order of predominance of each α1-AR subtype in terms of vasoconstrictor role and mRNA expression level, as follows: α1D-AR>α1B-AR>α1A-AR in the mouse thoracic aorta and α1D-AR>α1A-AR>α1B-AR in the mouse mesenteric artery (Figure 7). Furthermore, the mouse thoracic aorta scored more highly than the mesenteric artery in terms of both sensitivity to NAd and total copy number of α1-AR mRNAs.
Recent studies in animal vessels have investigated the correlation between mRNA or protein expression levels of α1-AR subtypes and the functional roles of these receptors in vasoconstriction (Guarino et al., 1996; Miller et al., 1996). In most cases, although all α1-AR subtypes are expressed at the protein level (Hrometz et al., 1999) or mRNA level (Xu et al., 1997), the correlation between protein or mRNA expression of one α1-AR subtype and the functional roles of these receptors in vasoconstriction has been elusive (Piascik et al., 1997; Hussain & Marshall, 2000). Use of the quantitative technique of real-time PCR enabled us to quantify the mRNA expression of each α1-AR subtype. Although the mRNAs of all three α1-AR subtypes were expressed in the mouse thoracic aorta and mesenteric artery, it appears that not all α1-AR subtypes mediate NAd-induced contraction in these vessels. In terms of compensatory expression of mRNA of α1-AR subtypes, the level of expression of α1D-AR mRNA in the thoracic aortas of α1B-KO mice tended to be higher than that in WT mice (P=0.066) (Figure 8). This tendency was in good agreement with the tendency toward a higher pA2 value for BMY7378 in the thoracic aortas of α1B-KO mice (P=0.062) than those of WT mice (Table 1). However, in each mutant strain, no dramatic compensatory change in expression of the other α1-AR subtypes was observed. This result was in good agreement with that of our previous report (Tanoue et al., 2002) and suggests that the difference in sensitivity to NAd in these vessels reflects the deletion of α1B- and/or α1D-AR.
In conclusion, we were able to evaluate the rank orders of the vasoconstrictor role and mRNA expression level of each α1-AR subtype in these mouse vessels. Our resulting pharmacological analysis and mRNA expression profile correlated well and demonstrated that α1D-AR is primarily predominant and that other α1-AR subtypes are secondary and differ between vessels in terms of both NAd-stimulated vasoconstriction and mRNA expression level.
Acknowledgments
We are grateful to M. Narutomi for expert technical assistance.
Abbreviations
- α1-AR
α1-adrenoceptor
- BMY7378
8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]-ethyl]-8-azaspiro[4,5]decane-7,9-dione dihydrochloride
- CRCs
concentration–response curves
- EC50
50% effective concentration
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- KMD-3213
(−)-1-(3-hydroxypropyl)-5-((2R)-2-{[2-({2-[(2,2,2-trifluoroethyl) propyl)-2,3-dihydro-1H-indole-7-carboxamide
- NAd
noradrenaline
- pA2 value
negative logarithm of dissociation constant, obtained from mechanical response
- pKB
negative logarithm of dissociation constant, obtained from Scatchard plot
- pKi value
negative logarithm of dissociation constant, obtained from Hill plot of displacement curve
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonist. Br. J. Chemother. 1959;14:48–52. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLI A., LATTION A.-L., HUMMLER E., NENNIGER M., PEDRAZZINI T., AUBERT J.-F., MICHEL M.C., YANG M., LEMBO G., VECCHIONE C., MOSTARDINI M., SCHMIDT A., BEERMANN F., COTECCHIA S. Decreased blood pressure response in mice deficient of the α1b-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTECCHIA S., SCHWINN D.A., RANDALL R.R., LEFKOWITZ R.J., CARON M.G., KOBILKA B.K. Molecular cloning and expression of the cDNA for hamster α1-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALY C.J., DEIGHAN C., McGEE A., MENNIE D., ALI Z., McBRIDE M., McGRATH J.C. A knockout approach indicates a minor vasoconstrictor role for vascular α1B-adrenoceptors in mouse. Physiol. Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- ESBENSHADE T.A., HIRASAWA A., TSUJIMOTO G., YANO J., MINNEMAN K.P., MURPHY T.J. Cloning of the human alpha 1D-adrenergic receptor and inducible expression of three human subtypes in SK-N-MC cells. Mol. Pharmacol. 1995;47:977–985. [PubMed] [Google Scholar]
- FOGLAR R., SHIBATA K., HORIE K., HIRASAWA A., TSUJIMOTO G. Use of recombinant α1-adrenoceptors to characterize subtype selectivity of drugs for the treatment of prostatic hypertrophy. Eur. J. Pharmacol. 1995;288:201–207. doi: 10.1016/0922-4106(95)90195-7. [DOI] [PubMed] [Google Scholar]
- FORD A.P.D., ARREDONDO N.F., BLUE D.R., BONHAUS D.W., JR, JASPER J., KAVA M.S., LESNICK T.J., PFISTER J.R., SHIEF I.A., VIMONT R.L., WILLIAMS T.J., MCNEAL J.E., STAMY T.A., CLARKE D.E. RE17053 (N-[2-(2-cyclopropylmethoxyphenoxy) ethyl ]-5-chloro-α, α-dimethyl-1H-indole-3- ethanamine hydrochloride), a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1-adrenoceptors in human prostate: implication for adrenoceptor classification. Mol. Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D., TRUE T., RIMELE T.J., SAUSSY D.L., JR BMY 7378 is a selective antagonist of the D type of α1-adrenoceptors. Eur. J. Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- GUARINO R.D., PEREZ D.M., PIASCIK M.T. Recent advances in the molecular pharmacology of the α1-adrenergic receptors. Cell Signal. 1996;8:323–333. doi: 10.1016/0898-6568(96)00066-6. [DOI] [PubMed] [Google Scholar]
- HAN C., ABEL P.W., MINNEMAN K.P. α1-Adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature. 1987;329:333–335. doi: 10.1038/329333a0. [DOI] [PubMed] [Google Scholar]
- HAN C., LI J., MINNEMAN K.P. Subtypes of α1-adrenoceptors in rat blood vessels. Eur. J. Pharmacol. 1990;190:97–104. doi: 10.1016/0014-2999(90)94116-f. [DOI] [PubMed] [Google Scholar]
- HARRISON D.C., MEDHURST A.D., BOND B.C., CAMPBELL R.P., DAVIS K.L., PHILPOTT K.L. The use of quantitative RT-PCR to measure mRNA expression in a rat model of focal ischemia–caspase-3 as a case study. Mol. Brain Res. 2000;75:143–149. doi: 10.1016/s0169-328x(99)00305-8. [DOI] [PubMed] [Google Scholar]
- HEID C.A., STEVENS J., LIVAK K.J., WILLIAMS P.M. Real-time quantitative PCR. Genome Methods. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., BONDINELL W.E., RUFFOLO R.R., JR α- and β-adrenoceptors: from gene to the clinic: 1. Molecular biopsy and adrenoceptor subclassification. J. Med. Chem. 1995a;38:3415–3444. doi: 10.1021/jm00018a001. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., BYLOUD D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R., JR International Union of Pharmacology. X. Recommendation for nomenclature of alpha1-adrenoceptor: consensus update. Pharmacol. Rev. 1995b;47:267–270. [PubMed] [Google Scholar]
- HIRASAWA A., HORIE K., TANAKA T., TAKAGI K., MURAI M., YANO J., TSUJIMOTO G. Cloning, functional expression and tissue distribution of human cDNA for the alpha 1C-adrenergic receptor. Biochem. Biophys. Res. Commun. 1993;195:902–909. doi: 10.1006/bbrc.1993.2130. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B.B.Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists Goodman & Gilman's The Pharmacological Basis of Therapeutics 2001New York: McGraw-Hill; 215–268.ed. Hardman, J.G. & Limbird, L.E. pp [Google Scholar]
- HROMETZ S.L., EDELMANN S.E., MCCUNE D.F., OLGES J.R., HADLEY R.W., PEREZ D.M., PIASCIK M.T. Expression of multiple alpha1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J. Pharmacol. Exp. Ther. 1999;290:452–463. [PubMed] [Google Scholar]
- HUSSAIN M.B., MARSHALL I. Alpha(1)-adrenoceptor subtypes mediating contractions of the rat mesenteric artery. Eur. J. Pharmacol. 2000;395:69–76. doi: 10.1016/s0014-2999(00)00220-x. [DOI] [PubMed] [Google Scholar]
- KENNY B.A., CHALMERS D.H., PHILPOTT P.C., NAILOR A.M. Characterization of an α1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br. J. Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGRATH J.C. Evidence for more than one type of post-junctional α1-adrenoceptor. Biochem. Pharmacol. 1982;31:467–484. doi: 10.1016/0006-2952(82)90147-2. [DOI] [PubMed] [Google Scholar]
- MEDHURST A.D., HARRISON D.C., READ S.J., CAMPBELL C.A., ROBBINS M.J., PANGALOS M.N. The use of real-time PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J. Neurosci. Methods. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]
- MILLER J.W., HU Z.W., OKAZAKI M., FUJINAGA M., HOFFMAN B.B. Expression of alpha 1 adrenergic receptor subtype mRNAs in the rat cardiovascular system with aging. Mech. Ageing Dev. 1996;87:75–89. doi: 10.1016/0047-6374(96)01697-1. [DOI] [PubMed] [Google Scholar]
- MURATA S., TANIGUCHI T., MURAMATSU I. Pharmacological analysis of the novel, selective alpha1-adrenoceptor antagonist, KMD-3213, and its suitability as a tritiated radioligand. Br. J. Pharmacol. 1999;127:19–26. doi: 10.1038/sj.bjp.0702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMIYA M., YAMAGUCHI O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J. Urol. 2003;170:649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- PEREZ D.M., PIASCIK M.T., GRAHAM R.M. Solution-phase library screening for the identification of rare clones: isolation of an alpha 1D-adrenergic receptor cDNA. Mol. Pharmacol. 1991;40:876–883. [PubMed] [Google Scholar]
- PIASCIK M.T., HROMETZ S.L., EDELMANN S.E., GUARINO R.D., HADLEY R.W., BROWN R.D. Immunocytochemical localization of the alpha-1B adrenergic receptor and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J. Pharmacol. Exp. Ther. 1997;283:854–868. [PubMed] [Google Scholar]
- ROKOSH D.G., SIMPSON P.C. Knockout of the α1A/C adrenergic receptor subtype: the a1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc. Natl. Acad. Sci. U.S.A. 2001;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATH D.E., BROOME H.E., PRYSTOWSKY M.B. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- SAUSSY D.L., JR, GOETZ A.S., QUEEN K.L., KING H.K., LUTZ M.W., RIMELE T.J. Structure activity relationships of a series of buspirone analogs at alpha-1 adrenoceptors: further evidence that rat aorta alpha-1 adrenoceptors are of the alpha-1D-subtype. J. Pharmacol. Exp. Ther. 1996;278:136–144. [PubMed] [Google Scholar]
- SCHWINN D.A., LOMASNEY J.M., LORENZ W., SZKLUT P.J., FREMEAU RT J.R., YANG-FENG T.L., CARON M.G., LEFKOWITZ R.J., COTECCHIA S. Molecular cloning and expression of the cDNA for a novel alpha 1-adrenergic receptor subtype. J. Biol. Chem. 1990;265:8183–8189. [PubMed] [Google Scholar]
- SHI A.G., KWAN C.Y., DANIEL E.E. Relation between density (maximum binding) and alpha adrenoceptor binding sites and contractile response in four canine vascular tissues. J. Pharmacol. Exp. Ther. 1989;250:1119–1124. [PubMed] [Google Scholar]
- SHIBATA K., FOGLAR R., HORIE K., OBIKA K., SAKAMOTO A., OGAWA S., TSUJIMOTO G. KMD-3213, a novel, potent, alpha 1a-adrenoceptor-selective antagonist: characterization using recombinant human alpha 1-adrenoceptors and native tissues. Mol. Pharmacol. 1995;48:250–258. [PubMed] [Google Scholar]
- TALLARIDA R.J., COWAN A., ADLER M.W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979;25:637–754. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- TANOUE A., NASA Y., KOSHIMIZU T., SHINOURA H., OSHIKAWA S., KAWAI T., SUNADA S., TAKEO S., TSUJIMOTO G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 2002;109:767–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLGIN D.V., MACKIEWICZ M.J, KUBIN L. Alpha(1B) receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. Chem. Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- XU K.M., TANG F., HAN C. Alterations of mRNA levels of alpha 1-adrenoceptor subtypes with maturation and aging in different rat blood vessels. Clin. Exp. Pharmacol. Physiol. 1997;24:415–417. doi: 10.1111/j.1440-1681.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- YAMAGISHI R., AKIYAMA K., NAKAMURA S., HORA M., MASUDA M., KITAZAWA M. Effect of KMD-3213, an alpha 1a-adrenoceptor-selective antagonist, on the contractions of rabbit prostate and rabbit and rat aorta. Eur. J. Pharmacol. 1996;315:73–79. doi: 10.1016/s0014-2999(96)00589-4. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y, KOIKE K. α1-Adrenoceptor subtypes in the mouse mesenteric artery and abdominal aorta. Br. J. Pharmacol. 2001a;134:1045–1054. doi: 10.1038/sj.bjp.0704350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO Y., KOIKE K. Characterization of α1-adrenoceptor-mediated contraction in the mouse thoracic aorta. Eur. J. Pharmacol. 2001b;424:131–140. doi: 10.1016/s0014-2999(01)01134-7. [DOI] [PubMed] [Google Scholar]