Abstract
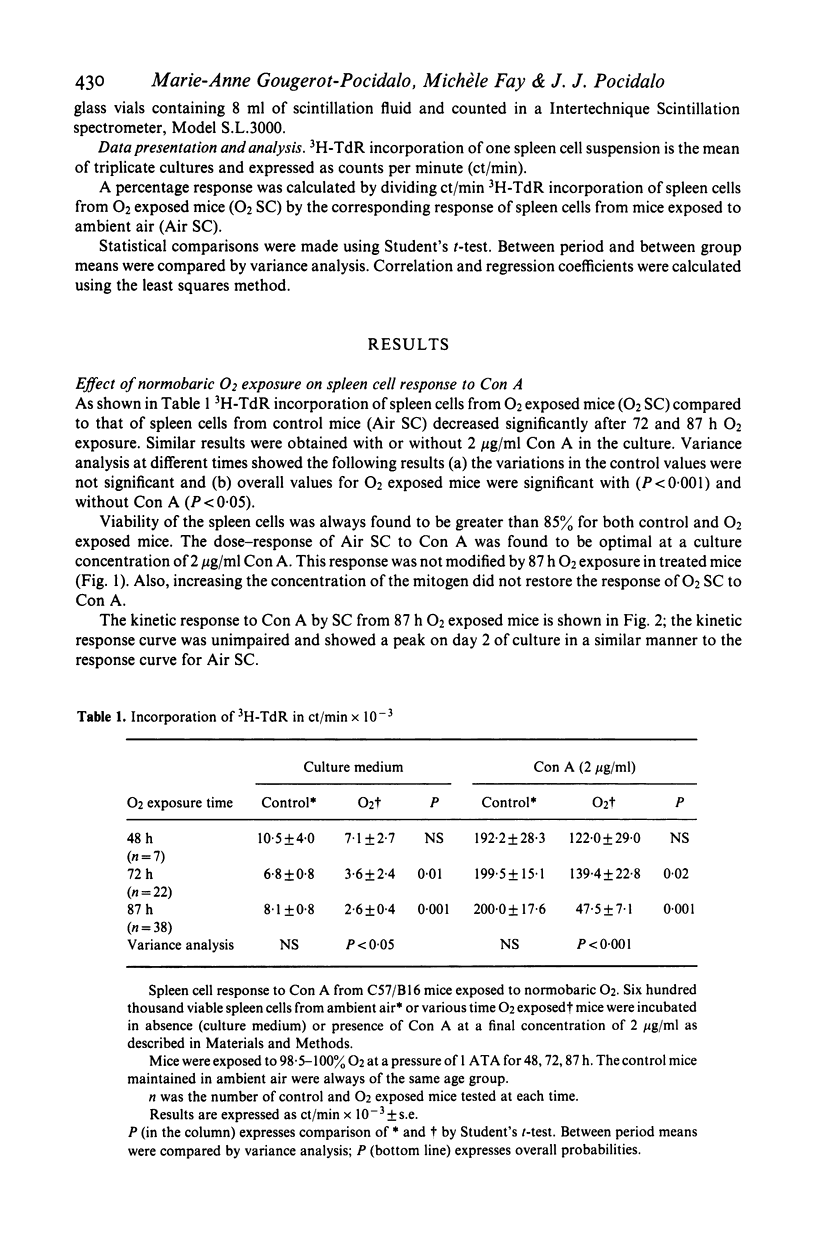
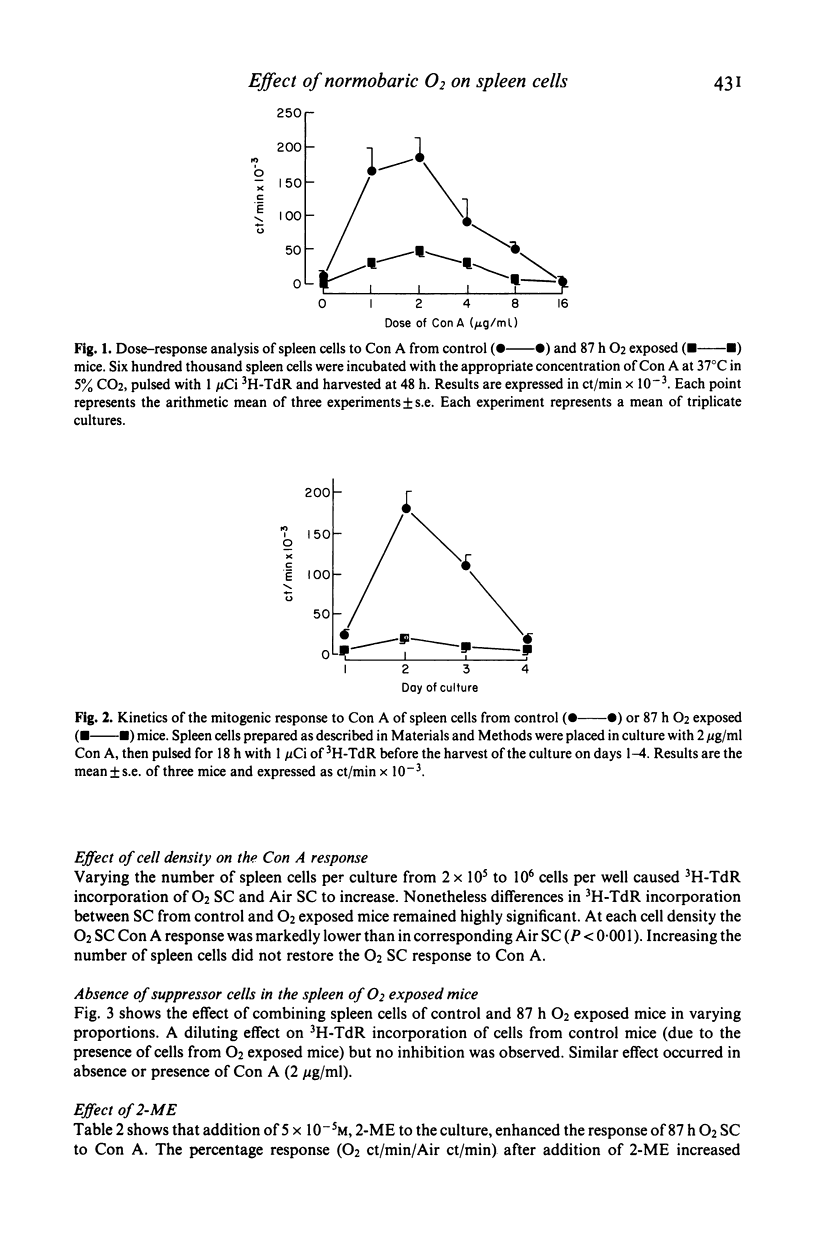
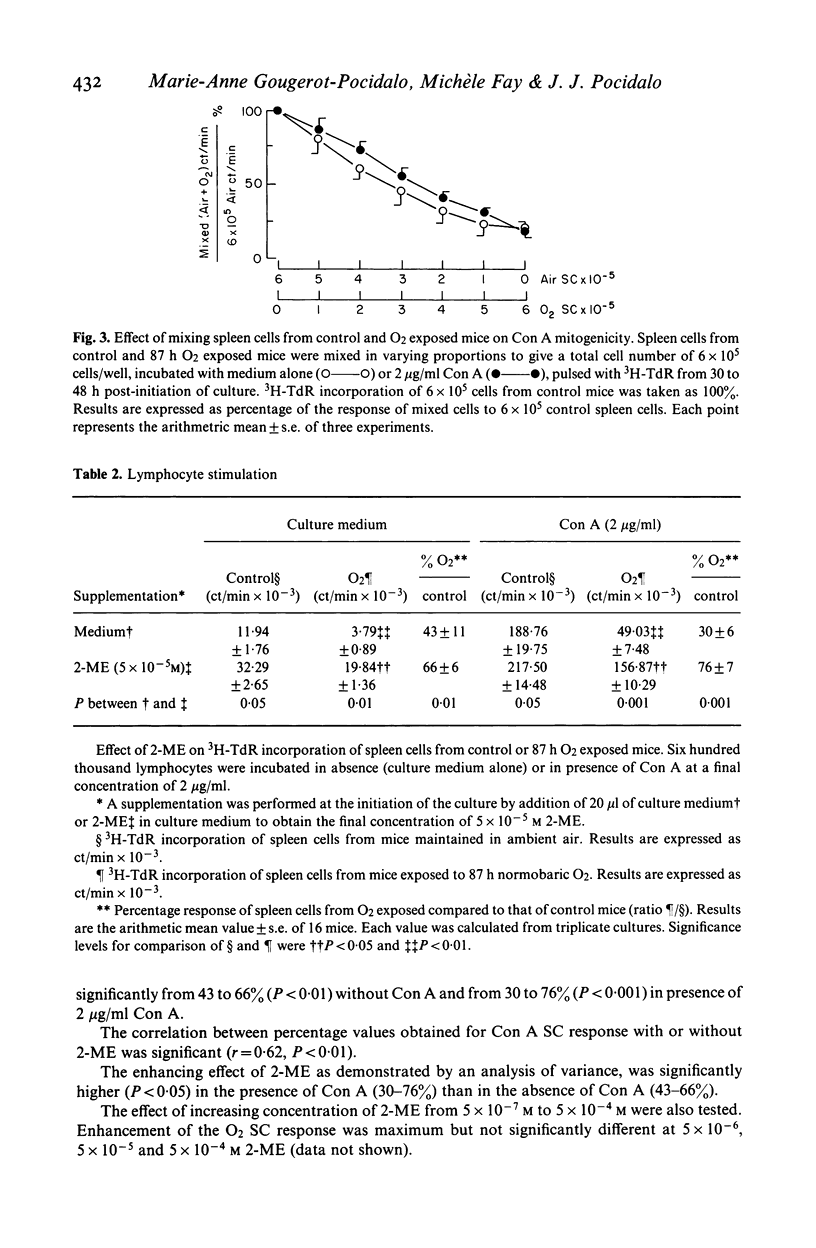
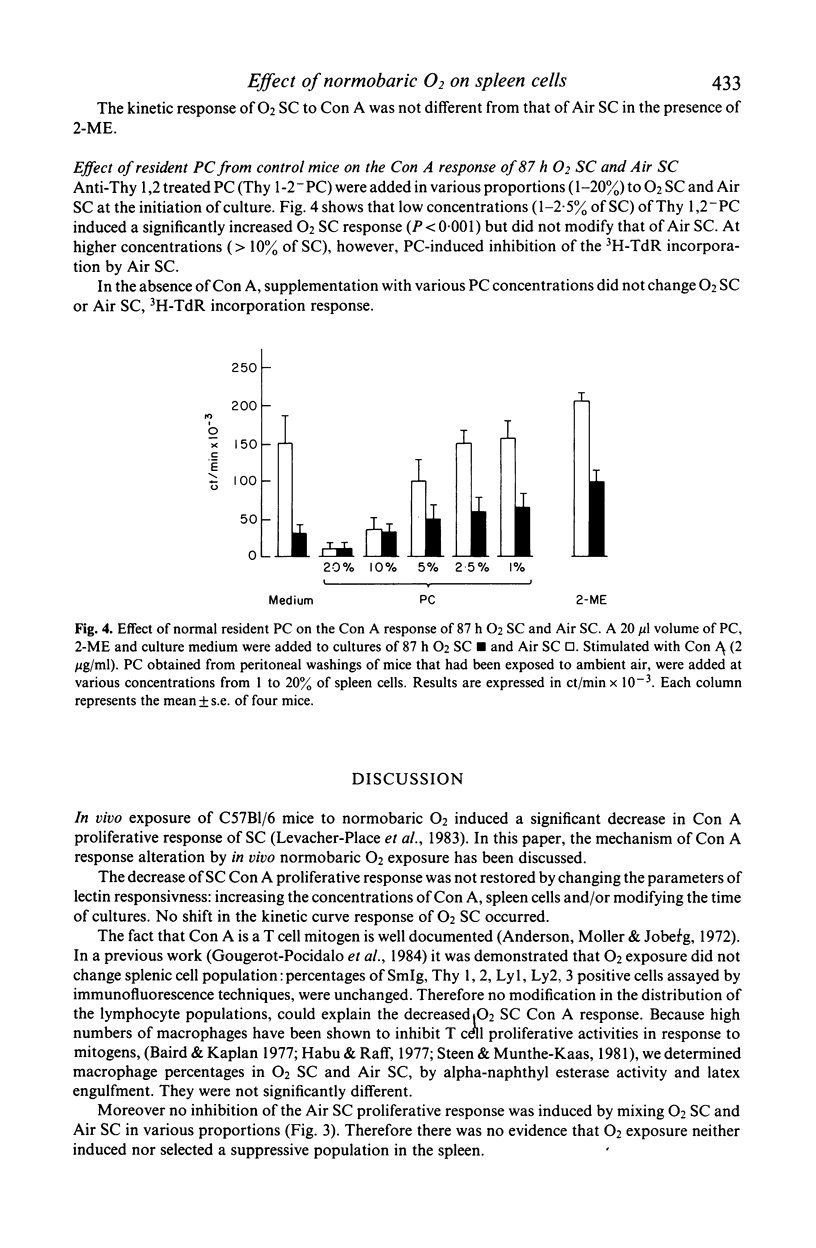
Normobaric O2 exposure decreased spleen cell (SC) response to T cell mitogen Con A. 3H-TdR incorporation of SC from O2 exposed mice (O2SC) compared to those of control mice (Air SC) decreased significantly after 72 and 87 h O2 exposure. The dose response kinetics to Con A were identical in O2SC or Air SC. Increasing SC number did not restore the response to Con A and the depressed hyperoxic effect was not related to suppressor cells in the spleen of O2 exposed mice. Response of O2SC to Con A was restored by the thiol compound 2-mercaptoethanol (2-ME), and the degree of restoration by 2-ME, was inversely proportional to the depressed response. Addition of intact peritoneal cells (PC) induced restoration within the same range as 2-ME. Restoration of the mitogenic response by 2-ME involved antioxidant properties and suggested that macrophages were functionally injured by O2 exposure. In cases where mitogen response was highly depressed, restoration was only partial; in these conditions in vivo O2 injury probably involved both macrophages and splenic T cells. The mechanisms of O2 toxicity have been discussed in terms of free radical generation under hyperoxic conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Baird L. G., Kaplan A. M. Macrophage regulation of mitogen-induced blastogenesis. I. Demonstration of inhibitory cells in the spleens and peritoneal exudates of mice. Cell Immunol. 1977 Jan;28(1):22–35. doi: 10.1016/s0008-8749(77)80003-8. [DOI] [PubMed] [Google Scholar]
- Bevan M. J., Epstein R., Cohn M. The effect of 2-mercaptoethanol on murine mixed lymphocyte cultures. J Exp Med. 1974 Apr 1;139(4):1025–1030. doi: 10.1084/jem.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau P., Brun M., Azoulay E., Bernaudin J. F., Blayo M. C., Pocidalo J. J. Respiratory and non-respiratory causes of death in the rat exposed to normobaric oxygen. Bull Eur Physiopathol Respir. 1982 Jul-Aug;18(4):633–642. [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Enhancement of antibody synthesis in vitro by mercaptoethanol. Cell Immunol. 1972 Jan;3(1):156–160. doi: 10.1016/0008-8749(72)90237-7. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Enhancement by reducing agents of the transformation of human and rabbit peripheral lymphocytes. J Immunol. 1970 Oct;105(4):1043–1045. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O. Nonspecific activation of murine lymphocytes. I. Proliferation and polyclonal activation induced by 2-mercaptoethanol and alpha-thioglycerol. J Exp Med. 1977 Mar 1;145(3):473–489. doi: 10.1084/jem.145.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O. Nonspecific activation of murine lymphocytes. II. Parameters of the interaction between splenic lymphocytes and radiolabeled 2-mercaptoethanol in vitro. J Immunol. 1978 May;120(5):1453–1459. [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O. Nonspecific activation of murine lymphocytes. VII. Functional correlates of molecular structure of thiol compounds. J Immunol. 1981 Jan;126(1):20–26. [PubMed] [Google Scholar]
- Habu S., Raff M. C. Accessory cell dependence of lectin-induced proliferation of mouse T lymphocytes. Eur J Immunol. 1977 Jul;7(7):451–457. doi: 10.1002/eji.1830070710. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Click R. E. Immune responses in vitro. V. Role of mercaptoethanol in the mixed-leukocyte reaction. Cell Immunol. 1972 Nov;5(3):410–418. doi: 10.1016/0008-8749(72)90067-6. [DOI] [PubMed] [Google Scholar]
- Igarashi T., Okada M., Kishimoto T., Yamamura Y. In vitro induction of polyclonal killer T cells with 2-mercaptoethanol and the essential role of macrophages in this process. J Immunol. 1977 May;118(5):1697–1703. [PubMed] [Google Scholar]
- Koren H. S., Hodes R. J. Effect of tumor cells on the generation of cytotoxic T lymphocytes in vitro. I. Accessory cell functions of mouse tumor cells in the generation of cytotoxic T lymphocytes in vitro: replacement of adherent phagocytic cells by tumor cells or 2-mercaptoethanol. Eur J Immunol. 1977 Jun;7(6):394–400. doi: 10.1002/eji.1830070613. [DOI] [PubMed] [Google Scholar]
- Lemke H., Opitz H. G. Function of 2-mercaptoethanol as a macrophage substitute in the primary immune response in vitro. J Immunol. 1976 Aug;117(2):388–395. [PubMed] [Google Scholar]
- Levacher-Place M., Gougerot-Pocidalo M. A., Rouveix B., Kraus L., Pocidalo J. J. Characterization of immunological depression in mice exposed to normobaric oxygen. Clin Exp Immunol. 1983 Nov;54(2):580–586. [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978 Jul;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Lawrence D. A. Modulation of T-cell function. II. Chemical basis for the involvement of cell surface thiol-reactive sites in control of T-cell proliferation. Cell Immunol. 1981 May 15;60(2):453–469. doi: 10.1016/0008-8749(81)90286-0. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Lawrence D. A. Modulation of T-cell functions. I. Effect of 2-mercaptoethanol and macrophages on T-cell proliferation. Cell Immunol. 1980 Mar 15;50(2):416–431. doi: 10.1016/0008-8749(80)90295-6. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. The participation of macrophages and macrophage cell lines in the activation of T lymphocytes by mitogens. Immunol Rev. 1978;40:102–135. doi: 10.1111/j.1600-065x.1978.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Munthe-Kaas A. C. Promotive and inhibitory effects of macrophages on the mitogen-induced blastogenesis of lymphocytes: a flow-cytometric study. Scand J Immunol. 1981 Jul;14(1):39–46. doi: 10.1111/j.1365-3083.1981.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]


