Abstract
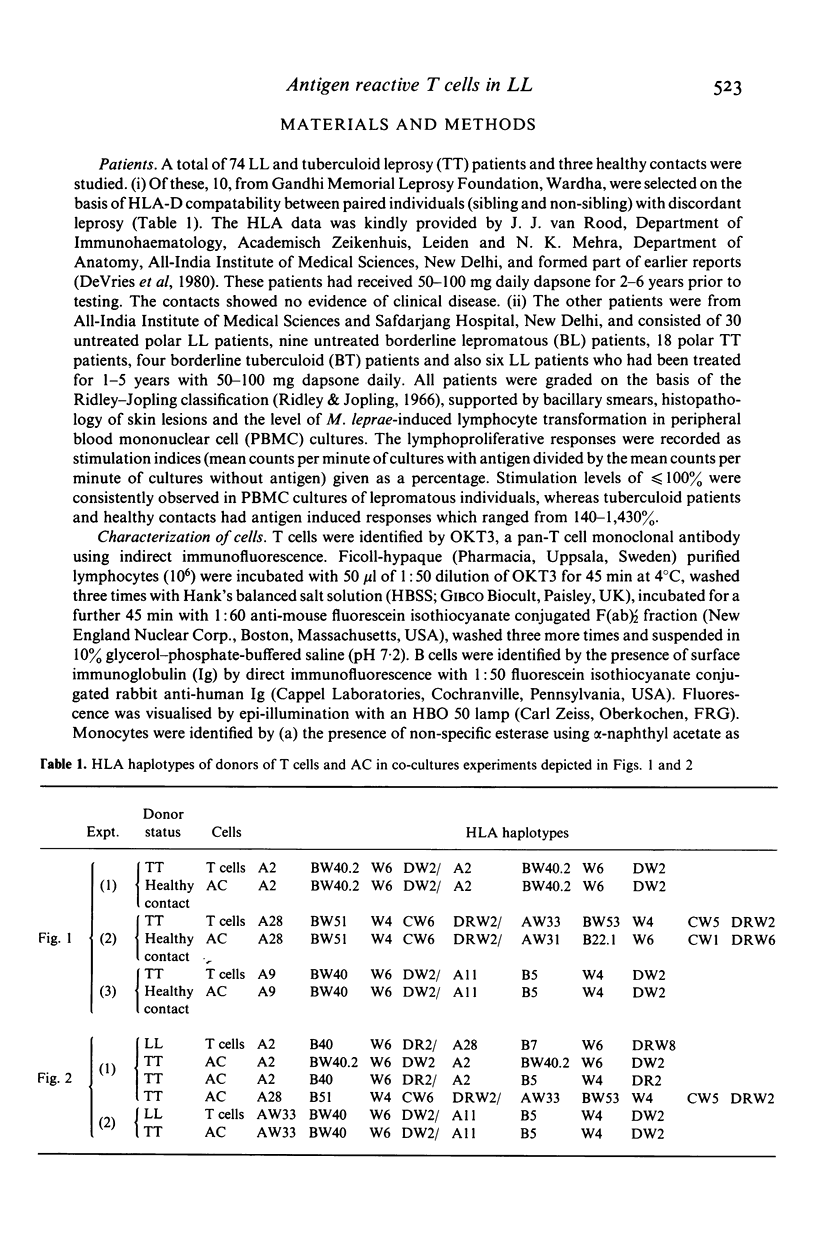
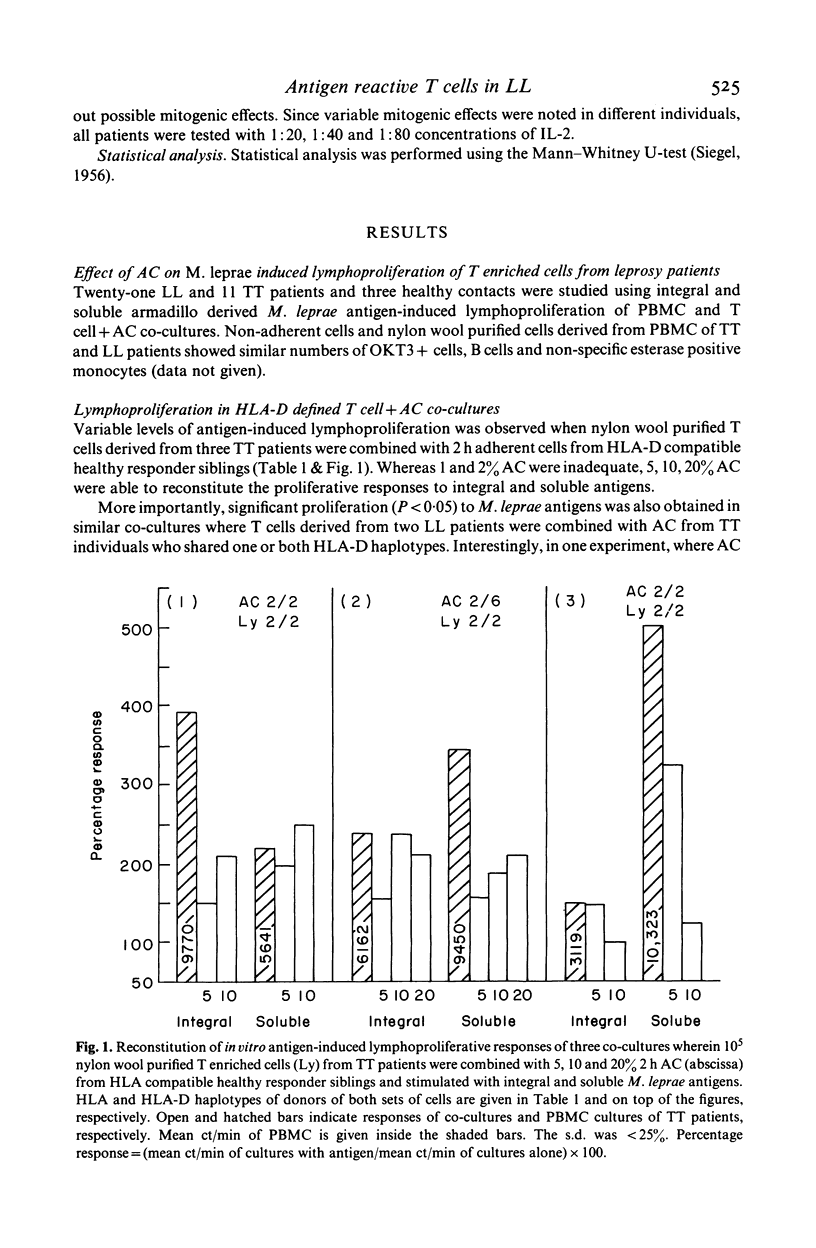
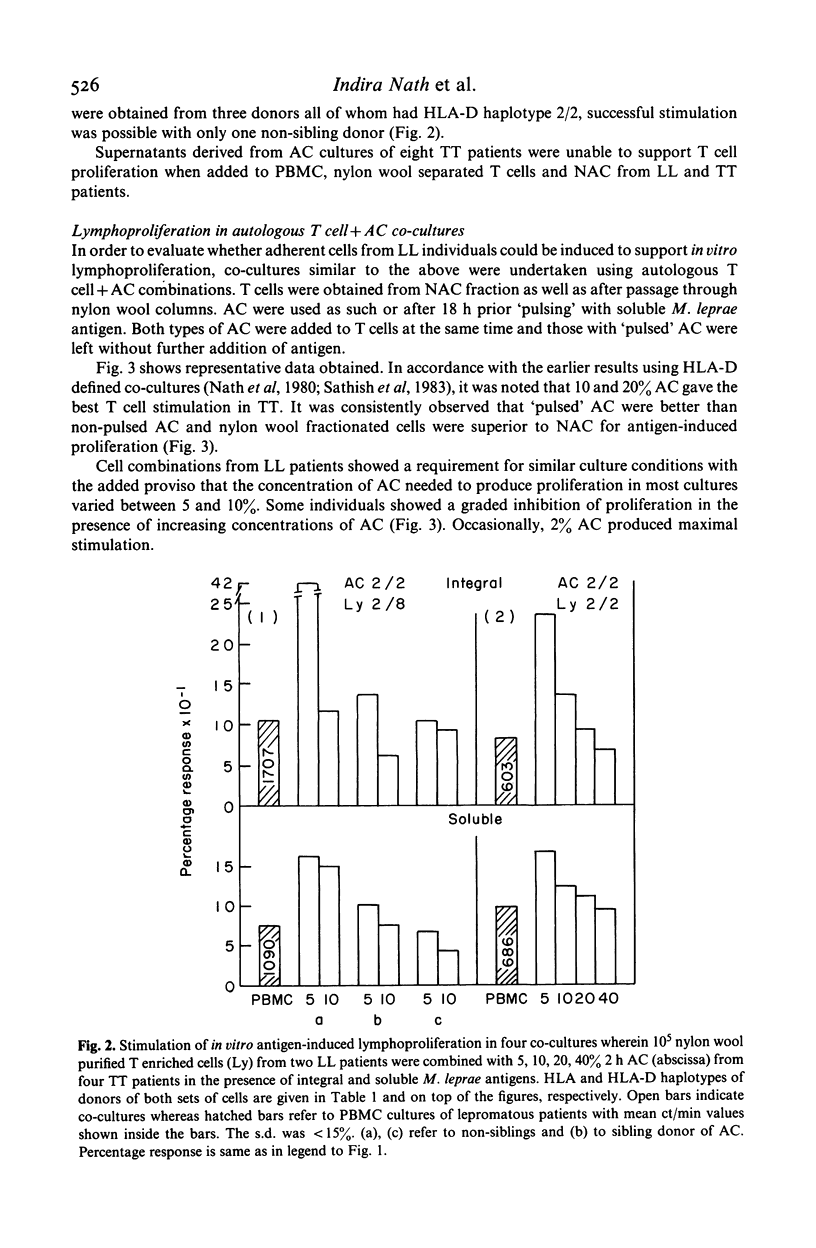
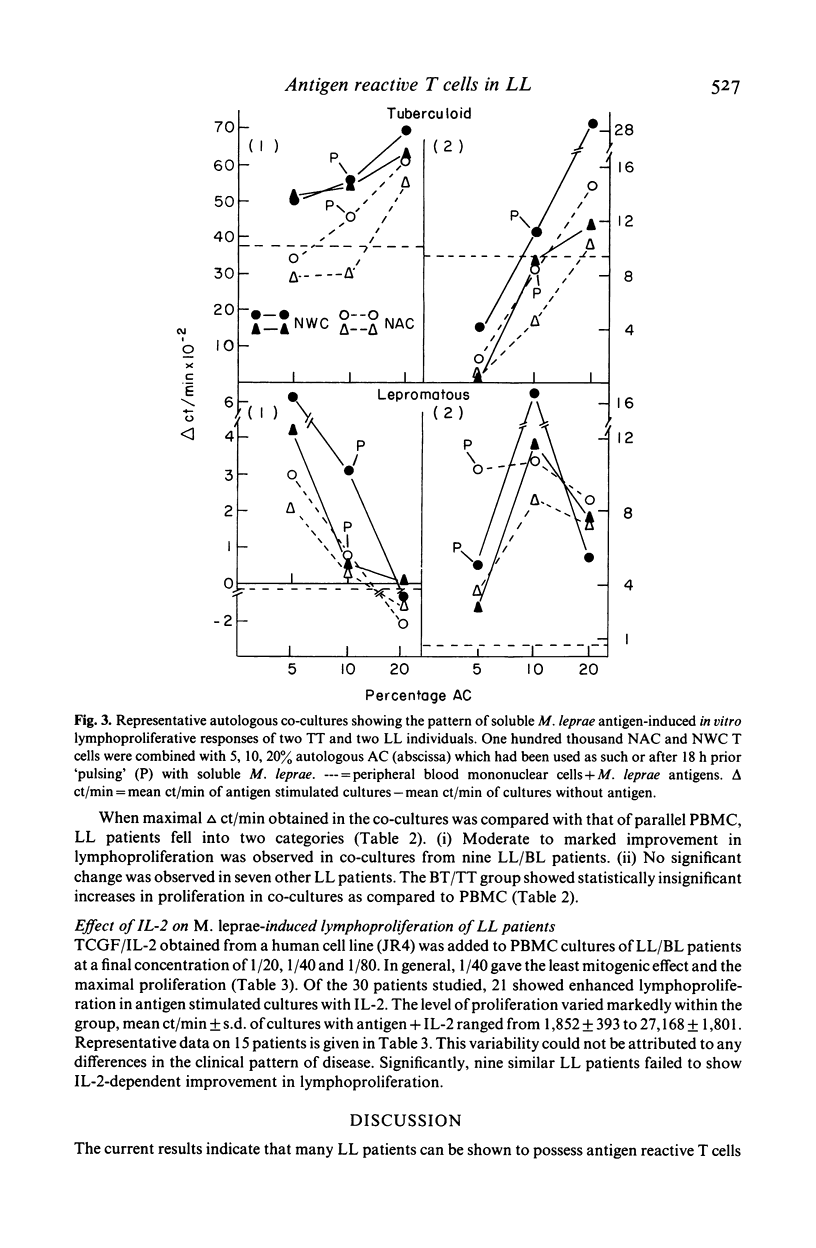
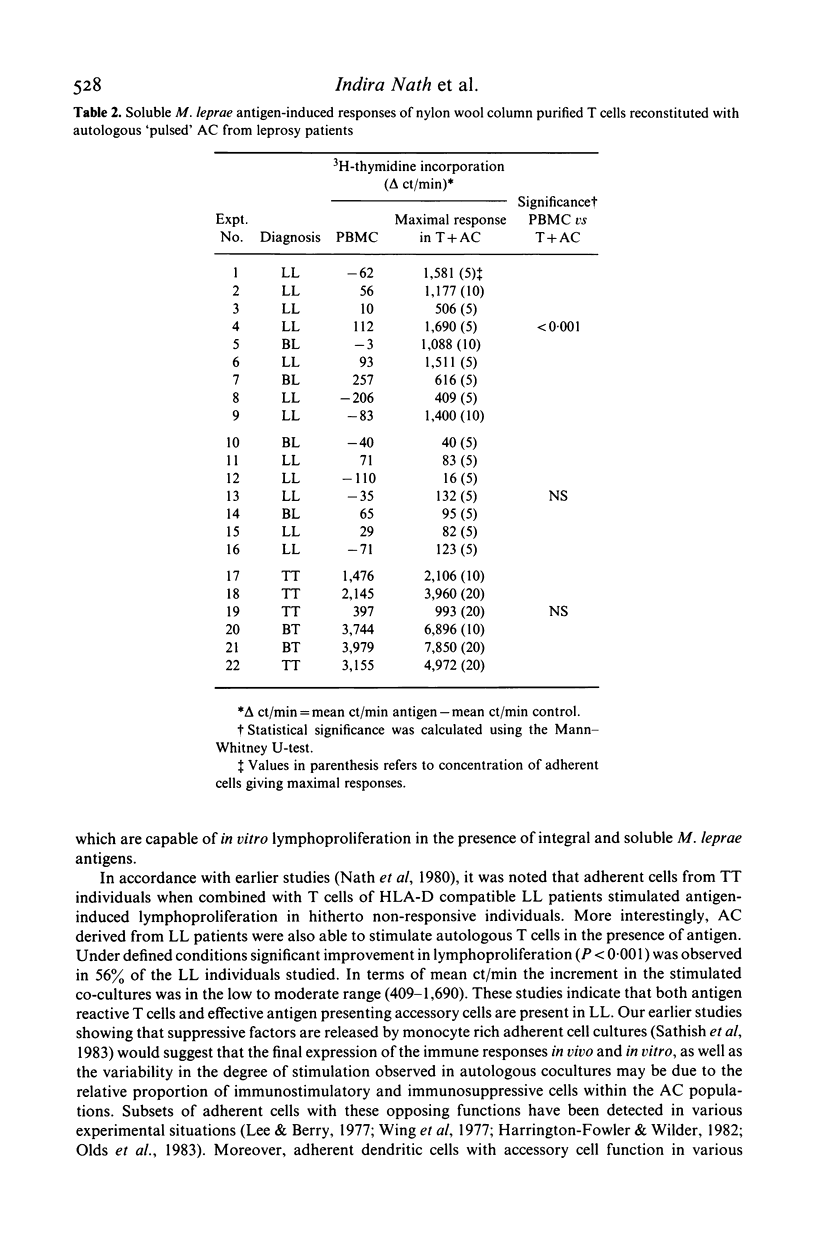
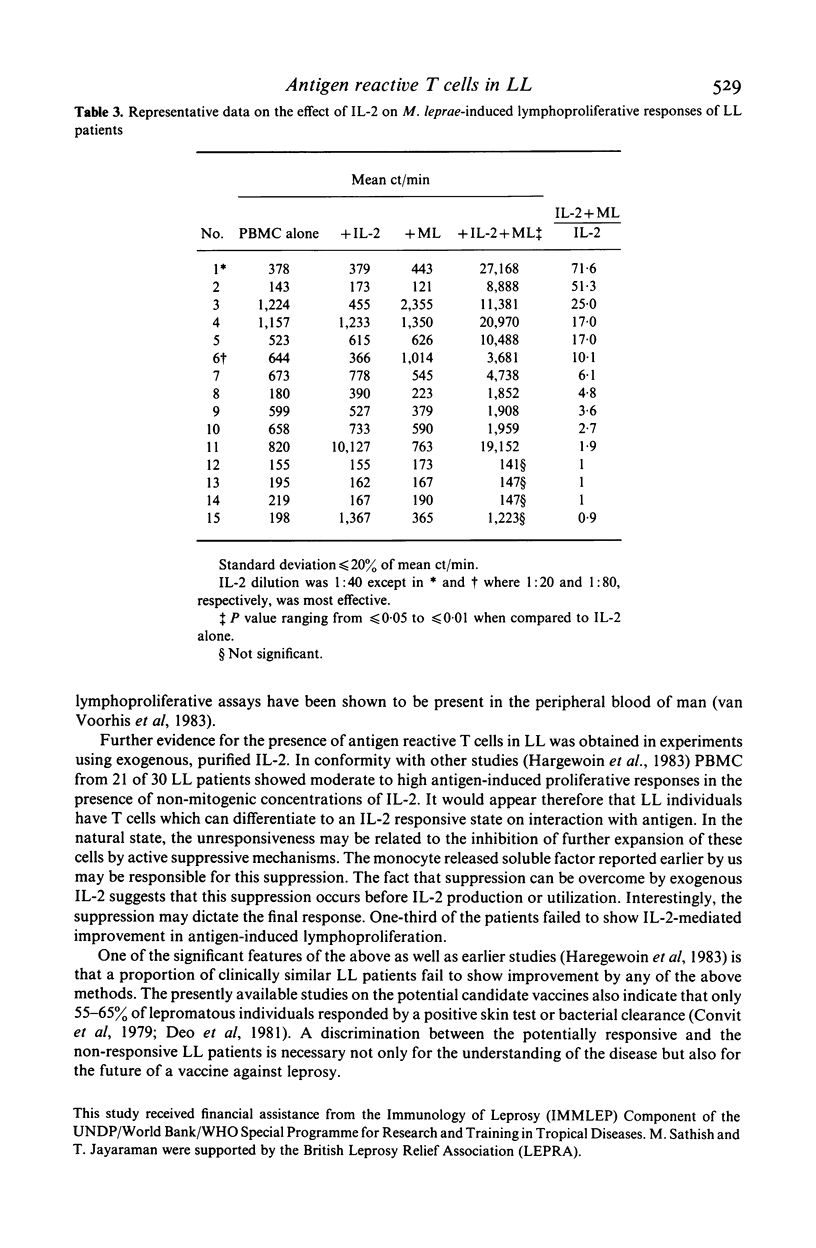
Evidence for the presence of Mycobacterium leprae reactive T cells in many lepromatous leprosy (LL) patients was obtained using in vitro antigen-induced lymphoproliferative responses. (1) Co-cultures of T enriched cells from LL patients when combined with 2 h adherent cells (AC) from HLA-D compatible tuberculoid leprosy individuals showed significant levels of 3H-thymidine incorporation in the presence of soluble and integral M. leprae antigens. (2) More interestingly, autologous T cell + AC co-cultures also showed significant improvement in antigen-induced lymphoproliferation in nine of 16 lepromatous patients. Insignificant improvement was observed in similar co-cultures of tuberculoid leprosy patients. (3) Addition of exogenous, purified human interleukin-2 (IL-2) to antigen stimulated PBMC from some lepromatous patients showed the best improvement in terms of overall 3H-thymidine incorporation, indicating that lepromatous patients possess T cells which can differentiate to an IL-2 responsive state. Significantly, the level of proliferation varied within the group. A proportion of clinically similar lepromatous patients failed to show improvement by any of the above methods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Grönvik K. O., Larsson E. L., Coutinho A. Studies on T lymphocyte activation. I. Requirements for the mitogen-dependent production of T cell growth factors. Eur J Immunol. 1979 Aug;9(8):581–587. doi: 10.1002/eji.1830090802. [DOI] [PubMed] [Google Scholar]
- Convit J., Aranzazu N., Pinardi M., Ulrich M. Immunological changes observed in indeterminate and lepromatous leprosy patients and Mitsuda-negative contacts after the inoculation of a mixture of Mycobacterium leprae and BCG. Clin Exp Immunol. 1979 May;36(2):214–220. [PMC free article] [PubMed] [Google Scholar]
- Deo M. G., Bapat C. V., Chullawalla R. G., Bhatki W. S. Potential anti-leprosy vaccine from killed ICRC bacilli--a clinicopathological study. Indian J Med Res. 1981 Aug;74:164–177. [PubMed] [Google Scholar]
- Gillis S., Baker P. E., Ruscetti F. W., Smith K. A. Long-term culture of human antigen-specific cytotoxic T-cell lines. J Exp Med. 1978 Oct 1;148(4):1093–1098. doi: 10.1084/jem.148.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Harrington-Fowler L., Wilder M. S. Fate of Listeria monocytogenes in murine peritoneal macrophage subpopulations. Infect Immun. 1982 Jan;35(1):124–132. doi: 10.1128/iai.35.1.124-132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Berry D. Functional heterogeneity in macrophages activated by Corynebacterium parvum: characterization of subpopulations with different activities in promoting immune responses and suppressing tumor cell growth. J Immunol. 1977 May;118(5):1530–1540. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nath I., Narayanan R. B., Mehra N. K., Sharma A. K., Gupte M. D. Concanavalin A induced suppressor activity in human leprosy. J Clin Lab Immunol. 1979 Nov;2(4):319–324. [PubMed] [Google Scholar]
- Nath I., Singh R. The suppressive effect of M. leprae on the in vitro proliferative responses of lymphocytes from patients with leprosy. Clin Exp Immunol. 1980 Sep;41(3):406–414. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Van Rood J. J., Mehra N. K., Vaidya M. C. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin Exp Immunol. 1980 Nov;42(2):203–210. [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Kholy A. E., Ellner J. J. Two distinctive patterns of monocyte immunoregulatory and effector functions in heavy human infections with Schistosoma mansoni. J Immunol. 1983 Aug;131(2):954–958. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Sathish M., Bhutani L. K., Sharma A. K., Nath I. Monocyte-derived soluble suppressor factor(s) in patients with lepromatous leprosy. Infect Immun. 1983 Dec;42(3):890–899. doi: 10.1128/iai.42.3.890-899.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- de Vries R. R., Mehra N. K., Vaidya M. C., Gupte M. D., Meera Khan P., Van Rood J. J. HLA-linked control of susceptibility to tuberculoid leprosy and association with HLA-DR types. Tissue Antigens. 1980 Oct;16(4):294–304. doi: 10.1111/j.1399-0039.1980.tb00309.x. [DOI] [PubMed] [Google Scholar]


