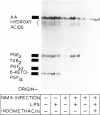
Abstract
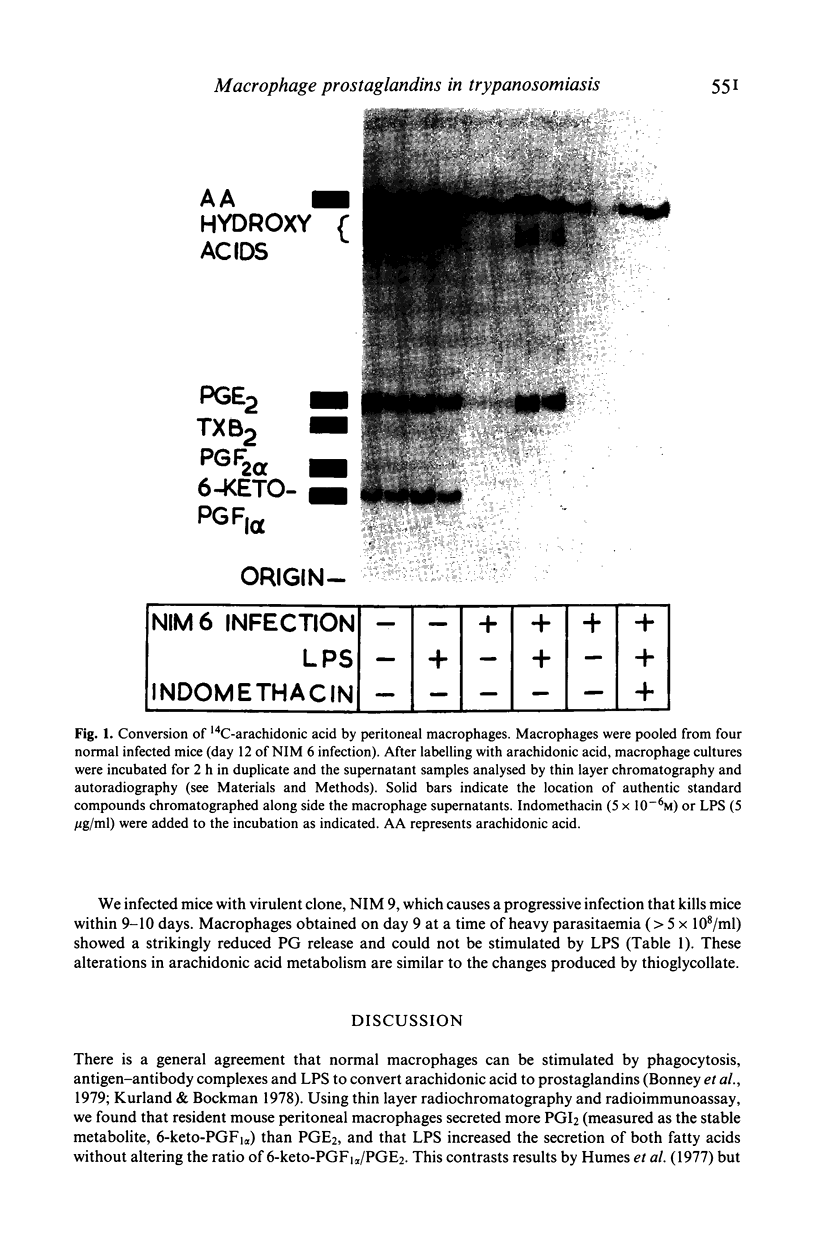
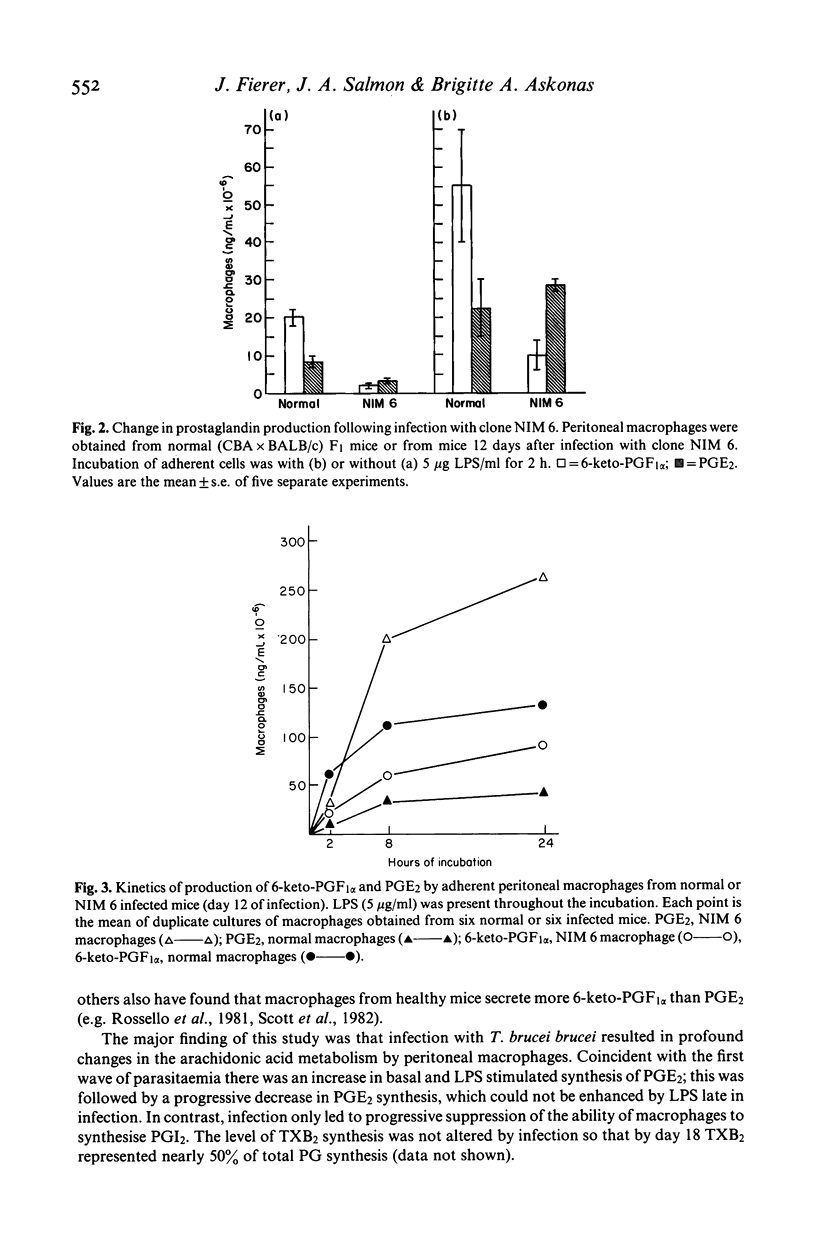
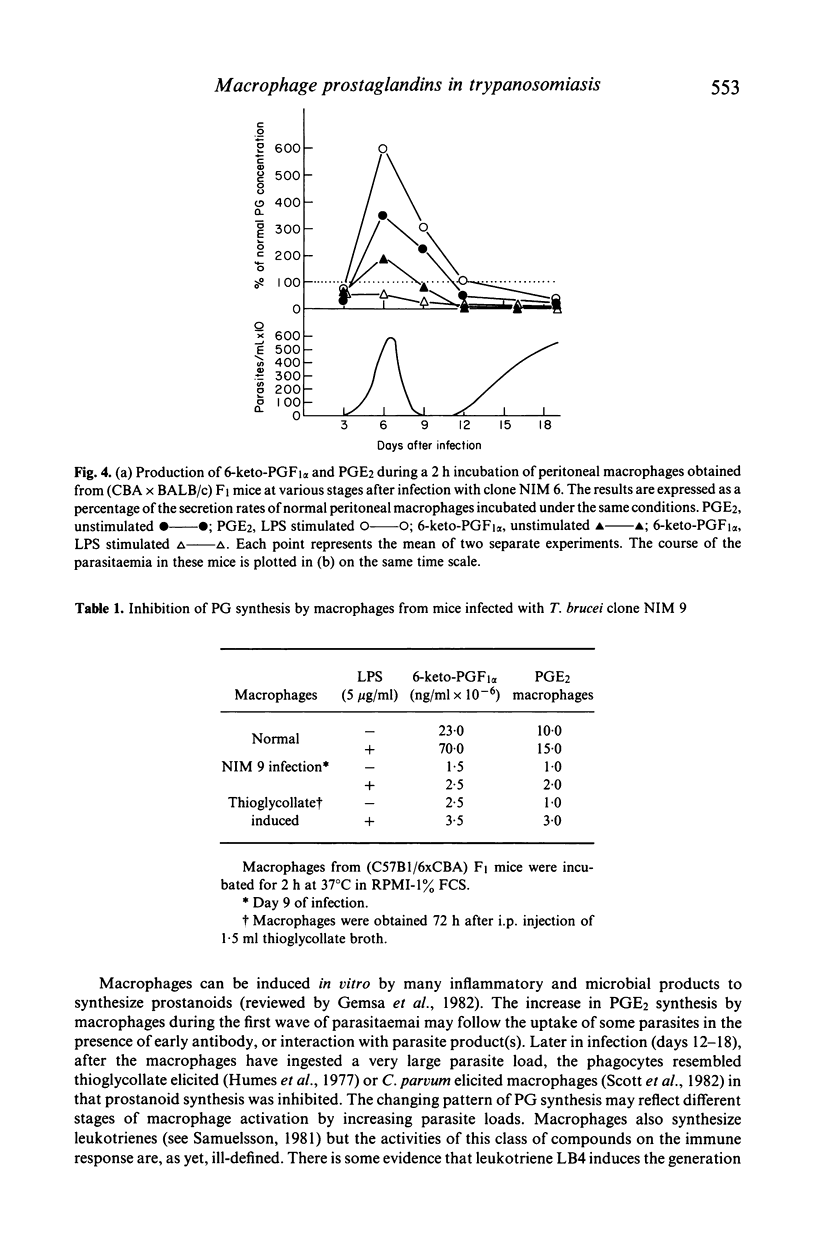
Many factors contribute to the severe immunosuppression associated with African trypanosomiasis. Macrophages have been shown to be important target cells which after uptake of parasites, mediate immune dysfunction in vivo. We observed that infection of mice with Trypanosome brucei brucei (clone NIM 6) induces profound changes in arachidonic acid metabolism and prostaglandin (PG) secretion by macrophages. Normal macrophages release more PGI2 than PGE2 and production of both these prostaglandins is stimulated equally by endotoxin (LPS). Macrophages taken from NIM 6 infected mice at the peak of the first parasitaemia, release increased amounts of PGE2 and are hyperresponsive to LPS stimulation, while PGI2 secretion remains normal. As the infection progresses, there is a striking decrease in both basal PGE2 and PGI2 secretion and the ability of macrophages to respond to LPS. By the third week of infection, shortly before death, peritoneal macrophages resemble thioglycollate elicited macrophages in their inability to be stimulated by LPS to synthesize prostaglandins. Infection with a more virulent clone of T. brucei (NIM9) results in suppression of both PGE2 and PGI2 release by day 9 of infection. The increased production of PGE2 by macrophages during the height of infection is likely to contribute to the general immunosuppression associated with African trypanosomiasis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boreham P. F. Kinin release and the immune reaction in human trypanosomiasis caused by Trypanosoma rhodesiense. Trans R Soc Trop Med Hyg. 1970;64(3):394–400. doi: 10.1016/0035-9203(70)90175-6. [DOI] [PubMed] [Google Scholar]
- Cahill J., Hopper K. E. Immunoregulation by macrophages: differential secretion of prostaglandin E and interleukin 1 during infection with Salmonella enteritidis. Cell Immunol. 1982 Mar 1;67(2):229–240. doi: 10.1016/0008-8749(82)90216-7. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Ogilvie B. M., Askonas B. A. Trypanosoma brucei infection in nude mice: B lymphocyte function is suppressed in the absence of T lymphocytes. Parasite Immunol. 1979 Spring;1(1):39–48. doi: 10.1111/j.1365-3024.1979.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Fierer J., Askonas B. A. Trypanosoma brucei infection stimulates receptor-mediated phagocytosis by murine peritoneal macrophages. Infect Immun. 1982 Sep;37(3):1282–1284. doi: 10.1128/iai.37.3.1282-1284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarre L. C., Hug K., Louis J. Murine T lymphocyte specificity for African trypanosomes. II. Suppression of the T lymphocyte proliferative response to Trypanosoma brucei by systemic trypanosome infection. Clin Exp Immunol. 1981 Jul;45(1):165–172. [PMC free article] [PubMed] [Google Scholar]
- Gemsa D., Leser H. G., Seitz M., Deimann W., Bärlin E. Membrane perturbation and stimulation of arachidonic acid metabolism. Mol Immunol. 1982 Oct;19(10):1287–1296. doi: 10.1016/0161-5890(82)90295-4. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Goodwin L. G., Green D. G., Guy M. W., Voller A. Immunosuppression during trypanosomiasis. Br J Exp Pathol. 1972 Feb;53(1):40–43. [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. G. The pathology of African trypanosomiasis. Trans R Soc Trop Med Hyg. 1970;64(6):797–817. doi: 10.1016/0035-9203(70)90096-9. [DOI] [PubMed] [Google Scholar]
- Grosskinsky C. M., Askonas B. A. Macrophages as primary target cells and mediators of immune dysfunction in African trypanosomiasis. Infect Immun. 1981 Jul;33(1):149–155. doi: 10.1128/iai.33.1.149-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskinsky C. M., Ezekowitz R. A., Berton G., Gordon S., Askonas B. A. Macrophage activation in murine African trypanosomiasis. Infect Immun. 1983 Mar;39(3):1080–1086. doi: 10.1128/iai.39.3.1080-1086.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham E. A., Egan R. W., Soderman D. D., Gale P. H., Kuehl F. A., Jr Peroxidase-dependent deactivation of prostacyclin synthetase. J Biol Chem. 1979 Apr 10;254(7):2191–2194. [PubMed] [Google Scholar]
- Hsueh W., Desai U., Gonzalez-Crussi F., Lamb R., Chu A. Two phospholipase pools for prostaglandin synthesis in macrophages. Nature. 1981 Apr 23;290(5808):710–713. doi: 10.1038/290710a0. [DOI] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H., Eardley D. D. Activation of distinct helper and suppressor T cells in experimental trypanosomiasis. J Immunol. 1978 Aug;121(2):622–628. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield J. M. Immunobiology of African trypanosomiasis. Cell Immunol. 1978 Aug;39(1):204–210. doi: 10.1016/0008-8749(78)90094-1. [DOI] [PubMed] [Google Scholar]
- Moncada S., Ferreira S. H., Vane J. R. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature. 1973 Nov 23;246(5430):217–219. doi: 10.1038/246217a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R. J., Bunting S., Vane J. R. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976 Nov;12(5):715–737. doi: 10.1016/0090-6980(76)90048-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Roelants G. E., Lundin L. B., Mayor-Withey K. S. Immune depression in trypanosome-infected mice. I. Depressed T lymphocyte responses. Eur J Immunol. 1978 Oct;8(10):723–727. doi: 10.1002/eji.1830081010. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Pinder M. Immunobiology of African trypanosomiasis. Contemp Top Immunobiol. 1984;12:225–274. doi: 10.1007/978-1-4684-4571-8_7. [DOI] [PubMed] [Google Scholar]
- Roselló J., Gelpi E., Rigaud M., Durand J., Breton J. C. Quantitative profiling of the metabolic cascade of arachidonic acid by capillary gas chromatography mass spectrometry. Biomed Mass Spectrom. 1981 Apr;8(4):149–154. doi: 10.1002/bms.1200080404. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., EARLE W. R., EVANS V. J., WALTZ H. K., SHANNON J. E. The measurement of proliferation in tissue cultures by enumeration of cell nuclei. J Natl Cancer Inst. 1951 Feb;11(4):773–795. [PubMed] [Google Scholar]
- Sacks D. L., Askonas B. A. Trypanosome-induced suppression of anti-parasite responses during experimental African trypanosomiasis. Eur J Immunol. 1980 Dec;10(12):971–974. doi: 10.1002/eji.1830101216. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Selkirk M., Ogilvie B. M., Askonas B. A. Intrinsic immunosuppressive activity of different trypanosome strains varies with parasite virulence. Nature. 1980 Jan 31;283(5746):476–478. doi: 10.1038/283476a0. [DOI] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Smith D. R., Flower R. J., Moncada S., Vane J. R. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta. 1978 Mar 14;523(1):250–262. doi: 10.1016/0005-2744(78)90028-1. [DOI] [PubMed] [Google Scholar]
- Salzman P. M., Salmon J. A., Moncada S. Prostacyclin and thromboxane A2 synthesis by rabbit pulmonary artery. J Pharmacol Exp Ther. 1980 Oct;215(1):240–247. [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: a novel group of compounds including SRS-A. Prog Lipid Res. 1981;20:23–30. doi: 10.1016/0163-7827(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Pawlowski N. A., Andreach M., Cohn Z. A. Resting macrophages produce distinct metabolites from exogenous arachidonic acid. J Exp Med. 1982 Feb 1;155(2):535–547. doi: 10.1084/jem.155.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Wellhausen S. R., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. II. Splenic suppressor cell activity. J Immunol. 1979 Mar;122(3):818–824. [PubMed] [Google Scholar]