Abstract
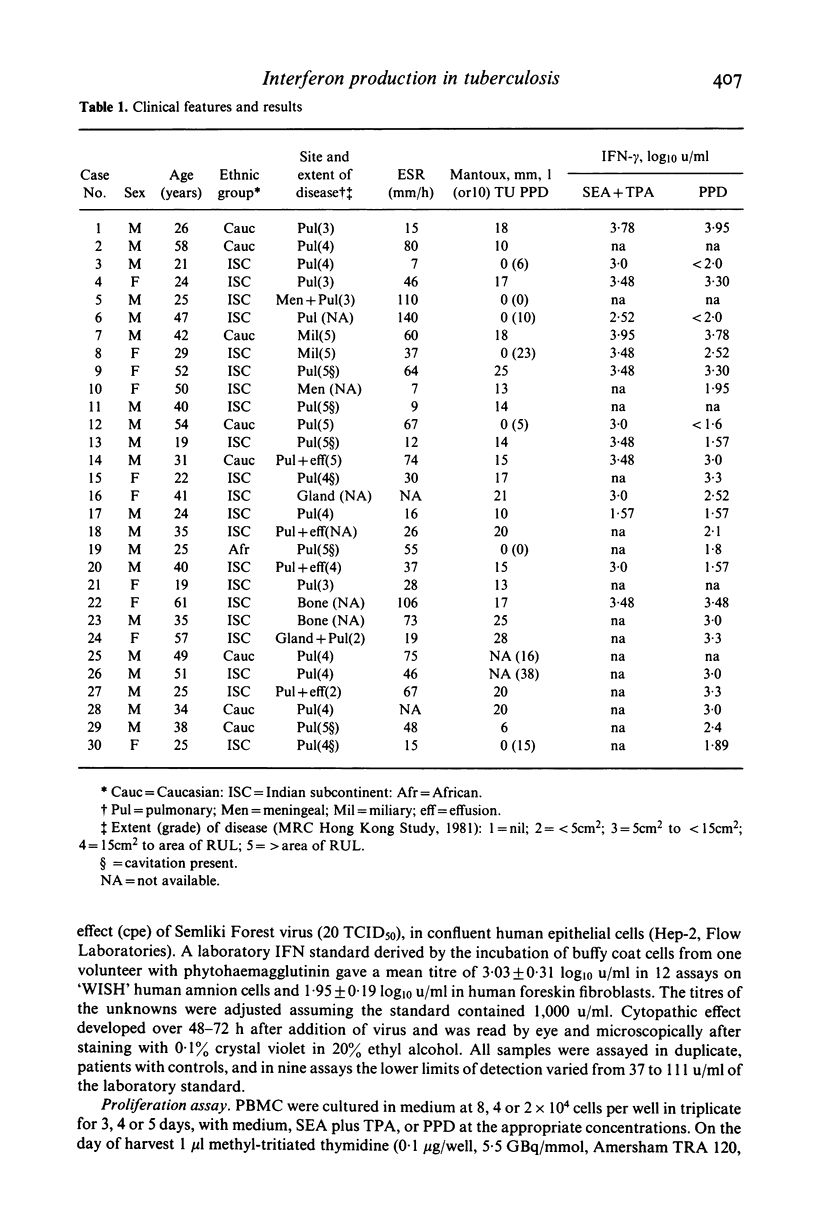
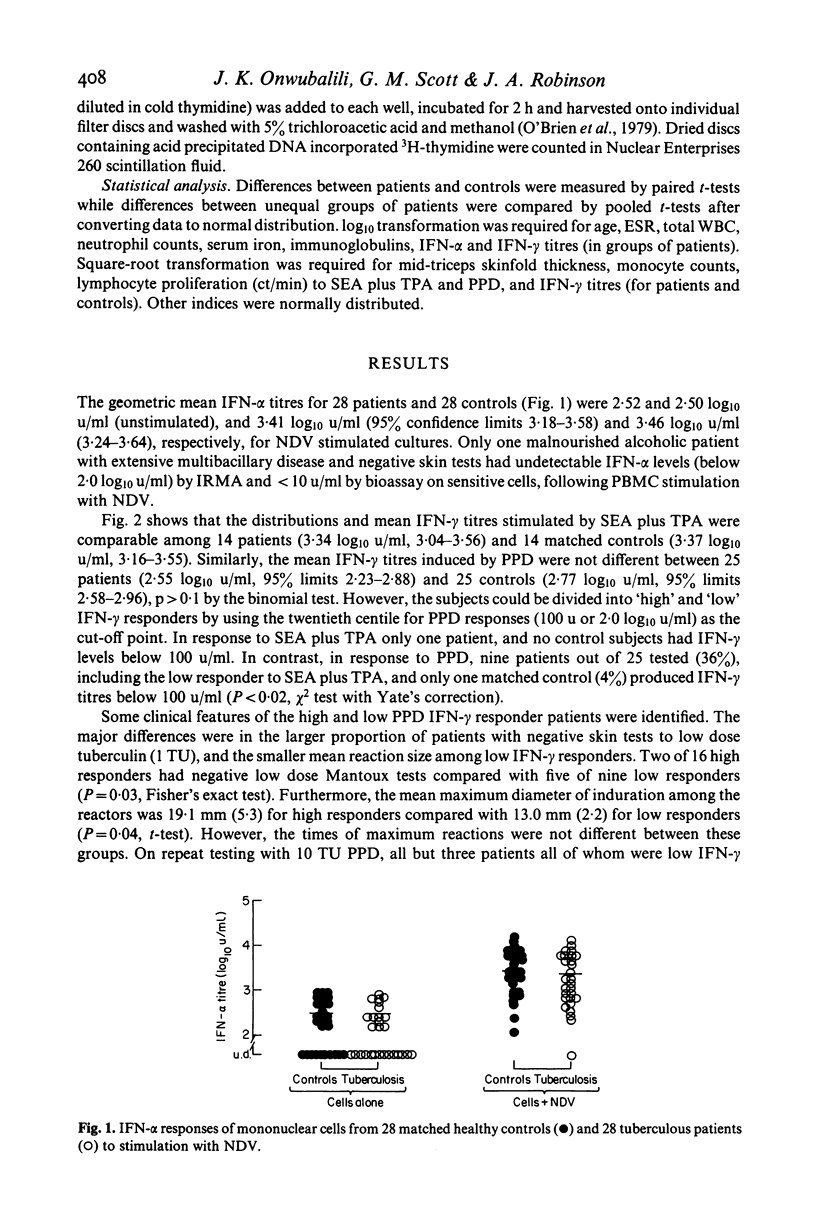
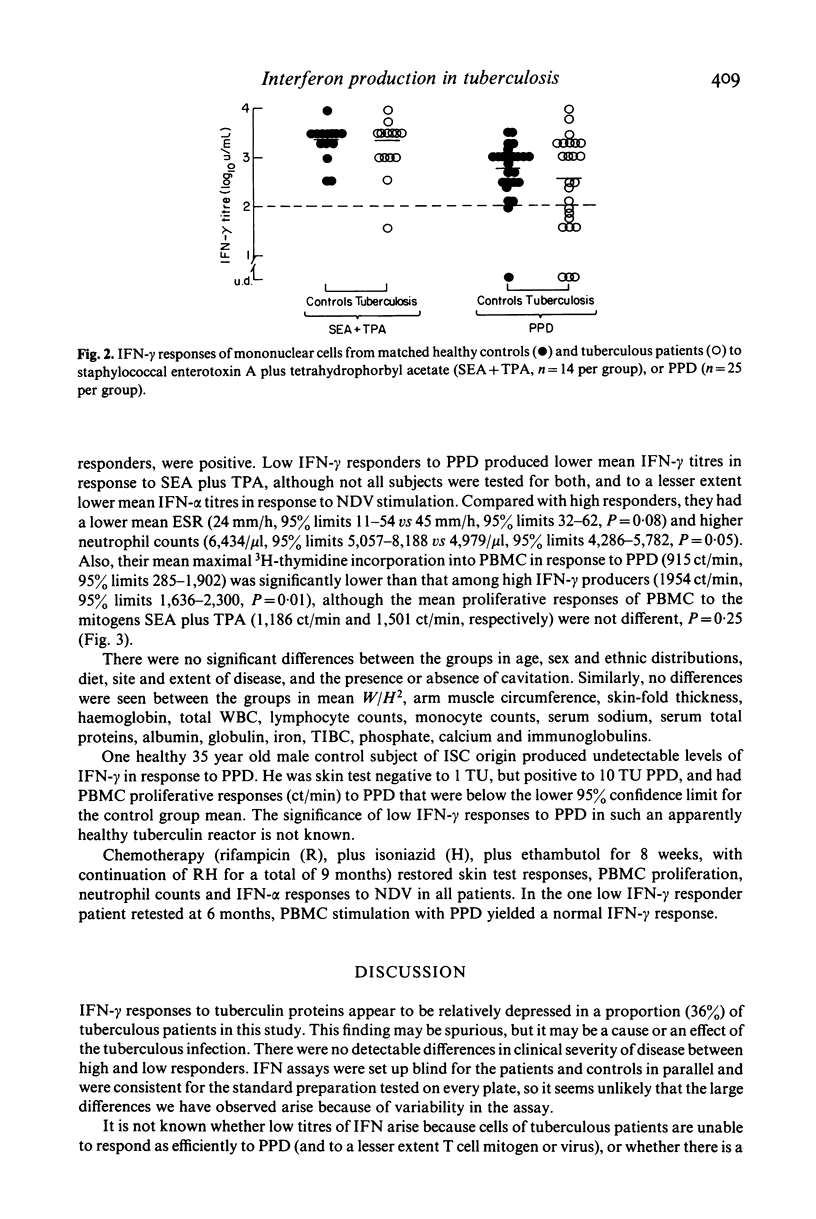
Production of interferon (IFN)-alpha and -gamma by peripheral blood mononuclear cells (PBMC) were studied in 28 patients with active tuberculosis and 28 healthy control subjects matched for age, sex, ethnic origin and diet. No significant differences were found between patients and matched controls in mean titres of IFN-alpha induced by Newcastle disease virus, IFN-gamma induced by staphylococcal enterotoxin A with tetrahydrophorbyl acetate, and IFN-gamma induced by purified protein derivative (PPD). However, a subset of nine out of 25 tuberculosis patients tested produced low titres (less than 100 u/ml) of IFN-gamma in response to PBMC stimulation with PPD. In comparison to other patients, this group was characterized by lower IFN-alpha and IFN-gamma responses to virus and mitogens respectively, relative anergy to tuberculin skin testing, depressed in vitro PBMC proliferative responses to PPD, and neutrophil leucocytosis. In all nine patients effective chemotherapy restored cutaneous reactivity, PBMC proliferative responses, neutrophil counts and IFN-alpha responses to virus by 6 months, and also IFN-gamma responses to PPD in one patient re-tested.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Arif L. I., Goldstein R. A., Affronti L. F., Janicki B. W. HLA-Bw15 and tuberculosis in a North American black population. Am Rev Respir Dis. 1979 Dec;120(6):1275–1278. doi: 10.1164/arrd.1979.120.6.1275. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Leary P. L., Levin M. J. Gamma interferon production by combinations of human peripheral blood lymphocytes, monocytes, and cultured macrophages. Infect Immun. 1982 Feb;35(2):383–390. doi: 10.1128/iai.35.2.383-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Malaviya A. N., Narayanan S., Rajgopalan P., Kumar R., Bharadwaj O. P. Spectrum of immune response abnormalities in different clinical forms of tuberculosis. Am Rev Respir Dis. 1977 Feb;115(2):207–212. doi: 10.1164/arrd.1977.115.2.207. [DOI] [PubMed] [Google Scholar]
- Campbell P. A. Immunocompetent cells in resistance to bacterial infections. Bacteriol Rev. 1976 Jun;40(2):284–313. doi: 10.1128/br.40.2.284-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D. Immunity in tuberculosis. Bull World Health Organ. 1982;60(4):447–462. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. The interaction of human macrophages and lymphocytes in the phytohemagglutinin-stimulated production of interferon. J Clin Invest. 1971 Apr;50(4):744–753. doi: 10.1172/JCI106545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Okuda Y., Fukukawa T., Tsuyuguchi I. In vitro tuberculin reactivity of lymphocytes from patients with tuberculous pleurisy. Infect Immun. 1982 Feb;35(2):402–409. doi: 10.1128/iai.35.2.402-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966 Jun;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J. M., Aber V. R., Allen B. W., Mitchison D. A., Mikhail J. R., McSwiggan D. A., Collins C. H. Comparison of strains of Mycobacterium tuberculosis from British, Ugandan and Asian immigrant patients: a study in bacteriophage typing, susceptibility to hydrogen peroxide and sensitivity to thiophen-2-carbonic acid hydrazide. Tubercle. 1977 Dec;58(4):207–215. doi: 10.1016/0041-3879(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Green J. A., Cooperband S. R., Kibrick S. Immune specific induction of interferon production in cultures of human blood lymphocytes. Science. 1969 Jun 20;164(3886):1415–1417. doi: 10.1126/science.164.3886.1415. [DOI] [PubMed] [Google Scholar]
- Gresser I., Maury C., Kress C., Blangy D., Maunoury M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. VI. Polyoma virus infection. Int J Cancer. 1979 Aug;24(2):178–183. doi: 10.1002/ijc.2910240209. [DOI] [PubMed] [Google Scholar]
- Heilman D. H., McFarland W. Inhibition of tuberculin-induced mitogenesis in cultures of lymphocytes from tuberculous donors. Int Arch Allergy Appl Immunol. 1966;30(1):58–66. doi: 10.1159/000229793. [DOI] [PubMed] [Google Scholar]
- Hinz C. F., Jr, Daniel T. M., Baum G. L. Quantitative aspects of the stimulation of lymphocytes by tuberculin purified protein derivative. Int Arch Allergy Appl Immunol. 1970;38(2):119–129. doi: 10.1159/000230265. [DOI] [PubMed] [Google Scholar]
- Isaacs D., Clarke J. R., Tyrrell D. A., Webster A. D., Valman H. B. Deficient production of leucocyte interferon (interferon-alpha) in vitro and in vivo in children with recurrent respiratory tract infections. Lancet. 1981 Oct 31;2(8253):950–952. doi: 10.1016/s0140-6736(81)91153-3. [DOI] [PubMed] [Google Scholar]
- Katz P., Goldstein R. A., Fauci A. S. Immunoregulation in infection caused by Mycobacterium tuberculosis: the presence of suppressor monocytes and the alteration of subpopulations of T lymphocytes. J Infect Dis. 1979 Jul;140(1):12–21. doi: 10.1093/infdis/140.1.12. [DOI] [PubMed] [Google Scholar]
- Kinnman J., Frydén A., Eriksson S., Möller E., Link H. Tuberculous meningitis: immune reactions within the central nervous system. Scand J Immunol. 1981;13(3):289–296. doi: 10.1111/j.1365-3083.1981.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Kleinhenz M. E., Ellner J. J., Spagnuolo P. J., Daniel T. M. Suppression of lymphocyte responses by tuberculous plasma and mycobacterial arabinogalactan. Monocyte dependence and indomethacin reversibility. J Clin Invest. 1981 Jul;68(1):153–162. doi: 10.1172/JCI110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J., Knight S., Quick N. A., Moore E. H., Platt A. S. A simple technique for harvesting lymphocytes cultured in Terasaki plates. J Immunol Methods. 1979;27(3):219–223. doi: 10.1016/0022-1759(79)90219-9. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Carswell J. W., Stanford J. L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of 'anergic' tuberculosis patients. Clin Exp Immunol. 1976 Oct;26(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Levin H. A., Rocklin R. E., David J. R. The compartmentalization of antigen-reactive lymphocytes in desensitized guinea pigs. J Exp Med. 1971 Sep 1;134(3 Pt 1):741–750. doi: 10.1084/jem.134.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher D. S. Immunoradiometric assay of human leukocyte interferon using monoclonal antibody. Nature. 1981 Apr 9;290(5806):501–503. doi: 10.1038/290501a0. [DOI] [PubMed] [Google Scholar]
- Singh S. P., Mehra N. K., Dingley H. B., Pande J. N., Vaidya M. C. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis. 1983 Oct;148(4):676–681. doi: 10.1093/infdis/148.4.676. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Yip Y. K., Pang R. H., Oppenheim J. D., Nachbar M. S., Henriksen D., Zerebeckyj-Eckhardt I., Vilcek J. Stimulation of human gamma interferon production by diterpene esters. Infect Immun. 1981 Oct;34(1):131–139. doi: 10.1128/iai.34.1.131-139.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


