Abstract
Release of O2- and H2O2 from isolated rat liver Kupffer cells was studied by making use of the methods of SOD sensitive ferricytochrome c reduction and horseradish peroxidase catalysed scopoletin oxidation, respectively. Kupffer cells from BCG treated rats showed a 1.8 times significantly higher O2- release and a 2.4 times higher H2O2 release as compared to the controls. Moreover the yield of Kupffer cells was also increased with administration of BCG. These results suggest that Kupffer cells can be immunologically activated to secrete larger amounts of O2- and H2O2.
Full text
PDF

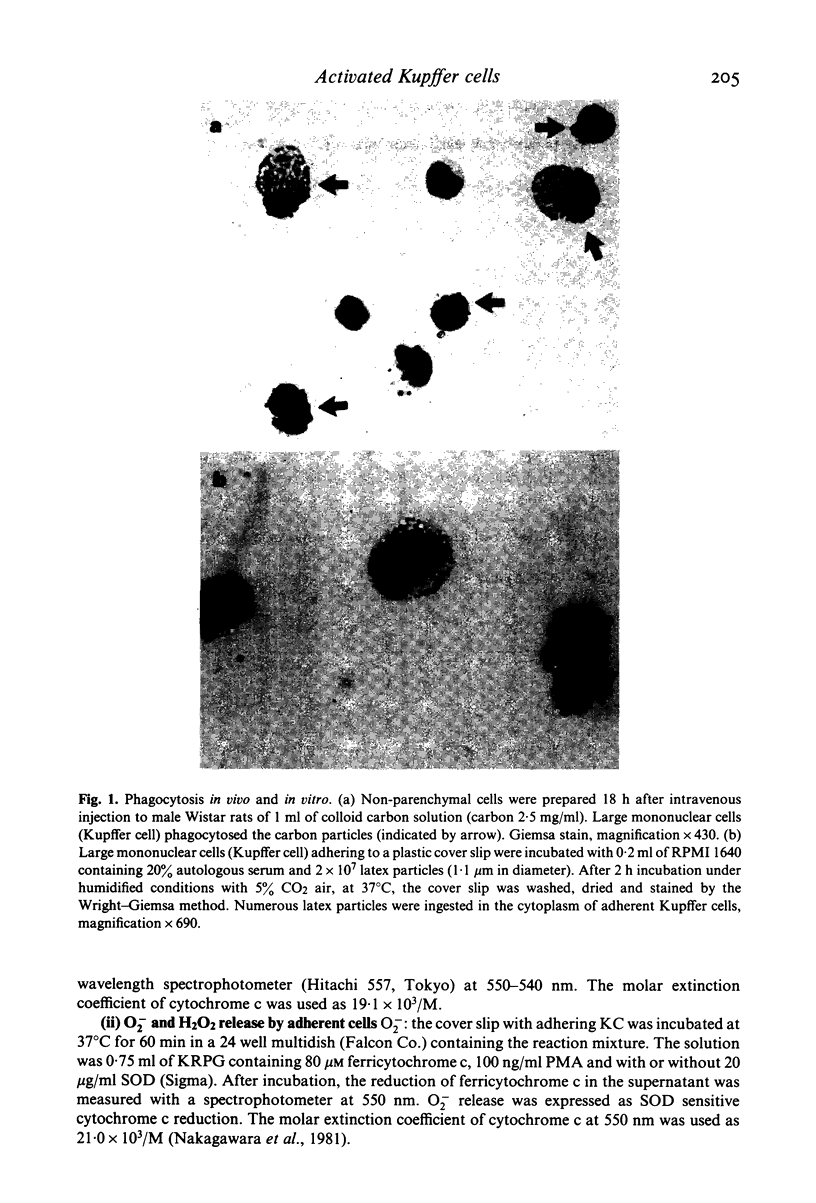



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatnagar R., Schirmer R., Ernst M., Decker K. Superoxide release by zymosan-stimulated rat Kupffer cells in vitro. Eur J Biochem. 1981 Sep;119(1):171–175. doi: 10.1111/j.1432-1033.1981.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesselhoff-den Dulk M. M., Crofton R. W., van Furth R. Origin and kinetics of Kupffer cells during an acute inflammatory response. Immunology. 1979 May;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C. Phagocytosis in rat Kupffer cells in vitro. Exp Cell Res. 1976 May;99(2):319–327. doi: 10.1016/0014-4827(76)90589-9. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Mononuclear phagocyte antimicrobial and antitumor activity: the role of oxygen intermediates. J Invest Dermatol. 1980 May;74(5):285–288. doi: 10.1111/1523-1747.ep12543457. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Kayashima K., Tamada R., Onoue K., Ikeda K., Inokuchi K. Sensitive and rapid method for determination of superoxide-generating activity of blood monocytes and its use as a probe for monocyte function in cancer patients. Gan. 1979 Dec;70(6):829–833. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Antigen presentation by isolated guinea pig Kupffer cells. J Immunol. 1980 Apr;124(4):1740–1744. [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Characterization of isolated guinea pig Kupffer cells: accessory cell function in mitogen-induced T lymphocyte activation. J Immunol. 1979 Nov;123(5):1920–1927. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh K., Yamamoto N., Kikuchi K. Modulation of cell growth by isolated Kupffer cells. J Reticuloendothel Soc. 1981 Apr;29(4):265–274. [PubMed] [Google Scholar]