Abstract
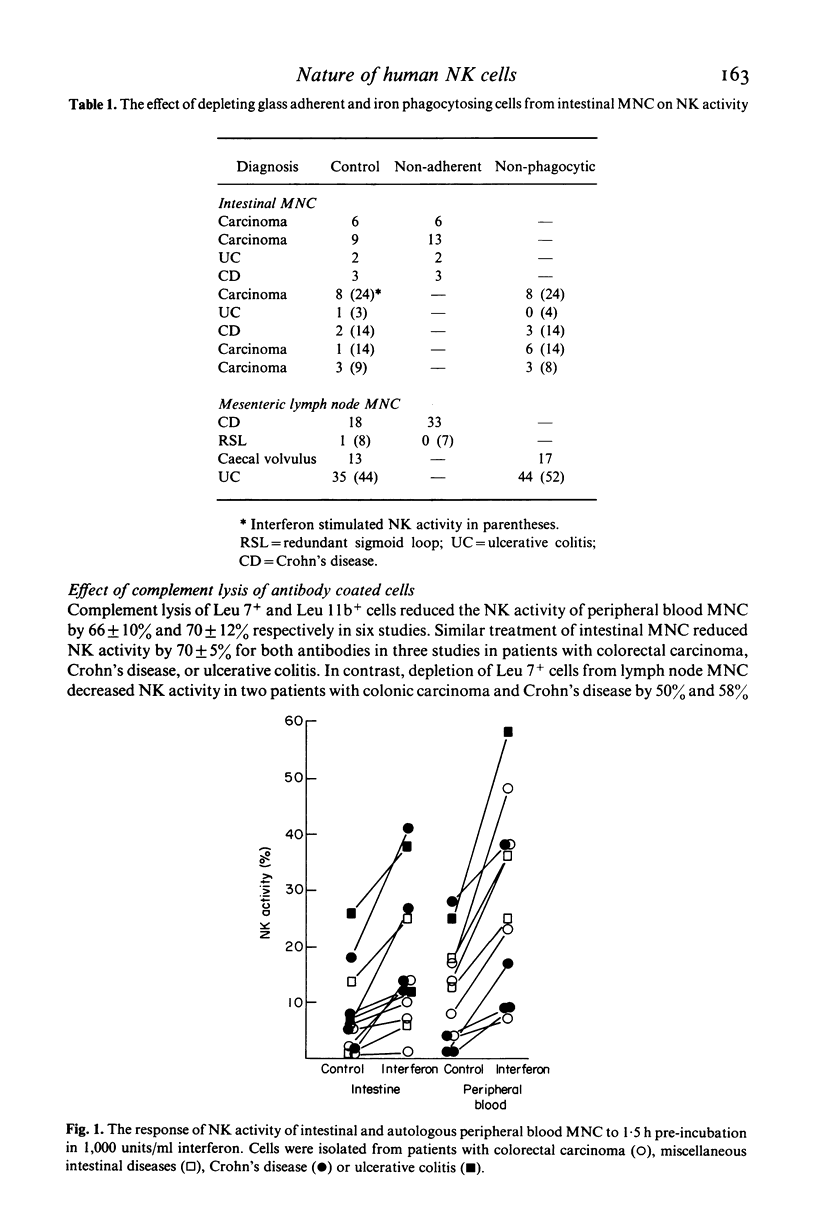
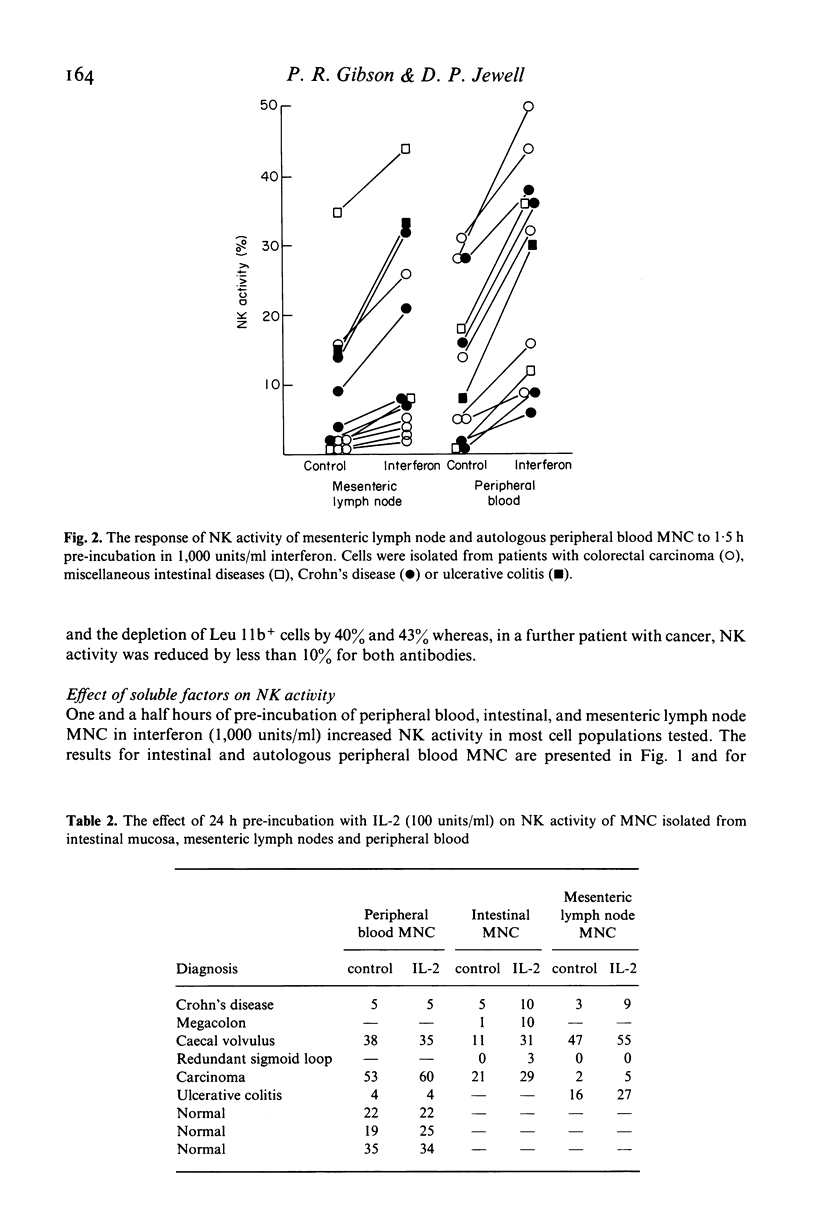
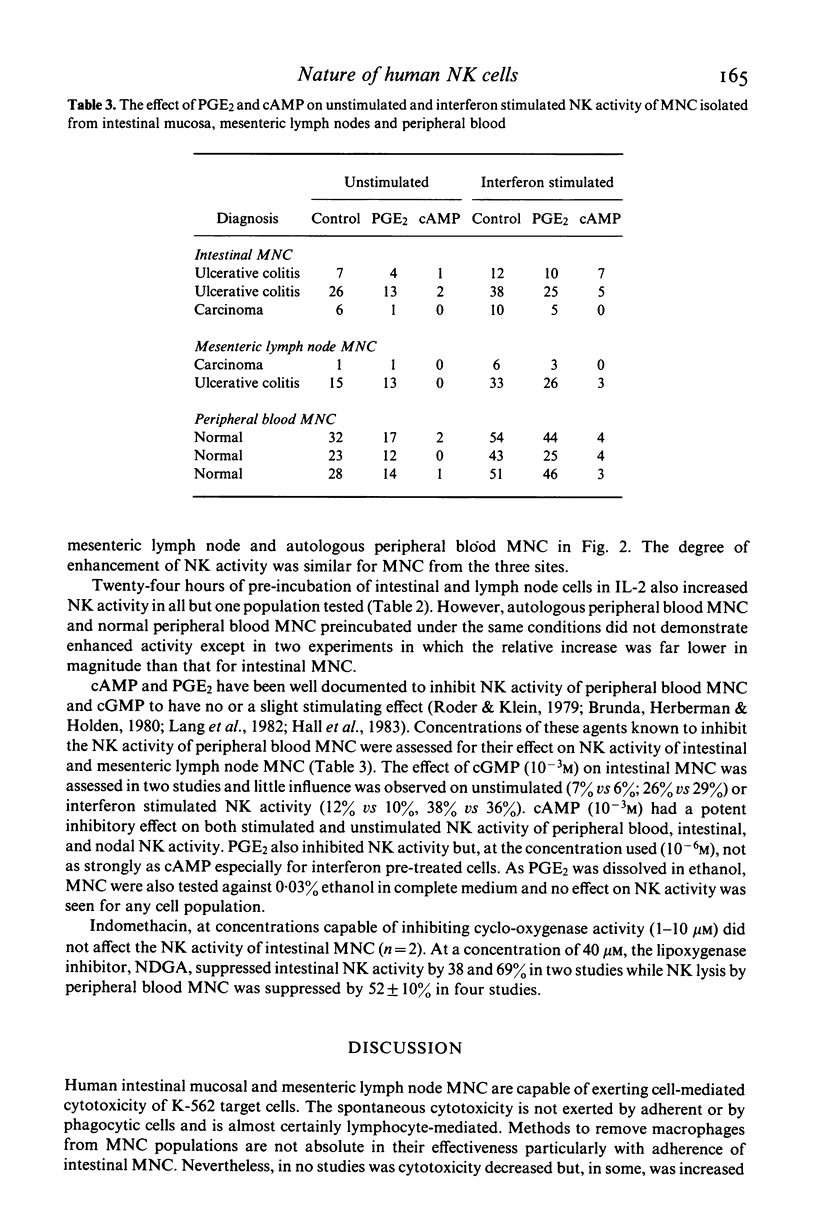
The relationship of the mononuclear cell (MNC) from human intestinal mucosa and mesenteric lymph node mediating anti-K-562 activity with that of peripheral blood has been assessed. Depletion of macrophages did not alter the measured cytotoxicity confirming that the effector cells were lymphocytes. Complement lysis of Leu 7 and Leu 11b coated cells reduced intestinal natural killer (NK) activity by a similar degree to that of peripheral blood but mesenteric lymph node NK activity was affected to a lesser extent. The response in NK activity of mucosal and nodal MNC to short incubation with lymphoblastoid interferon was similar to that for peripheral blood MNC. Twenty-four hours incubation of MNC with low concentrations of purified interleukin-2 (IL-2) consistently augmented intestinal and nodal NK activity but failed to augment that of peripheral blood MNC. No differences between the inhibitory effects of cAMP and prostaglandin E2 on NK activity from the three sites were seen. In addition, inhibition of cyclo-oxygenase activity with indomethacin had no effect on NK activity of intestinal and peripheral blood MNC while the lipoxygenase inhibitor, nordihydroguaiaretic acid, suppressed intestinal and peripheral blood NK activity similarly. In conclusion, anti-K-562 activity by intestinal MNC is mediated by NK cells with similar phenotypic and functional properties to those of peripheral blood. However, the increased sensitivity of mucosal NK cells to IL-2 suggests that higher proportions of NK cell precursors may be present in intestinal MNC populations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeken W. L., St Andre-Ukena S., Gundel R. M. Comparative studies of mononuclear phagocyte function in patients with Crohn's disease and colon neoplasms. Gut. 1983 Nov;24(11):1034–1040. doi: 10.1136/gut.24.11.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Antibody-induced augmentation of murine natural killer cell activity. Int J Cancer. 1981 Feb 15;27(2):205–211. doi: 10.1002/ijc.2910270213. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domzig W., Stadler B. M., Herberman R. B. Interleukin 2 dependence of human natural killer (NK) cell activity. J Immunol. 1983 Apr;130(4):1970–1973. [PubMed] [Google Scholar]
- Eremin O., Ashby J., Stephens J. P. Human natural cytotoxicity in the blood and lymphoid organs of healthy donors and patients with malignant disease. Int J Cancer. 1978 Jan 15;21(1):35–41. doi: 10.1002/ijc.2910210108. [DOI] [PubMed] [Google Scholar]
- Eremin O., Coombs R. R., Plumb D., Ashby J. Characterization of the human natural killer (NK) cell in blood and lymphoid organs. Int J Cancer. 1978 Jan 15;21(1):42–50. doi: 10.1002/ijc.2910210109. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Hilfiker M. L., Youngman K. R., Doerder N. C., Finke J. H. Interleukin 2 activity of human intestinal mucosa mononuclear cells. Decreased levels in inflammatory bowel disease. Gastroenterology. 1984 Apr;86(4):734–742. [PubMed] [Google Scholar]
- Flexman J. P., Shellam G. R., Mayrhofer G. Natural cytotoxicity, responsiveness to interferon and morphology of intra-epithelial lymphocytes from the small intestine of the rat. Immunology. 1983 Apr;48(4):733–741. [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Dow E. L., Selby W. S., Strickland R. G., Jewell D. P. Natural killer cells and spontaneous cell-mediated cytotoxicity in the human intestine. Clin Exp Immunol. 1984 May;56(2):438–444. [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Hermanowicz A., Jewell D. P. Factors affecting the spontaneous cell-mediated cytotoxicity of intestinal mononuclear cells. Immunology. 1984 Oct;53(2):267–274. [PMC free article] [PubMed] [Google Scholar]
- Golder J. P., Doe W. F. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983 Apr;84(4):795–802. [PubMed] [Google Scholar]
- Hall T. J., Chen S. H., Brostoff J., Lydyard P. M. Modulation of human natural killer cell activity by pharmacological mediators. Clin Exp Immunol. 1983 Nov;54(2):493–500. [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Fischer D. G., Copeland C. S., Jensen P. J. Regulation of human natural killing. I. The role of monocytes, interferon, and prostaglandins. J Immunol. 1981 Nov;127(5):2007–2013. [PubMed] [Google Scholar]
- Lang N. P., Ortaldo J. R., Bonnard G. D., Herberman R. B. Interferon and prostaglandins: effects on human natural and lectin-induced cytotoxicity. J Natl Cancer Inst. 1982 Aug;69(2):339–343. [PubMed] [Google Scholar]
- Leung K. H., Koren H. S. Regulation of human natural killing. II. Protective effect of interferon on NK cells from suppression by PGE2. J Immunol. 1982 Oct;129(4):1742–1747. [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Crosby W. H. Histochemical characterization of cellular and structural elements of the human spleen. J Histochem Cytochem. 1972 Dec;20(12):1049–1058. doi: 10.1177/20.12.1049. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Darnell B., Baron S., Talal N. Interleukin 2 enhances natural killing of normal lymphocytes. Cell Immunol. 1984 Mar;84(1):154–162. doi: 10.1016/0008-8749(84)90086-8. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Tait R. C., MacKenzie S., Davies M. D., Parrott D. M. Analysis of natural killer effector and suppressor activity by intraepithelial lymphocytes from mouse small intestine. Clin Exp Immunol. 1983 Apr;52(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Nauss K. M., Pavlina T. M., Kumar V., Newberne P. M. Functional characteristics of lymphocytes isolated from the rat large intestine. Response to T-cell mitogens and natural killer cell activity. Gastroenterology. 1984 Mar;86(3):468–475. [PubMed] [Google Scholar]
- Roder J. C., Karre K., Kiessling R. Natural killer cells. Prog Allergy. 1981;28:66–159. [PubMed] [Google Scholar]
- Roder J. C., Klein M. Target-effector interaction in the natural killer cell system. IV. Modulation by cyclic nucleotides. J Immunol. 1979 Dec;123(6):2785–2790. [PubMed] [Google Scholar]
- Seaman W. E. Human natural killer cell activity is reversibly inhibited by antagonists of lipoxygenation. J Immunol. 1983 Dec;131(6):2953–2957. [PubMed] [Google Scholar]
- Suthanthiran M., Solomon S. D., Williams P. S., Rubin A. L., Novogrodsky A., Stenzel K. H. Hydroxyl radical scavengers inhibit human natural killer cell activity. Nature. 1984 Jan 19;307(5948):276–278. doi: 10.1038/307276a0. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Luini W., Soldateschi D., Boraschi D. Natural killer activity of gut mucosal lymphoid cells in mice. Eur J Immunol. 1981 Nov;11(11):919–922. doi: 10.1002/eji.1830111112. [DOI] [PubMed] [Google Scholar]
- Targan S., Britvan L., Kendal R., Vimadalal S., Soll A. Isolation of spontaneous and interferon inducible natural killer like cells from human colonic mucosa: lysis of lymphoid and autologous epithelial target cells. Clin Exp Immunol. 1983 Oct;54(1):14–22. [PMC free article] [PubMed] [Google Scholar]


