Abstract
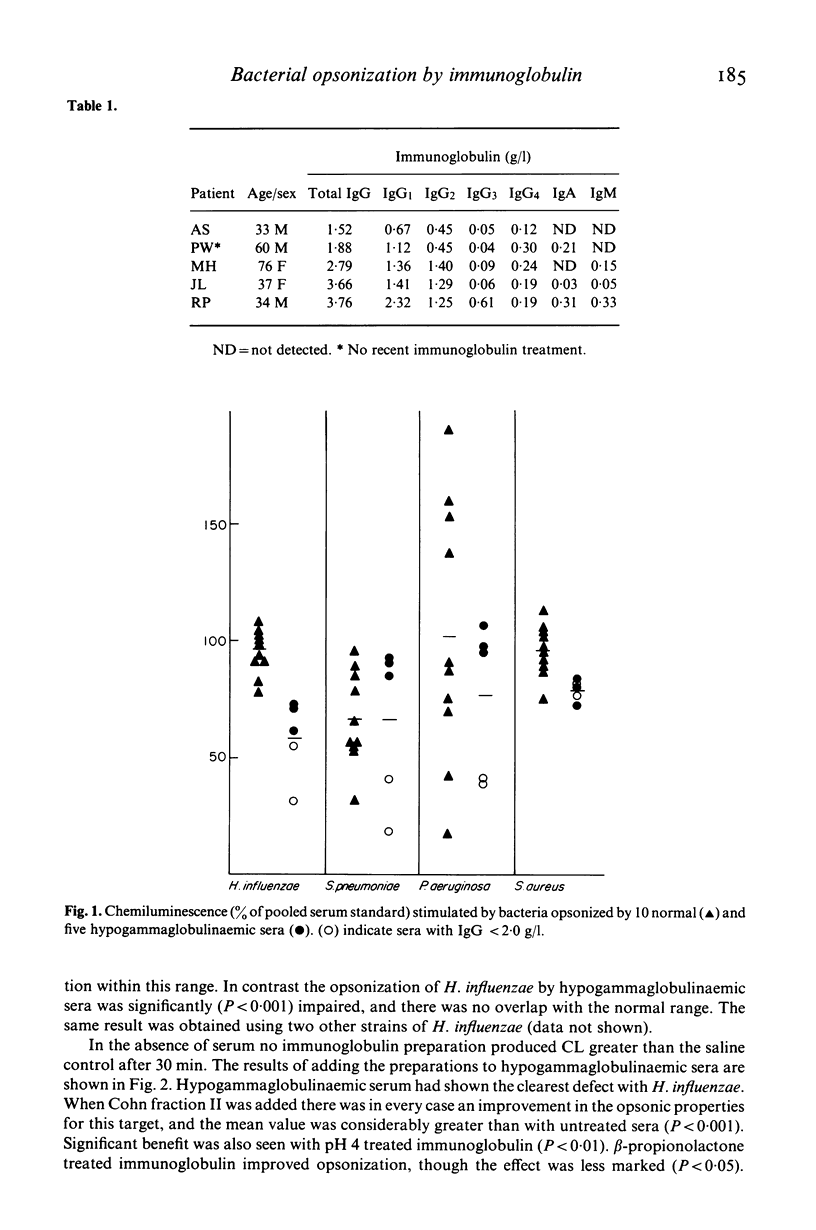
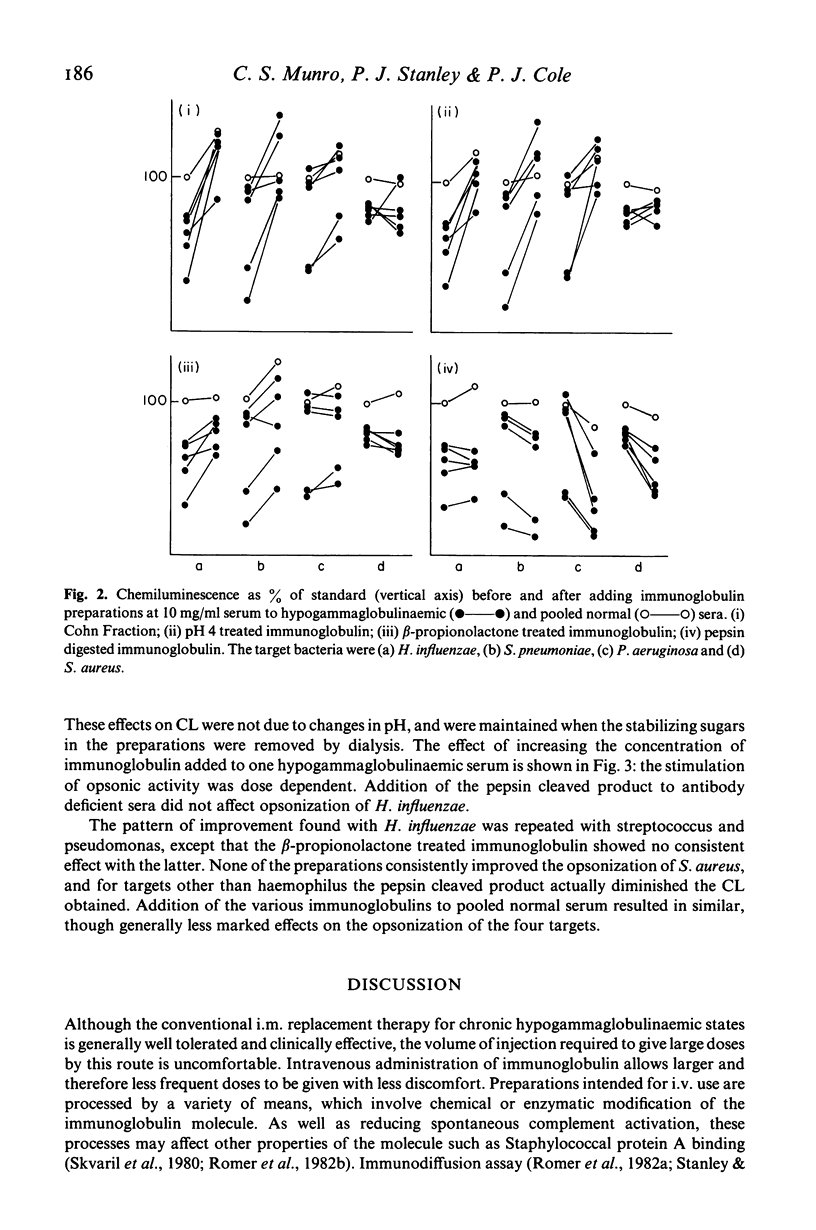
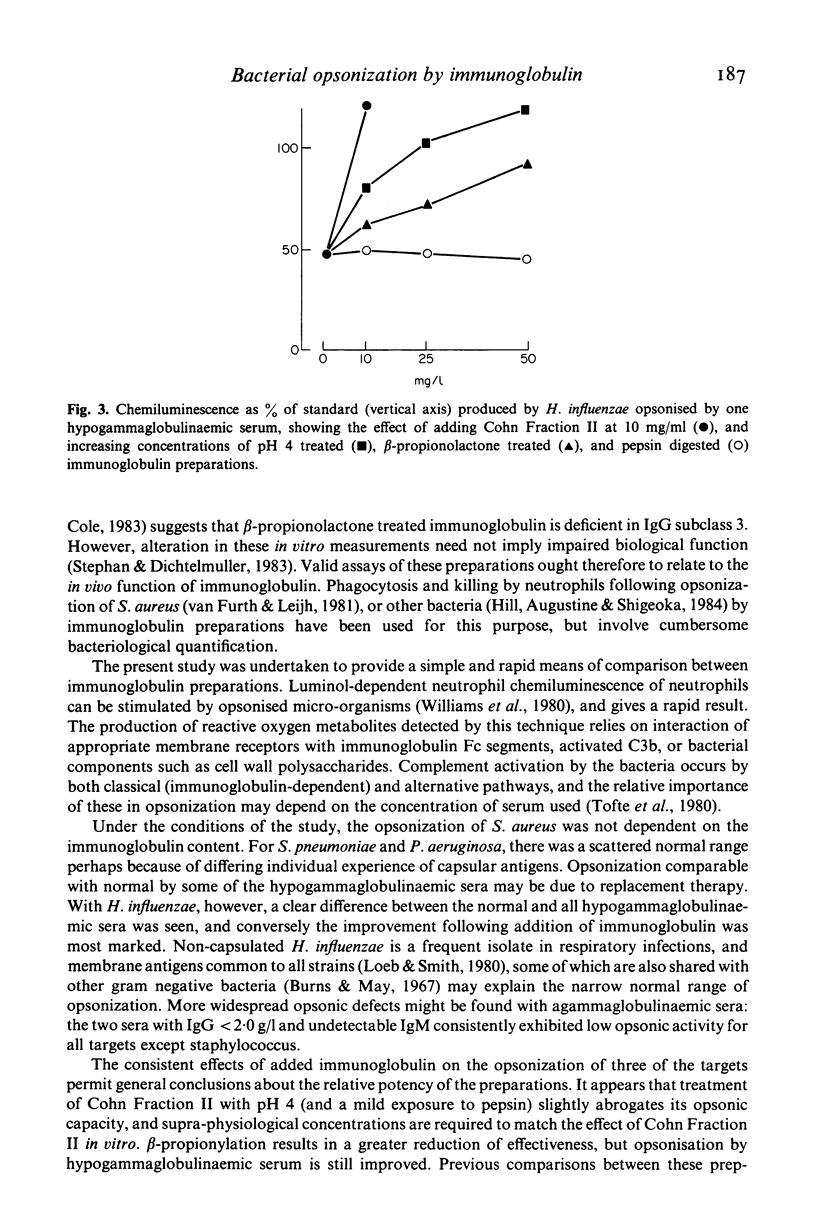
We have used the ability of opsonised bacteria to stimulate luminol enhanced chemiluminescence of human neutrophils to examine the opsonic capabilities of normal and hypogammaglobulinaemic sera for four common bacterial pathogens. Preparations of human immunoglobulin modified for i.v. use have then been compared with unmodified Cohn Fraction II for their effectiveness in improving opsonization when added to antibody deficient sera in vitro. Hypogammaglobulinaemic sera exhibited impaired opsonisation of Haemophilus influenzae, and severely antibody deficient sera also opsonized Streptococcus pneumoniae and Pseudomonas aeruginosa poorly. The opsonization of these organisms was improved by Cohn Fraction II, and by pH 4 and beta-propionolactone treated immunoglobulins, in descending order of effectiveness. Pepsin digested immunoglobulin was inactive, and in some cases impaired opsonic capacity. The opsonisation of Staphylococcus aureus by hypogammaglobulinaemic sera was near normal, and was not improved by any immunoglobulin. This technique, which assesses biological activity of immunoglobulin, is useful in comparing preparations, and may help to establish appropriate dosage and frequency for intravenous immunoglobulin replacement therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving B. M., Tankersley D. L., Mason B. L., Rossi F., Aronson D. L., Finlayson J. S. Contact-activated factors: contaminants of immunoglobulins preparations with coagulant and vasoactive properties. J Lab Clin Med. 1980 Aug;96(2):334–346. [PubMed] [Google Scholar]
- BARANDUN S., KISTLER P., JEUNET F., ISLIKER H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–174. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Burns M. W., May J. R. Haemophilus influenzae precipitins in the serum of patients with chronic bronchial disorders. Lancet. 1967 Feb 18;1(7486):354–358. doi: 10.1016/s0140-6736(67)92895-4. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Augustine N. H., Shigeoka A. O. Comparative opsonic activity of intravenous gamma globulin preparations for common bacterial pathogens. Am J Med. 1984 Mar 30;76(3A):61–66. doi: 10.1016/0002-9343(84)90321-8. [DOI] [PubMed] [Google Scholar]
- Lever A. M., Webster A. D., Brown D., Thomas H. C. Non-A, non-B hepatitis occurring in agammaglobulinaemic patients after intravenous immunoglobulin. Lancet. 1984 Nov 10;2(8411):1062–1064. doi: 10.1016/s0140-6736(84)91506-x. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Römer J., Morgenthaler J. J., Scherz R., Skvaril F. Characterization of various immunoglobulin preparations for intravenous application. I. Protein composition and antibody content. Vox Sang. 1982 Feb;42(2):62–73. doi: 10.1159/000460850. [DOI] [PubMed] [Google Scholar]
- Römer J., Späth P. J., Skvaril F., Nydegger U. E. Characterization of various immunoglobulin preparations for intravenous application. II. Complement activation and binding to staphylococcus protein A. Vox Sang. 1982 Feb;42(2):74–80. doi: 10.1159/000460851. [DOI] [PubMed] [Google Scholar]
- Skvaril F., Roth-Wicky B., Barandun S. IgG subclasses in human gamma-globulin preparations for intravenous use and their reactivity with staphylococcus protein A. Vox Sang. 1980;38(3):147–155. doi: 10.1111/j.1423-0410.1980.tb02342.x. [DOI] [PubMed] [Google Scholar]
- So A., Brenner M. K., Hill I. D., Asherson G. L., Webster A. D. Intravenous gammaglobulin treatment in patients with hypogammaglobulinaemia. Br Med J (Clin Res Ed) 1984 Nov 3;289(6453):1177–1178. doi: 10.1136/bmj.289.6453.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Cole P. Intravenous immunoglobulin preparations. Lancet. 1983 Apr 9;1(8328):829–829. doi: 10.1016/s0140-6736(83)91896-2. [DOI] [PubMed] [Google Scholar]
- Stephan W., Dichtelmüller H. Intravenous immunoglobulin preparations. Lancet. 1983 May 14;1(8333):1111–1111. doi: 10.1016/s0140-6736(83)91953-0. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Kim Y., Quie P. G. Influence of serum concentration on opsonization by the classical and alternative complement pathways. Infect Immun. 1980 Feb;27(2):693–696. doi: 10.1128/iai.27.2.693-696.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Hastings M. J., Easmon C. S., Cole P. J. Factor affecting the in vitro assessment of opsonization: a study of the kinetics of opsonization using the technique of phagocytic chemiluminescence. Immunology. 1980 Dec;41(4):903–911. [PMC free article] [PubMed] [Google Scholar]


