Abstract
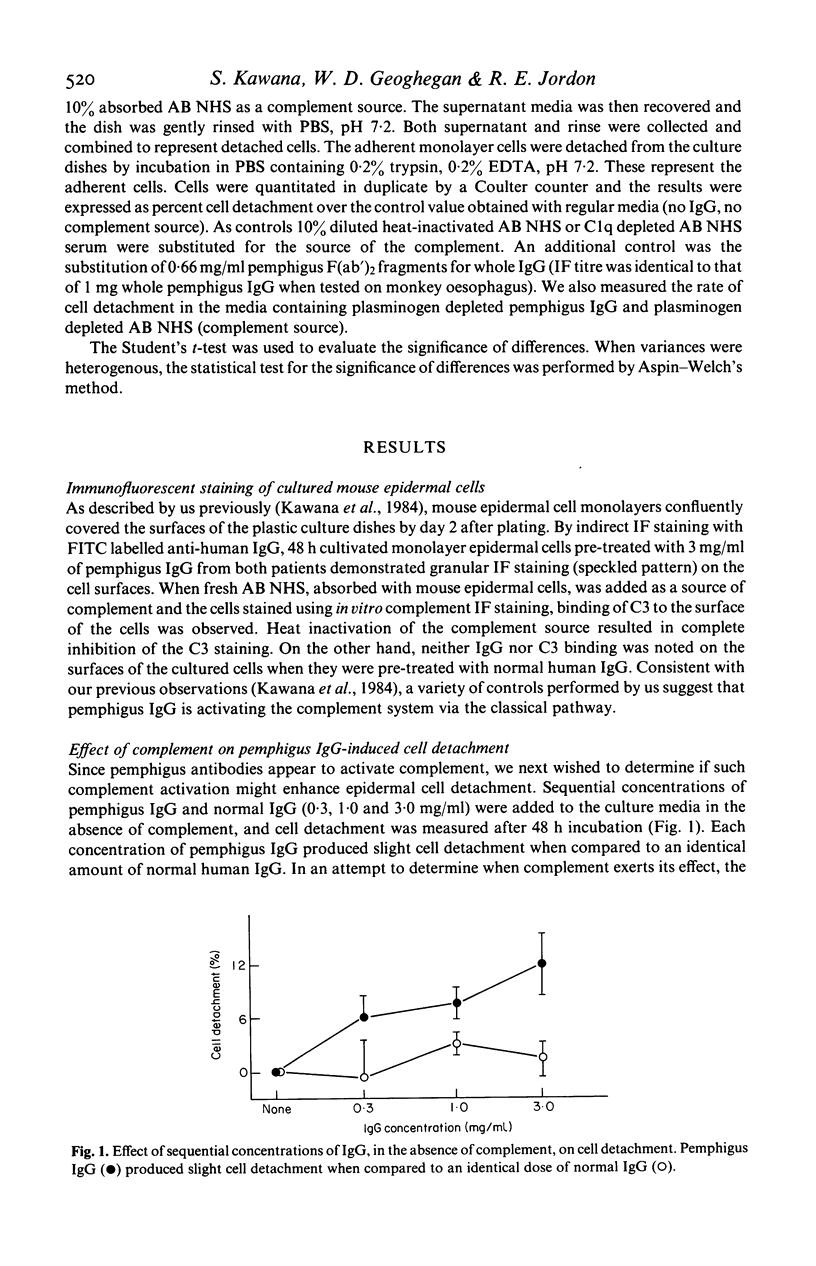
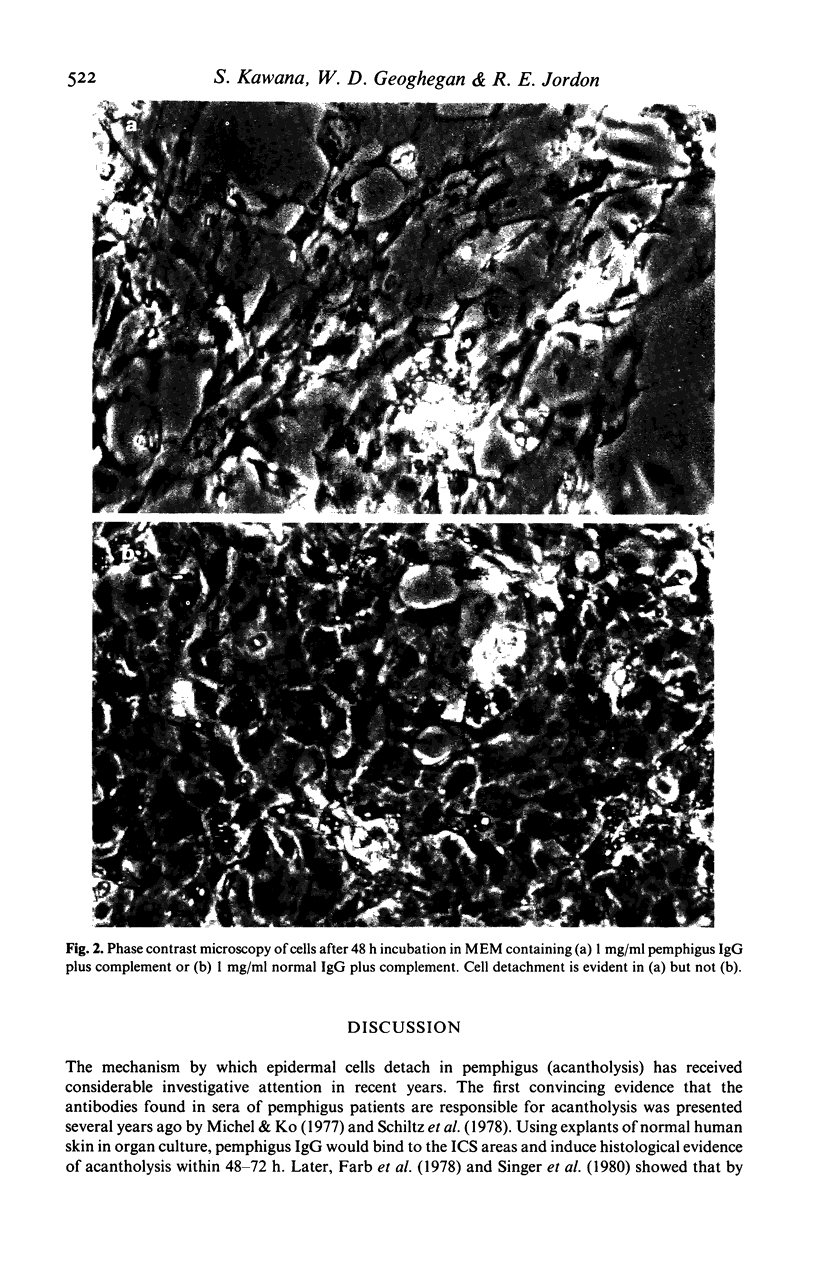
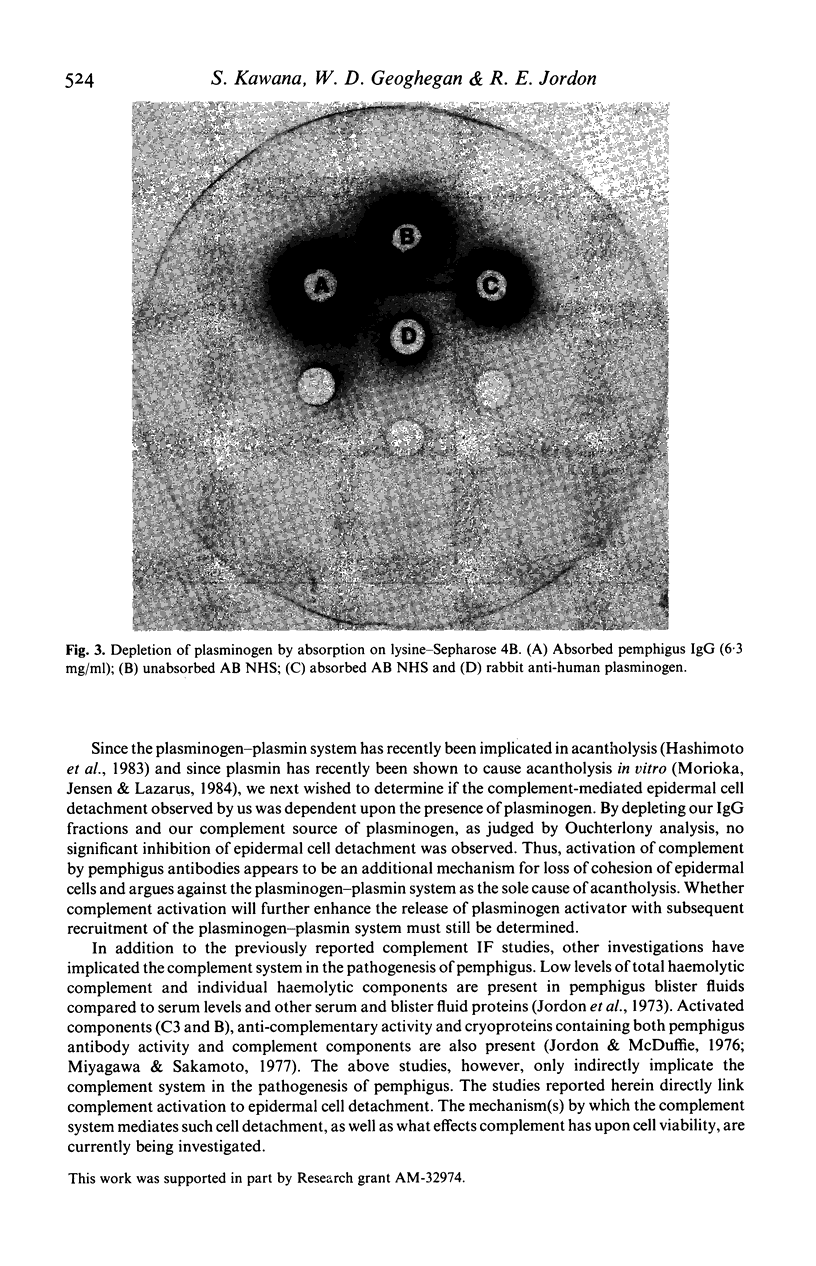
We have previously demonstrated that pemphigus antibodies will fix complement to organ and tissue cultured epidermal cells in vitro. In the present study, we sought to determine the role complement plays in the detachment of cultured murine epidermal cells by pemphigus antibody. Forty-eight hour cultivated epidermal monolayers from neonatal BALB/c mice were treated with purified IgG fractions of pemphigus sera in the presence or absence of complement. In the absence of complement, pemphigus IgG produced slight cell detachment when compared to an identical amount of normal IgG. When cells were maintained in media with pemphigus IgG plus complement for 48 h, the cell detachment was significantly higher than that obtained with pemphigus IgG alone, or with normal IgG plus complement. Heat inactivation or Clq depletion of the complement source resulted in complete inhibition of cell detachment. When pemphigus F(ab')2 plus complement was used instead of whole pemphigus IgG plus complement, the cell detachment rate was again lowered significantly. Both pemphigus IgG and the complement source were depleted of plasminogen by passage over a lysine-Sepharose 4B column, and tested for their effects on cell detachment depleted of plasminogen. Depletion of plasminogen failed to inhibit cell detachment induced by pemphigus IgG and complement. These results suggest that complement activated by pemphigus antibody binding to the epidermal cell surface induces epidermal cell detachment and would argue against the plasminogen-plasmin system as the sole cause of cell detachment in pemphigus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTNER E. H., JORDON R. E. DEMONSTRATION OF SKIN ANTIBODIES IN SERA OF PEMPHIGUS VULGARIS PATIENTS BY INDIRECT IMMUNOFLUORESCENT STAINING. Proc Soc Exp Biol Med. 1964 Nov;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Gordon J. M., Reddick M. E., Eisen A. Z. Quantitation and immunocytochemical localization of human skin collagenase in basal cell carcinoma. J Invest Dermatol. 1977 Oct;69(4):363–367. doi: 10.1111/1523-1747.ep12510240. [DOI] [PubMed] [Google Scholar]
- Cram D. L., Fukuyama K. Immunohistochemistry of ultraviolet-induced pemphigus and pemphigoid lesions. Arch Dermatol. 1972 Dec;106(6):819–824. [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Diaz L. A., Marcelo C. L. Pemphigoid and pemphigus antigens in cultured epidermal cells. Br J Dermatol. 1978 Jun;98(6):631–637. doi: 10.1111/j.1365-2133.1978.tb03581.x. [DOI] [PubMed] [Google Scholar]
- Farb R. M., Dykes R., Lazarus G. S. Anti-epidermal-cell-surface pemphigus antibody detaches viable epidermal cells from culture plates by activation of proteinase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):459–463. doi: 10.1073/pnas.75.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Shafran K. M., Webber P. S., Lazarus G. S., Singer K. H. Anti-cell surface pemphigus autoantibody stimulates plasminogen activator activity of human epidermal cells. A mechanism for the loss of epidermal cohesion and blister formation. J Exp Med. 1983 Jan 1;157(1):259–272. doi: 10.1084/jem.157.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Sugiura M., Kurihara S., Nishikawa T. In vitro complement activation by intercellular antibodies. J Invest Dermatol. 1982 Apr;78(4):316–318. doi: 10.1111/1523-1747.ep12507396. [DOI] [PubMed] [Google Scholar]
- Jordan R. E., Day N. K., Luckasen J. R., Good R. A. Complement activation in pemphigus vulgaris blister fluid. Clin Exp Immunol. 1973 Sep;15(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- Jordon R. E., McDuffie F. C. Serum and blister fluid anticomplementary activity in pemphigus and bullous pemphigoid. Sucrose density gradient studies. Proc Soc Exp Biol Med. 1976 Mar;151(3):594–598. doi: 10.3181/00379727-151-39267. [DOI] [PubMed] [Google Scholar]
- Jordon R. E., Sams W. M., Jr, Diaz G., Beutner E. H. Negative complement immunofluorescence in pemphigus. J Invest Dermatol. 1971 Dec;57(6):407–410. doi: 10.1111/1523-1747.ep12293273. [DOI] [PubMed] [Google Scholar]
- Jordon R. E., Schroeter A. L., Rogers R. S., 3rd, Perry H. O. Classical and alternate pathway activation of complement in pemphigus vulgaris lesions. J Invest Dermatol. 1974 Sep;63(3):256–259. doi: 10.1111/1523-1747.ep12680098. [DOI] [PubMed] [Google Scholar]
- Kawana S., Janson M., Jordon R. E. Complement fixation by pemphigus antibody. I. In vitro fixation to organ and tissue culture skin. J Invest Dermatol. 1984 May;82(5):506–510. doi: 10.1111/1523-1747.ep12261058. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Kolb L. M., Podack E. R. C1q: isolation from human serum in high yield by affinity chromatography and development of a highly sensitive hemolytic assay. J Immunol. 1979 May;122(5):2103–2111. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Michel B., Ko C. S. An organ culture model for the study of pemphigus acantholysis. Br J Dermatol. 1977 Mar;96(3):295–302. doi: 10.1111/j.1365-2133.1977.tb06141.x. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Kurihara S., Harada T., Sugawara M., Hatano H. Capability of complement fixation of pemphigus antibodies in vitro. Arch Dermatol Res. 1977 Oct 27;260(1):1–6. doi: 10.1007/BF00558008. [DOI] [PubMed] [Google Scholar]
- Patel H., Marcelo C., Voorhees J. J., Diaz L. A. In vitro alterations of epidermal cell adhesion induced by temperature, substrate, and cations. J Invest Dermatol. 1981 Jun;76(6):474–479. doi: 10.1111/1523-1747.ep12521148. [DOI] [PubMed] [Google Scholar]
- Pemphigus: current concepts. Ann Intern Med. 1980 Mar;92(3):396–405. doi: 10.7326/0003-4819-92-3-396. [DOI] [PubMed] [Google Scholar]
- Pochi P. E. Endocrinology of acne. J Invest Dermatol. 1983 Jul;81(1):1–1. doi: 10.1111/1523-1747.ep12537361. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Schur P. H. Studies of the antibodies in pemphigoid and pemphigus. J Lab Clin Med. 1973 Aug;82(2):249–254. [PubMed] [Google Scholar]
- Schiltz J. R., Michel B., Papay R. Pemphigus antibody interaction with human epidermal cells in culture. J Clin Invest. 1978 Oct;62(4):778–788. doi: 10.1172/JCI109189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer K. H., Sawka N. J., Samowitz H. R., Lazarus G. S. Proteinase activation: a mechanism for cellular dyshesion in pemphigus. J Invest Dermatol. 1980 May;74(5):363–367. doi: 10.1111/1523-1747.ep12543780. [DOI] [PubMed] [Google Scholar]
- van Joost T., Cormane R. H., Pondman K. W. Direct immunofluorescent study of the skin on occurrence of complement in pemphigus. Br J Dermatol. 1972 Nov;87(5):466–474. doi: 10.1111/j.1365-2133.1972.tb01595.x. [DOI] [PubMed] [Google Scholar]




