Abstract
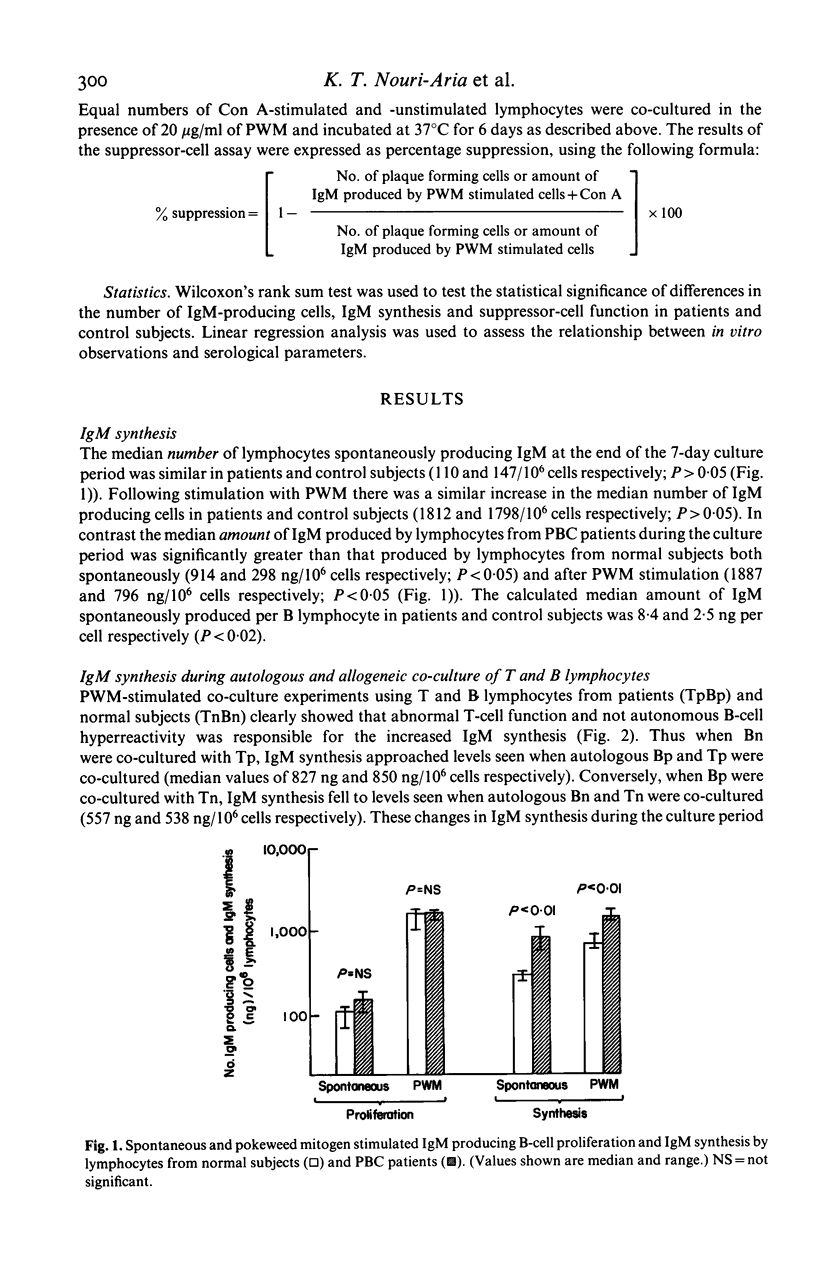
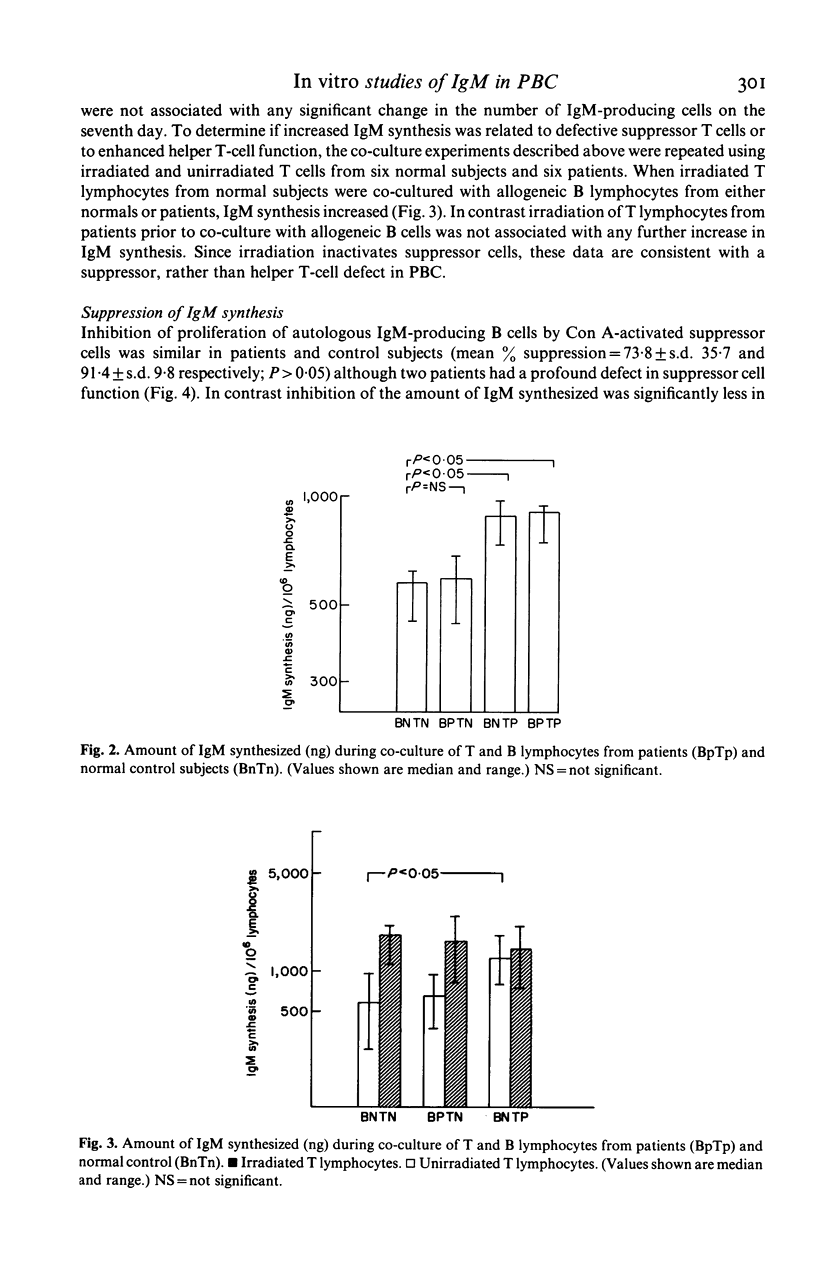
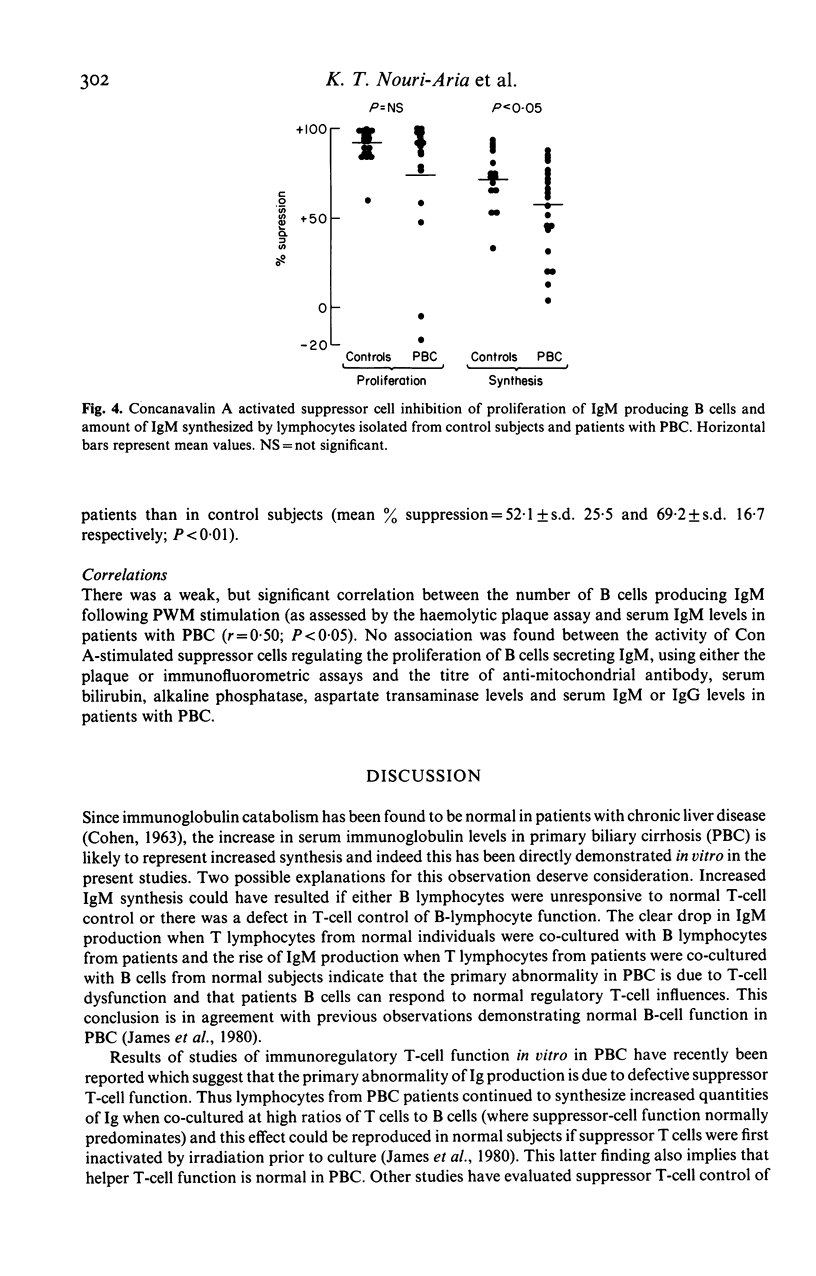
To evaluate the mechanisms underlying the increase in serum IgM in primary biliary cirrhosis (PBC) studies were designed to examine IgM production in vitro and to assess the relative contribution of intrinsic B cell activity and immunoregulatory T cell balance to IgM synthesis. The number of peripheral blood lymphocytes (PBL) producing IgM (spontaneous and pokeweed mitogen (PWM) stimulated) at the end of a seven day culture period was similar in PBC patients and control subjects while the amount of IgM synthesized (spontaneous and PWM stimulated) during this period was significantly greater in the patient group, implying that the amount of IgM produced per B cell was increased in PBC. Co-culture of autologous and allogeneic T and B lymphocytes and irradiation of T lymphocytes from patients and normal subjects clearly implicated abnormal suppressor T cell function, rather than autonomous B cell hyperactivity, as the cause of the increased IgM synthesis. Direct studies of T cell function indicated that although concanavalin A (Con A) activated suppressor cells inhibited proliferation of IgM producing B cells in the majority of PBC patients, they were unable to inhibit IgM synthesis. The demonstration of a disparity between IgM synthesis and the proliferation of IgM-producing B cells, together with the observation that the abnormality of T cell function is largely confined to the control of IgM secretion, is consistent with the presence of at least two different suppressor subpopulations regulating IgM production. In PBC the main suppressor cell abnormality seems to affect regulation of IgM secretion rather than B cell proliferation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- COHEN S. GAMMA-GLOBULIN METABOLISM. Br Med Bull. 1963 Sep;19:202–206. doi: 10.1093/oxfordjournals.bmb.a070057. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Weake J. R., Wands J. R. Abnormalities of mononuclear cell regulation in vitro in primary biliary cirrhosis. Liver. 1981 Sep;1(3):230–243. doi: 10.1111/j.1600-0676.1981.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Doniach D., Roitt I. M., Walker J. G., Sherlock S. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other liver diseases and their clinical implications. Clin Exp Immunol. 1966 Jul;1(3):237–262. [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Shore A., Green B., Lin M. T., Dosch H. M. The E-rosette assay: a cautionary note. Clin Immunol Immunopathol. 1979 Jan;12(1):119–123. doi: 10.1016/0090-1229(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Bird A. G., Britton S., Smith C. I. Pokeweed mitogen induced differentiation of human B cells: evaluation by a protein A haemolytic plaque assay. Immunology. 1979 Sep;38(1):181–189. [PMC free article] [PubMed] [Google Scholar]
- James S. P., Elson C. O., Jones E. A., Strober W. Abnormal regulation of immunoglobulin synthesis in vitro in primary biliary cirrhosis. Gastroenterology. 1980 Aug;79(2):242–254. [PubMed] [Google Scholar]
- James S. P., Vierling J. M., Strober W. The role of the immune response in the pathogenesis of primary biliary cirrhosis. Semin Liver Dis. 1981 Nov;1(4):322–337. doi: 10.1055/s-2008-1040735. [DOI] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. II. Pokeweed mitogen-responsive B cells belong to a surface immunoglobulin D-negative subpopulation. J Exp Med. 1982 May 1;155(5):1561–1566. doi: 10.1084/jem.155.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- McFarlane I. G., Wojcicka B. M., Tsantoulas D. C., Portmann B. C., Eddleston A. L., Williams R. Leukocyte migration inhibition in response to biliary antigens in primary biliary cirrhosis, sclerosing cholangitis, and other chronic liver diseases. Gastroenterology. 1979 Jun;76(6):1333–1340. [PubMed] [Google Scholar]
- Nouri-Aria K. T., Hegarty J. E., Alexander G. J., Eddleston A. L., Williams R. Effect of corticosteroids on suppressor-cell activity in "autoimmune" and viral chronic active hepatitis. N Engl J Med. 1982 Nov 18;307(21):1301–1304. doi: 10.1056/NEJM198211183072102. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Schaffner F., Popper H. Clinical-pathologic relations in primary biliary cirrhosis. Prog Liver Dis. 1982;7:529–554. [PubMed] [Google Scholar]
- Schwartz S. A. Heavy chain-specific suppression of immunoglobulin synthesis and secretion by lymphocytes from patients with selective IgA deficiency. J Immunol. 1980 Apr;124(4):2034–2041. [PubMed] [Google Scholar]
- Sherlock S., Scheuer P. J. The presentation and diagnosis of 100 patients with primary biliary cirrhosis. N Engl J Med. 1973 Sep 27;289(13):674–678. doi: 10.1056/NEJM197309272891306. [DOI] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., Holden R., Jones J. V., Peacock D. B. Immune response to phi X 174 in man. 5. Primary and secondary antibody production in primary biliary cirrhosis. Gut. 1976 Nov;17(11):844–848. doi: 10.1136/gut.17.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsantoulas D., Perperas A., Portmann B., Eddleston A. L., Williams R. Antibodies to a human liver membrane lipoprotein (LSP) in primary biliary cirrhosis. Gut. 1980 Jul;21(7):557–560. doi: 10.1136/gut.21.7.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling J. M., Nelson D. L., Strober W., Bundy D. M., Jones E. A. In vitro cell-mediated cytotoxicity in primary biliary cirrhosis and chronic hepatitis. Dysfunction of spontaneous cell-mediated cytotoxicity in primary biliary cirrhosis. J Clin Invest. 1977 Nov;60(5):1116–1128. doi: 10.1172/JCI108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands J. R., Dienstag J. L., Bhan A. K., Feller E. R., Isselbacher K. J. Circulating immune complexes and complement activation in primary biliary cirrhosis. N Engl J Med. 1978 Feb 2;298(5):233–237. doi: 10.1056/NEJM197802022980502. [DOI] [PubMed] [Google Scholar]


