Abstract
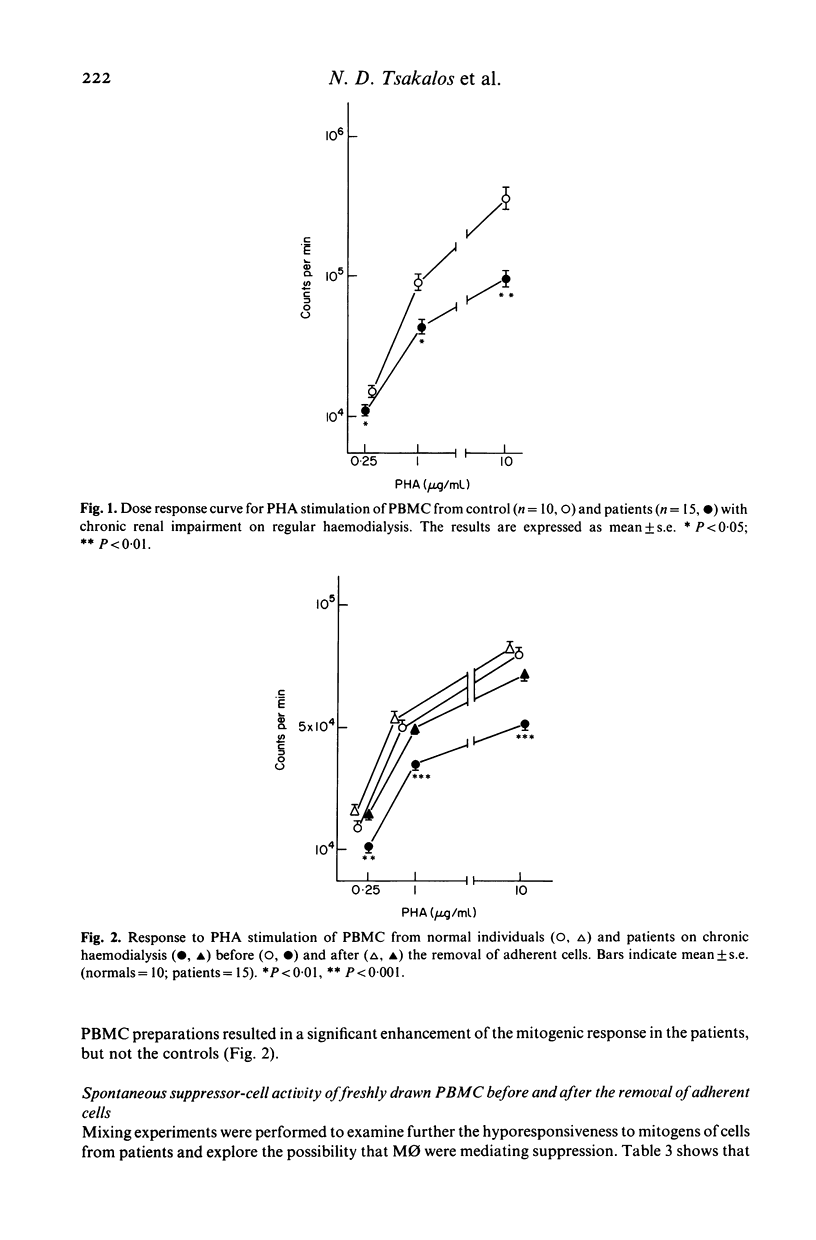
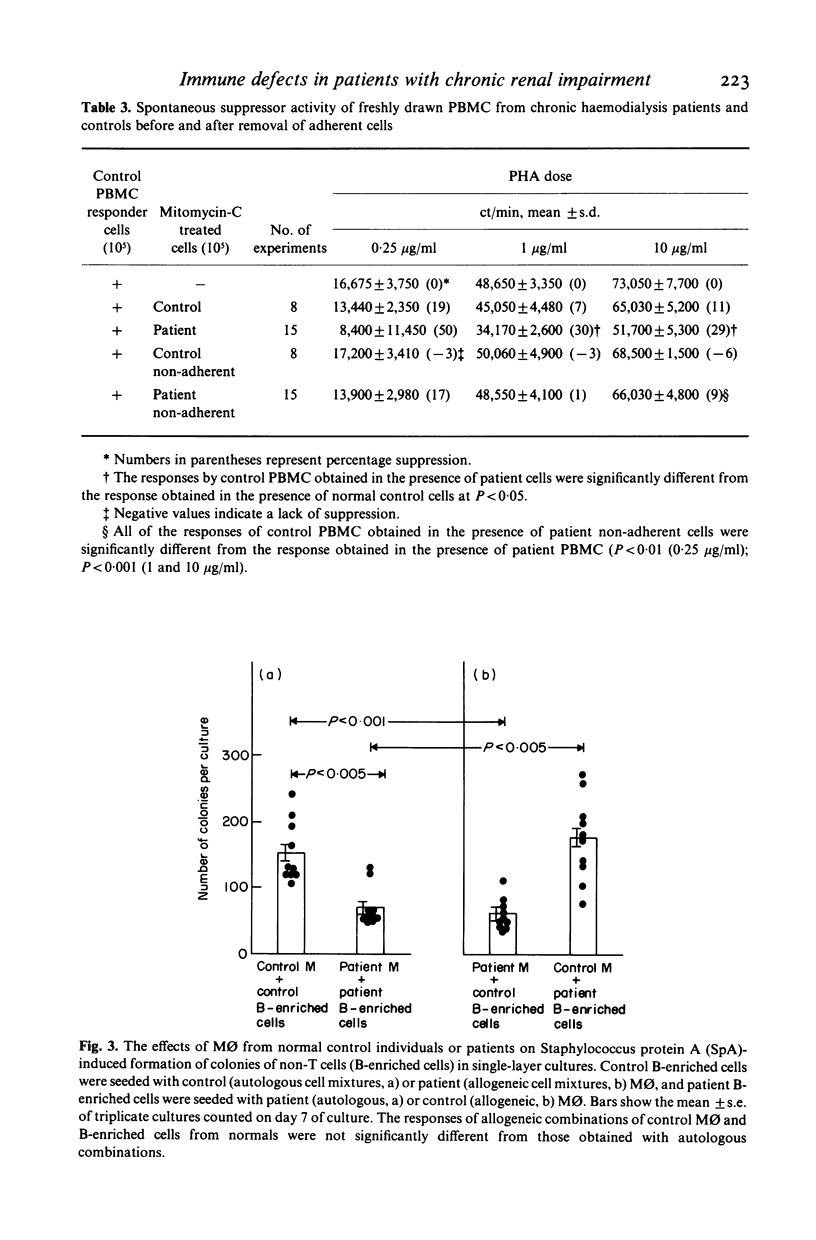
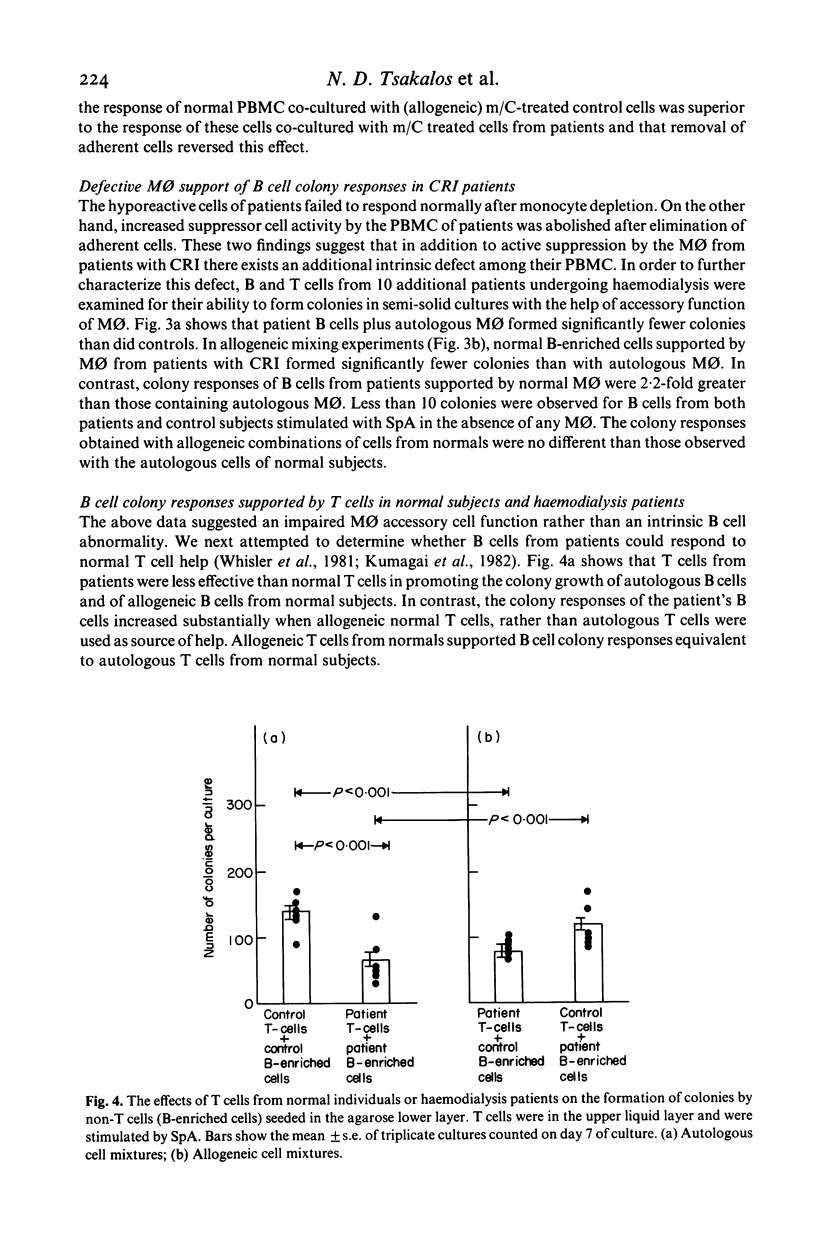
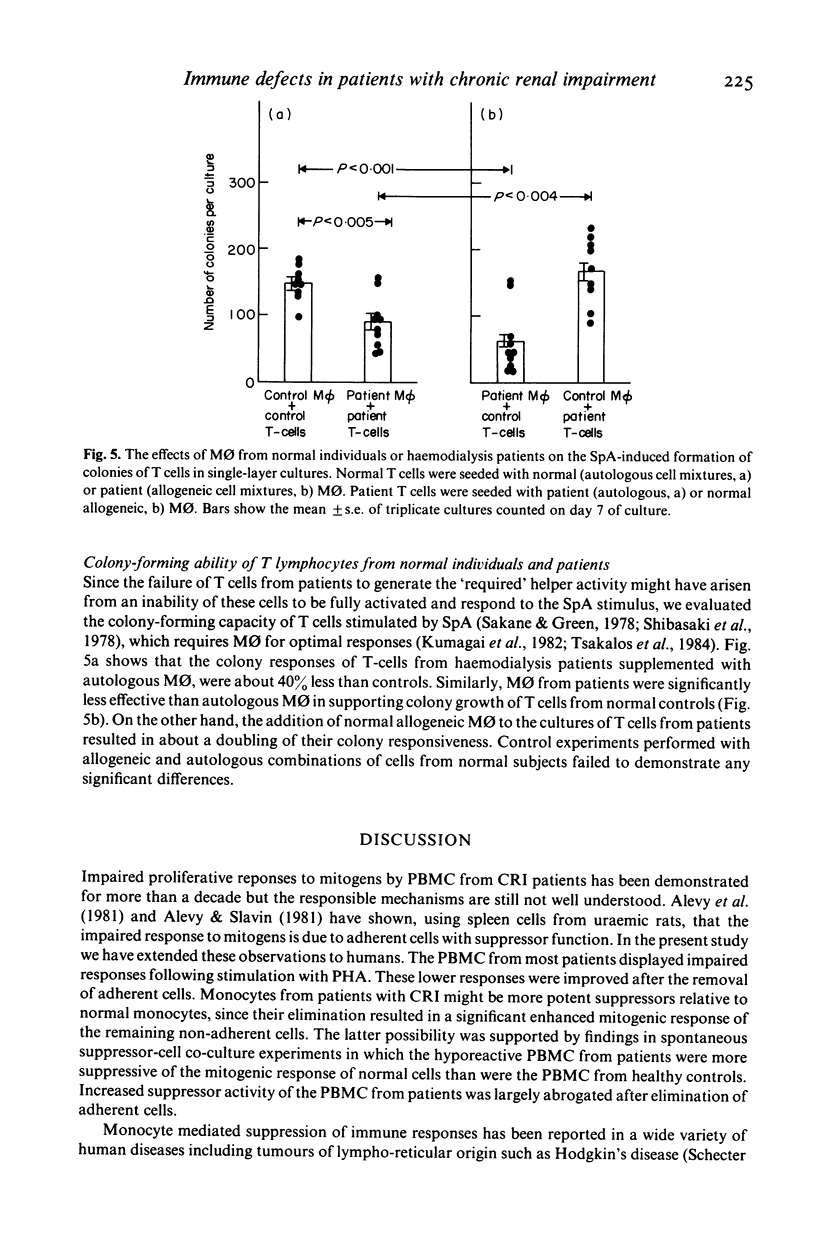
Cellular mechanisms contributing to impaired lymphocyte proliferative responses in chronic renal impairment (CRI) were investigated using peripheral blood mononuclear cells (PBMC) from 25 patients receiving haemodialysis. Impaired T cell proliferative responses to phytohaemagglutinin were demonstrated. The hyporeactive PBMC from patients with CRI suppressed the responses of PBMC from normals to a greater degree than did control PBMC. This immunosuppression was reversed significantly by depleting adherent monocytes (M phi). To further determine if these impairments might be critically dependent on cell-cell contact, M phi from an additional 10 patients on haemodialysis were examined for ability to support B and T cell colony formation in semi-solid cultures stimulated by Staphylococcus protein A (SpA). When compared to normal controls, significantly fewer B and T cell colonies were observed with M phi from CRI patients than when autologous M phi were used. Also, T cells from patients were significantly less effective than controls in supporting B cell colony growth. Decreased T and B cell colony responses in patients were not due to a primary abnormality of these cells, since allogeneic mixing experiments showed that B and T cells from patients were able to form a sufficient number of colonies when control M phi or T cells from normals were used as accessory and helper cells. These findings suggest that although M phi-mediated suppressor activity is an important mechanism contributing to impaired lymphocyte responsiveness in patients with chronic renal impairment on haemodialysis, additional or related abnormalities in M phi 'accessory' function may also exist.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alevy Y. G., Slavin R. G., Hutcheson P. Immune response in experimentally induced uremia. I. Suppression of mitogen responses by adherent cells in chronic uremia. Clin Immunol Immunopathol. 1981 Apr;19(1):8–18. doi: 10.1016/0090-1229(81)90043-x. [DOI] [PubMed] [Google Scholar]
- Alevy Y. G., Slavin R. G. Immune response in experimentally induced uremia. II. Suppression of PHA response in uremia is mediated by an adherent, Ia-negative and indomethacin-insensitive suppressor cell. J Immunol. 1981 May;126(5):2007–2010. [PubMed] [Google Scholar]
- Beer D. J., Osband M. E., McCaffrey R. P., Soter N. A., Rocklin R. E. Abnormal histamine-induced suppressor-cell function in atopic subjects. N Engl J Med. 1982 Feb 25;306(8):454–458. doi: 10.1056/NEJM198202253060804. [DOI] [PubMed] [Google Scholar]
- Bobak D., Whisler R. Human B lymphocyte colony responses. I. General characteristics and modulation by monocytes. J Immunol. 1980 Dec;125(6):2764–2769. [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelstein H. Immune system to uremia. Nephron. 1976;17(6):409–414. doi: 10.1159/000180749. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Johnson C. The regulation of T cell responses by spontaneously active suppressor cells. Clin Exp Immunol. 1982 Nov;50(2):406–415. [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Johnson C. The use of concanavalin A to study the immunoregulation of human T cells. Clin Exp Immunol. 1981 Nov;46(2):237–249. [PMC free article] [PubMed] [Google Scholar]
- Elves M. W., Israëls M. C., Collinge M. An assessment of the mixed leucocyte reaction in renal failure. Lancet. 1966 Mar 26;1(7439):682–685. doi: 10.1016/s0140-6736(66)91628-x. [DOI] [PubMed] [Google Scholar]
- Katz P., Fauci A. S. Inhibition of polyclonal B-cell activation by suppressor monocytes in patients with sarcoidosis. Clin Exp Immunol. 1978 Jun;32(3):554–562. [PMC free article] [PubMed] [Google Scholar]
- Kumagai S., Sredni B., House S., Steinberg A. D., Green I. Defective regulation of B lymphocyte colony formation in patients with systemic lupus erythematosus. J Immunol. 1982 Jan;128(1):258–262. [PubMed] [Google Scholar]
- Markenson J. A., Morgan J. W., Lockshin M. D., Joachim C., Winfield J. B. Responses of fractionated cells from patients with systemic lupus erythematosus and normals to plant mitogen: evidence for a suppressor population of monocytes. Proc Soc Exp Biol Med. 1978 May;158(1):5–9. doi: 10.3181/00379727-158-40127. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Kishimoto T., Kuritani T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. VII. Effect of anti-mu and anti-delta antibodies on B colony formation and detection of abnormal B cells in patients with juvenile rheumatoid arthritis. J Immunol. 1980 Dec;125(6):2638–2642. [PubMed] [Google Scholar]
- Newberry W. M., Sanford J. P. Defective cellular immunity in renal failure: depression of reactivity of lymphocytes to phytohemagglutinin by renal failure serum. J Clin Invest. 1971 Jun;50(6):1262–1271. doi: 10.1172/JCI106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadracci L. J., Ringdén O., Krzymanski M. The effect of uremia and transplantation on lymphocyte subpopulations. Kidney Int. 1976 Aug;10(2):179–184. doi: 10.1038/ki.1976.93. [DOI] [PubMed] [Google Scholar]
- RIIS P., STOUGAARD J. The peripheral blood leukocytes in chronic renal insufficiency. Dan Med Bull. 1959 May;6(3):85–90. [PubMed] [Google Scholar]
- Radnay J., Goldman I., Rozenszajn L. A. Growth of human B-lymphocyte colonies in vitro. Nature. 1979 Mar 22;278(5702):351–353. doi: 10.1038/278351a0. [DOI] [PubMed] [Google Scholar]
- Raskova J., Morrison A. B. A decrease in cell-mediated immunity in uremia associated with an increase in activity of suppressor cells. Am J Pathol. 1976 Jul;84(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Schechter G. P., Soehnlen F. Monocyte-mediated inhibition of lymphocyte blastogenesis in Hodgkin disease. Blood. 1978 Aug;52(2):261–271. [PubMed] [Google Scholar]
- Shibasaki M., Nemoto H., Suzuki S., Kuroume T. Induction of lymphocyte colony formation in vitro by protein A. J Immunol. 1978 Dec;121(6):2278–2281. [PubMed] [Google Scholar]
- Slavin R. G., Fitch C. D. Inhibition of lymphocyte transformation by guanidinosuccinic acid, a surplus metabolite in uremia. Experientia. 1971;27(11):1340–1341. doi: 10.1007/BF02136727. [DOI] [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Touraine J. L., Touraine F., Revillard J. P., Brochier J., Traeger J. T-lymphocytes and serum inhibitors of cell-mediated immunity in renal insufficiency. Nephron. 1975;14(2):195–208. doi: 10.1159/000180448. [DOI] [PubMed] [Google Scholar]
- Tsakalos N. D., Lachman L. B., Newhouse Y. G., Whisler R. L. Lipopolysaccharide (LPS) modulation of human monocyte accessory cell function in promoting T-cell colonies: inability of LPS and IL-1 to abrogate the need for monocytes with high HLA-DR expression. Cell Immunol. 1984 Feb;83(2):229–241. doi: 10.1016/0008-8749(84)90302-2. [DOI] [PubMed] [Google Scholar]
- Whisler R. L., Bobak D. A., Newhouse Y. G. Human B lymphocyte colony responses. II. The role of T cells in the enhancement of colony growth. J Immunol. 1981 Nov;127(5):1758–1762. [PubMed] [Google Scholar]
- Zembala M., Mytar B., Popiela T., Asherson G. L. Depressed in vitro peripheral blood lymphocyte response to mitogens in cancer patients: the role of suppressor cells. Int J Cancer. 1977 May 15;19(5):605–613. doi: 10.1002/ijc.2910190503. [DOI] [PubMed] [Google Scholar]


