Abstract
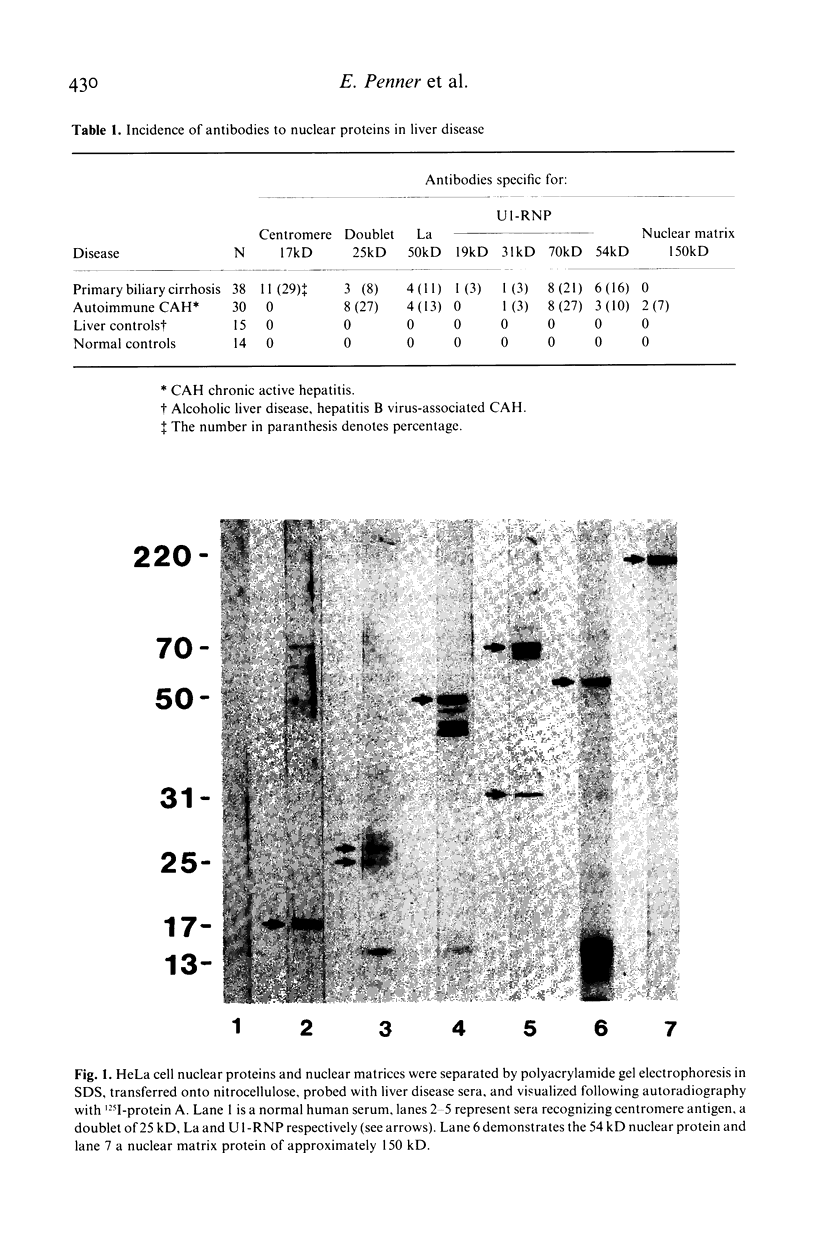
Nuclear and nuclear matrix proteins of HeLa cells were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and subsequently transferred onto nitrocellulose. Antibodies present in sera of patients with primary biliary cirrhosis and autoimmune chronic active hepatitis reacted with some of the blotted proteins. The antibodies were mainly directed against chromatin-associated proteins and protein constituents of discrete RNP particles. In addition, antibodies found in autoimmune liver disease sera detected a hitherto undescribed nuclear protein of 54 kD, and a nuclear matrix protein of approximately 150 kD. Antibodies recognizing a nuclear 25 kD doublet apparently constituted a marker antibody for autoimmune liver disease. Those directed at the 17 kD centromere protein were associated with the primary biliary cirrhosis-related CREST syndrome, while those recognizing La antigen were related to cases of sicca syndrome associated with autoimmune liver diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein R. M., Callender M. E., Neuberger J. M., Hughes G. R., Williams R. Anticentromere antibody in primary biliary cirrhosis. Ann Rheum Dis. 1982 Dec;41(6):612–614. doi: 10.1136/ard.41.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. M., Neuberger J. M., Bunn C. C., Callender M. E., Hughes G. R., Williams R. Diversity of autoantibodies in primary biliary cirrhosis and chronic active hepatitis. Clin Exp Immunol. 1984 Mar;55(3):553–560. [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Douvas A. S., Achten M., Tan E. M. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem. 1979 Oct 25;254(20):10514–10522. [PubMed] [Google Scholar]
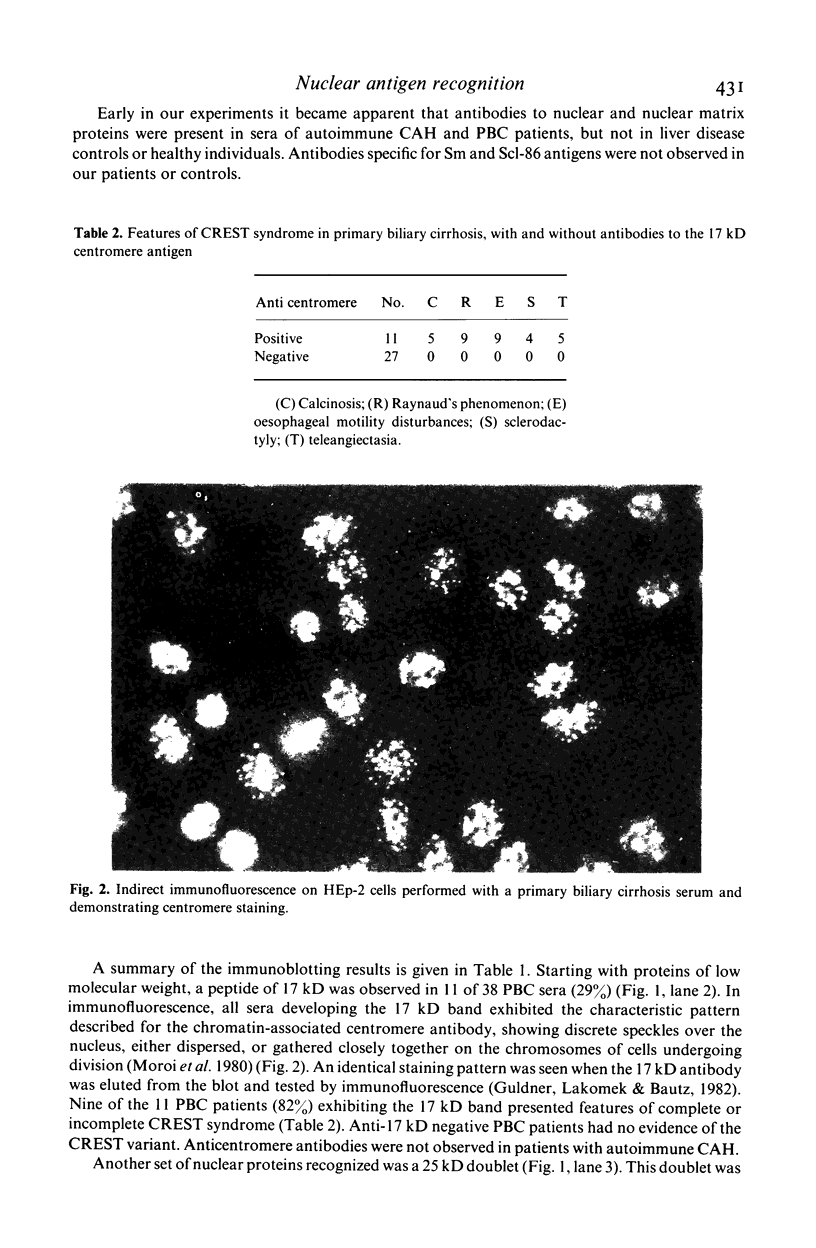
- Guldner H. H., Lakomek H. J., Bautz F. A. Human anti-centromere sera recognise a 19.5 kD non-histone chromosomal protein from HeLa cells. Clin Exp Immunol. 1984 Oct;58(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Guldner H. H., Lakomek H. J., Bautz F. A. Identification of human Sm and (U1) RNP antigens by immunoblotting. J Immunol Methods. 1983 Nov 11;64(1-2):45–59. doi: 10.1016/0022-1759(83)90383-6. [DOI] [PubMed] [Google Scholar]
- Habets W. J., de Rooij D. J., Salden M. H., Verhagen A. P., van Eekelen C. A., van de Putte L. B., van Venrooij W. J. Antibodies against distinct nuclear matrix proteins are characteristic for mixed connective tissue disease. Clin Exp Immunol. 1983 Oct;54(1):265–276. [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., den Brok J. H., Boerbooms A. M., van de Putte L. B., van Venrooij W. J. Characterization of the SS-B (La) antigen in adenovirus-infected and uninfected HeLa cells. EMBO J. 1983;2(10):1625–1631. doi: 10.1002/j.1460-2075.1983.tb01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge L. D., Mancini P., Davis F. M., Heywood P. Nuclear matrix of HeLa S3 cells. Polypeptide composition during adenovirus infection and in phases of the cell cycle. J Cell Biol. 1977 Jan;72(1):194–208. doi: 10.1083/jcb.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay I. R., Whittingham S., Mathews J. D., Tait B. D. Genetic determinants of autoimmune chronic active hepatitis. Springer Semin Immunopathol. 1980 Dec;3(3):285–296. doi: 10.1007/BF02054105. [DOI] [PubMed] [Google Scholar]
- Makinen D., Fritzler M., Davis P., Sherlock S. Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum. 1983 Jul;26(7):914–917. doi: 10.1002/art.1780260714. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974 Jul-Aug;17(4):421–429. doi: 10.1002/art.1780170413. [DOI] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., Salden M. H., Habets W. J., van de Putte L. B., van Venrooij W. J. On the existence of an internal nuclear protein structure in HeLa cells. Exp Cell Res. 1982 Sep;141(1):181–190. doi: 10.1016/0014-4827(82)90080-5. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Stapel S. O., Houben H., Habets W. J., Kallenberg C. G., Penner E., van de Putte L. B. Scl-86, a marker antigen for diffuse scleroderma. J Clin Invest. 1985 Mar;75(3):1053–1060. doi: 10.1172/JCI111767. [DOI] [PMC free article] [PubMed] [Google Scholar]