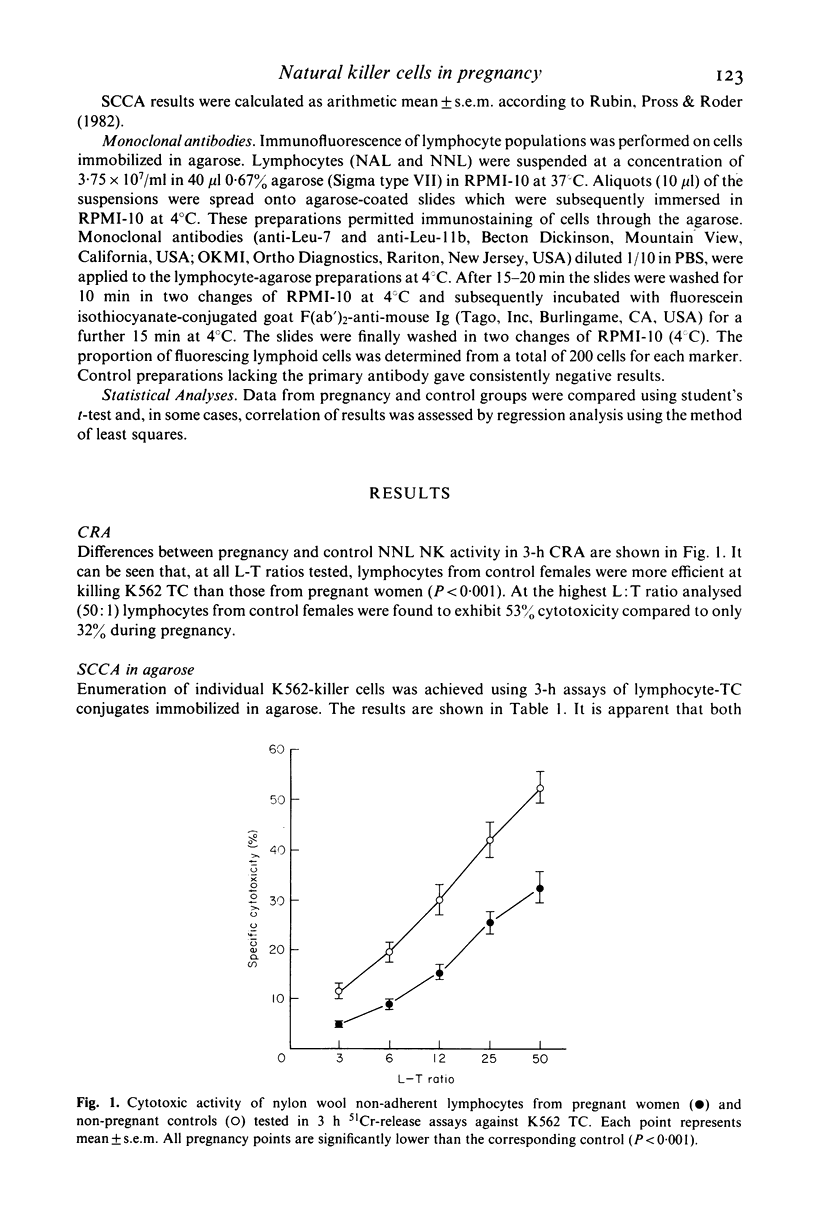
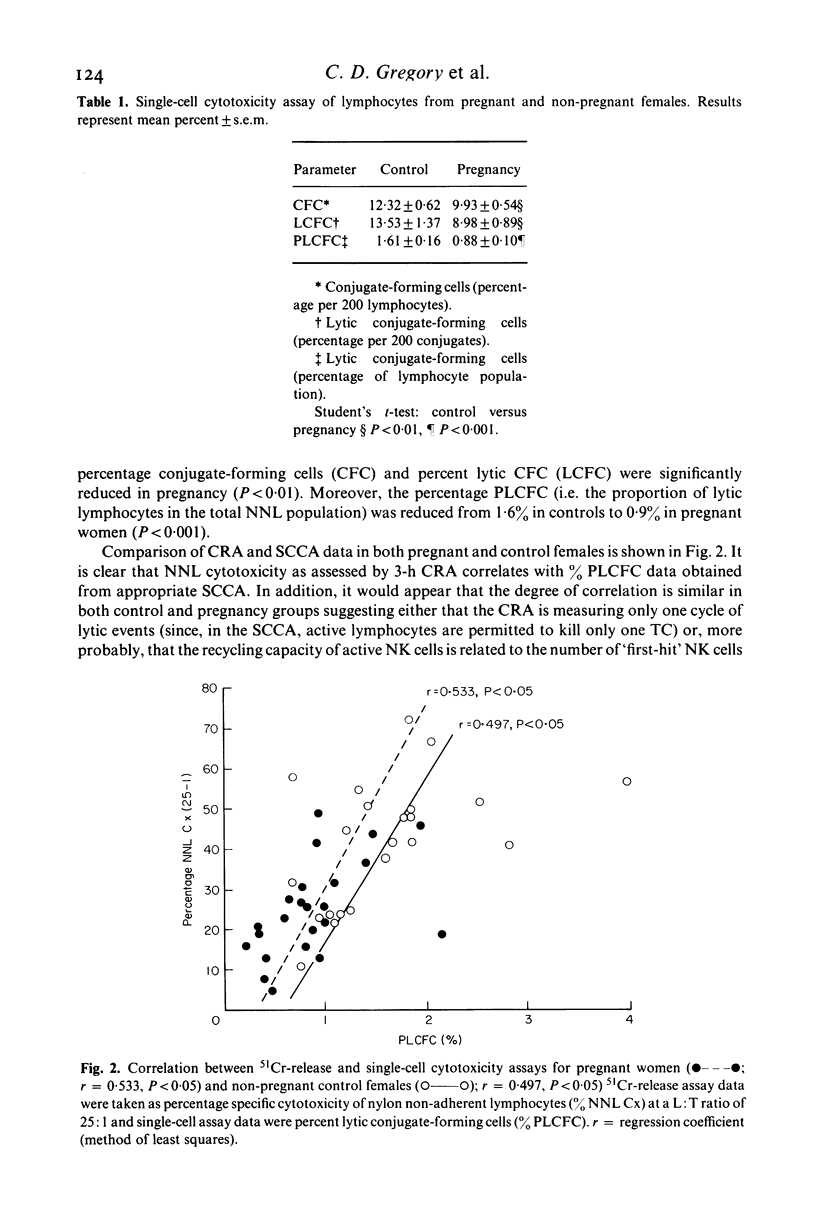
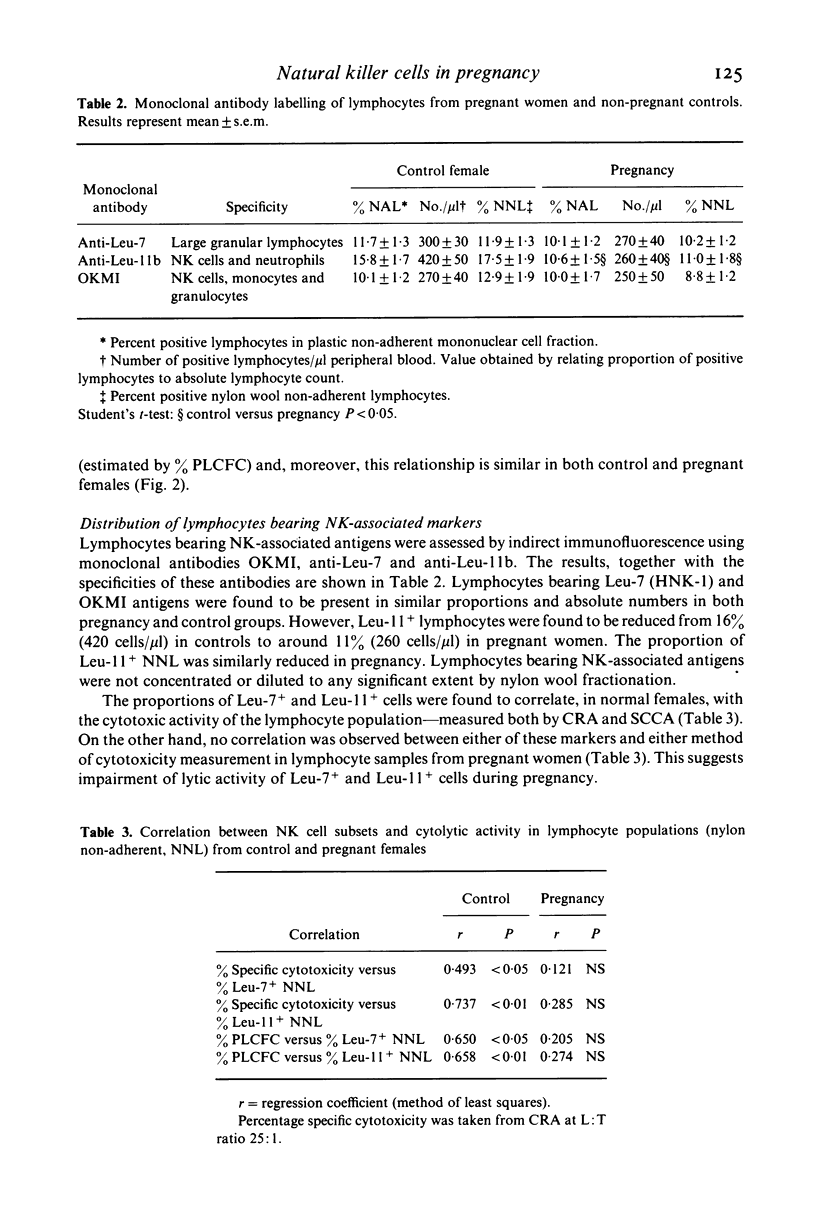
Abstract
Peripheral blood lymphocytes (nylon wool non-adherent) from healthy pregnant women and normal non-pregnant females were tested for natural killer (NK) cell-mediated cytotoxicity against K562 target cells both by 51Cr-release assay and single-cell cytotoxicity assay in agarose. The results indicated depression of NK cytotoxicity in pregnancy due to a decrease in the proportion of target-binding lymphocytes as well as a reduction in the lytic capacity of target-bound cells. The ability of active pregnancy-associated NK lymphocytes to recycle appeared to be unimpaired. Analysis of lymphocyte populations with monoclonal antibodies recognizing NK cell-associated antigens showed that the number of Leu-11+ lymphocytes was reduced in pregnancy. Enumeration of Leu-7+ cells and correlation of NK cell subpopulation data with cytotoxicity assay data suggest that pregnancy is associated with a reduction in the number of mature NK cells and probably also an inhibition of post-binding lytic activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Miller C. A., Balch C. M. Characterization of human granular lymphocyte subpopulations expressing HNK-1 (Leu-7) and Leu-11 antigens in the blood and lymphoid tissues from fetuses, neonates and adults. Eur J Immunol. 1984 Jul;14(7):616–623. doi: 10.1002/eji.1830140707. [DOI] [PubMed] [Google Scholar]
- Abo T., Miller C. A., Gartland G. L., Balch C. M. Differentiation stages of human natural killer cells in lymphoid tissues from fetal to adult life. J Exp Med. 1983 Jan 1;157(1):273–284. doi: 10.1084/jem.157.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Nilsson K., Gahmberg C. G. K562--a human erythroleukemic cell line. Int J Cancer. 1979 Feb;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- Baines M. G., Pross H. F., Millar K. G. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IV. The suppressive effect of normal pregnancy. Am J Obstet Gynecol. 1978 Apr 1;130(7):741–744. doi: 10.1016/0002-9378(78)90001-7. [DOI] [PubMed] [Google Scholar]
- Barrett D. S., Rayfield L. S., Brent L. Suppression of natural cell-mediated cytotoxicity in man by maternal and neonatal serum. Clin Exp Immunol. 1982 Mar;47(3):742–748. [PMC free article] [PubMed] [Google Scholar]
- Billingham R. E. Immunobiology of the maternal-fetal relationship. Prog Clin Biol Res. 1981;70:63–75. [PubMed] [Google Scholar]
- Bonavida B., Bradley T. P., Grimm E. A. Frequency determination of killer cells by a single-cell cytotoxic assay. Methods Enzymol. 1983;93:270–280. doi: 10.1016/s0076-6879(83)93049-5. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Atkinson M. E. Large agranular lymphocytes: early non-specific effector cells in allograft rejection in the mouse. Immunology. 1984 Oct;53(2):257–265. [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kalland T., Campbell T. Effects of diethylstilbestrol on human natural killer cells in vitro. Immunopharmacology. 1984 Aug;8(1):19–25. doi: 10.1016/0162-3109(84)90053-5. [DOI] [PubMed] [Google Scholar]
- Kolb J. P., Chaouat G., Chassoux D. Immunoactive products of placenta. III. Suppression of natural killing activity. J Immunol. 1984 May;132(5):2305–2310. [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Nair P. N., Fernandes G., Onoe K., Day N. K., Good R. A. Inhibition of effector cell functions in natural killer cell activity (NK) and antibody-dependent cellular cytotoxicity (ADCC) in mice by normal and cancer sera. Int J Cancer. 1980 May 15;25(5):667–677. doi: 10.1002/ijc.2910250518. [DOI] [PubMed] [Google Scholar]
- Nemlander A., Saksela E., Häyry P. Are "natural killer" cells involved in allograft rejection? Eur J Immunol. 1983 Apr;13(4):348–350. doi: 10.1002/eji.1830130415. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Babcock G. F. NKP-15: a monoclonal antibody reactive against purified human natural killer cells and granulocytes. Immunol Lett. 1983 Mar;6(3):143–149. doi: 10.1016/0165-2478(83)90096-2. [DOI] [PubMed] [Google Scholar]
- Rubin P., Pross H. F., Roder J. C. Studies of human natural killer cells. II. Analysis at the single cell level. J Immunol. 1982 Jun;128(6):2553–2558. [PubMed] [Google Scholar]
- Seaman W. E., Blackman M. A., Gindhart T. D., Roubinian J. R., Loeb J. M., Talal N. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978 Dec;121(6):2193–2198. [PubMed] [Google Scholar]
- Steinhauer E. H., Doyle A. T., Reed J., Kadish A. S. Defective natural cytotoxicity in patients with cancer: normal number of effector cells but decreased recycling capacity in patients with advanced disease. J Immunol. 1982 Nov;129(5):2255–2259. [PubMed] [Google Scholar]
- Tanaka K., Chang K. S. Modulation of natural killer sensitivity of murine trophoblast cells by tumor promoter and interferon. Int J Cancer. 1982 Mar 15;29(3):315–321. doi: 10.1002/ijc.2910290315. [DOI] [PubMed] [Google Scholar]
- Toder V., Nebel L., Elrad H., Blank M., Durdana A., Gleicher N. Studies of natural killer cells in pregnancy. II. The immunoregulatory effect of pregnancy substances. J Clin Lab Immunol. 1984 Jul;14(3):129–133. [PubMed] [Google Scholar]
- Toder V., Nebel L., Gleicher N. Studies of natural killer cells in pregnancy. I. Analysis at the single cell level. J Clin Lab Immunol. 1984 Jul;14(3):123–127. [PubMed] [Google Scholar]
- Zarling J. M., Clouse K. A., Biddison W. E., Kung P. C. Phenotypes of human natural killer cell populations detected with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2575–2580. [PubMed] [Google Scholar]


