Abstract
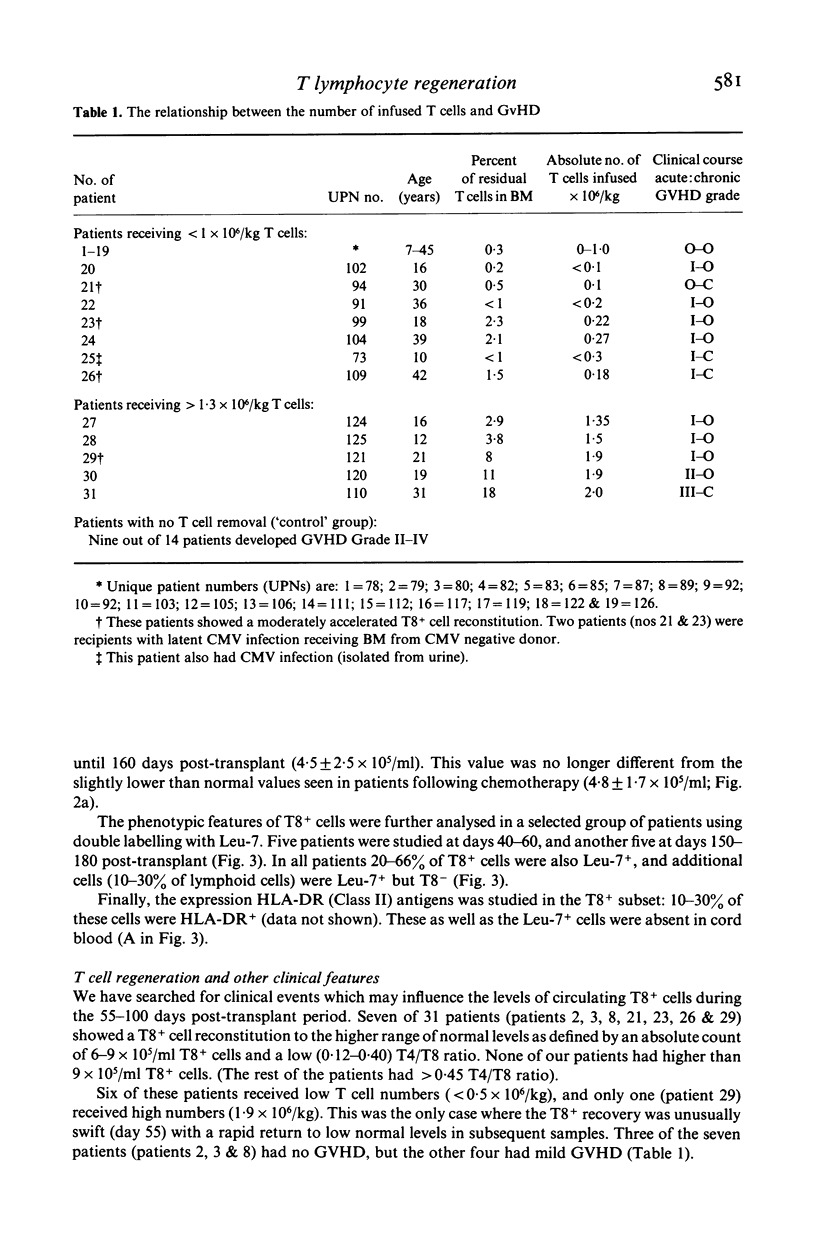
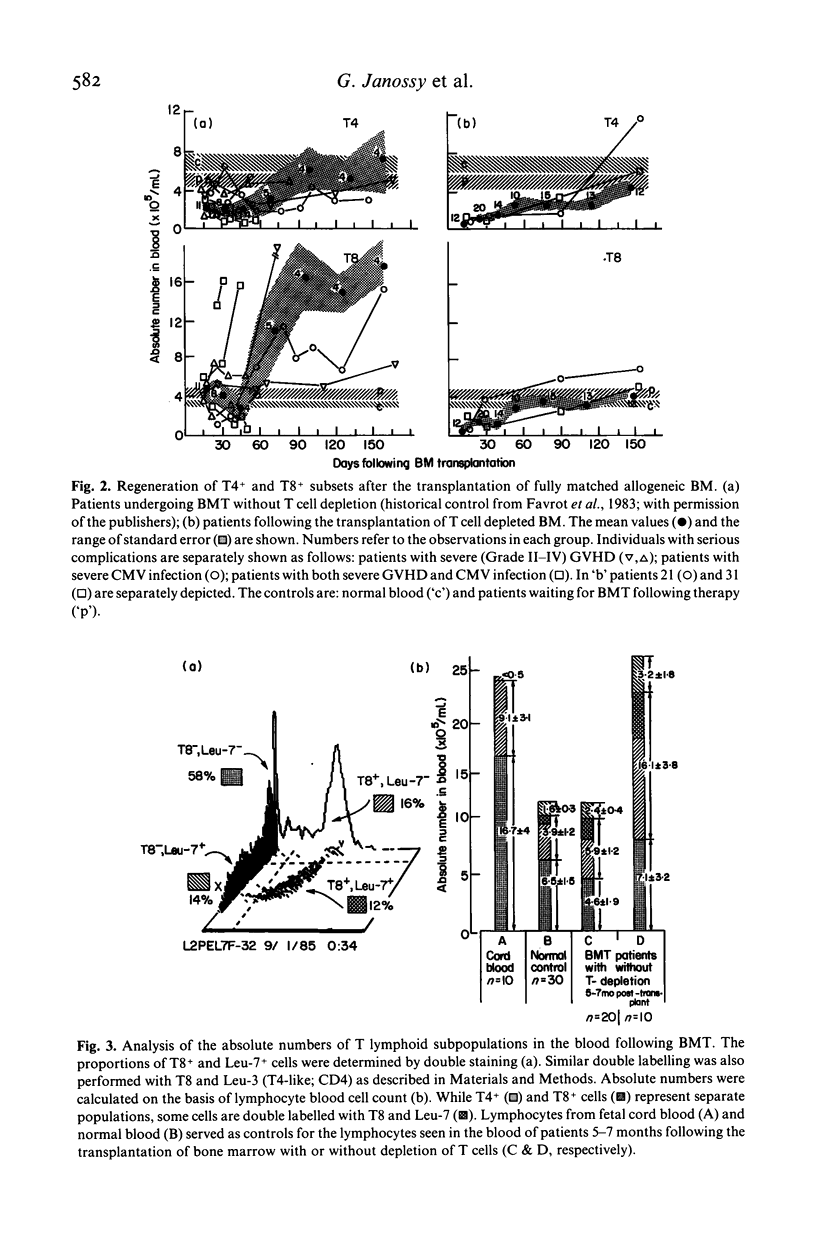
The regeneration of T cell subsets was studied with double immunofluorescence marker methods in 37 patients who received HLA matched T lymphocyte depleted bone marrow transplants (BMT) as part of the treatment for their haematological disease. A cocktail of anti-pan-T (CD6: MBG6) and anti-suppressor/cytotoxic-T cell (CD8: RFT8) monoclonal antibodies was used with rabbit serum as a source of cytolytic complement to achieve selective T cell lysis. The T8+ cells reached low normal values around 60 days post-transplant and remained within the normal range throughout the study (>150 days). This observation is in contrast to our previously published results in patients who, after receiving BMT without efficient T cell depletion, had increased numbers of circulation T8+ cells from 60 days post-transplant. In the present study Leu-7+, RFT8- cells reached normal values rapidly but the reconstitution of T4+ lymphocytes was slow: low normal levels were reached only around day 150 following BMT. The degree of graft-versus-host disease (GVHD) seemed to be related to the number of residual T cells infused: two of the three patients who received the highest numbers of T cells developed Grade II and III; otherwise GVHD was minimal. Among the clinical parameters studied cytomegalovirus (CMV) immune status moderately influenced reconstitution: at 55—90 days post-transplant T8+ cells were present at the upper normal levels in seven out of 15 patients receiving BMT from CMV seronegative donors, but in none of the 16 individuals receiving BMT from seropositive donors. CMV related complications were relatively uncommon. Thus the most significant factor in preventing `T8+ cell overshoot' and T cell imbalance during regeneration appears to be the depletion of T (including T8+) lymphocytes from marrow. The difference of T8+ cell reconstitution in this and previous studies may reflect a different regeneration pattern from T cell precursors as opposed to inoculated mature T cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Atkinson K., Hansen J. A., Storb R., Goehle S., Goldstein G., Thomas E. D. T-cell subpopulations identified by monoclonal antibodies after human marrow transplantation. I. Helper-inducer and cytotoxic-suppressor subsets. Blood. 1982 Jun;59(6):1292–1298. [PubMed] [Google Scholar]
- Bastin J. M., Granger S., Tidman N., Janossy G., McMichael A. J. Recognition of a human T-lymphocyte differentiation antigen by an IgM monoclonal antibody. Clin Exp Immunol. 1981 Dec;46(3):597–606. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Hijmans W., Haaijman J. J. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981 Oct;46(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Favrot M., Janossy G., Tidman N., Blacklock H., Lopez E., Bofill M., Lampert I., Morgenstein G., Powles R., Prentice H. G. T cell regeneration after allogeneic bone marrow transplantation. Clin Exp Immunol. 1983 Oct;54(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Gilmore M. J., Prentice H. G., Blacklock H. A., Janossy G., Hoffbrand A. V. A technique for rapid isolation of bone marrow mononuclear cells using Ficoll-Metrizoate and the IBM 2991 blood cell processor. Br J Haematol. 1982 Apr;50(4):619–626. doi: 10.1111/j.1365-2141.1982.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Granger S., Janossy G., Francis G., Blacklock H., Poulter L. W., Hoffbrand A. V. Elimination of T-lymphocytes from human bone marrow with monoclonal T-antibodies and cytolytic complement. Br J Haematol. 1982 Feb;50(2):367–374. doi: 10.1111/j.1365-2141.1982.tb01928.x. [DOI] [PubMed] [Google Scholar]
- Gratama J. W., Naipal A., Oljans P., Zwaan F. E., Verdonck L. F., de Witte T., Vossen J. M., Bolhuis R. L., de Gast G. C., Jansen J. T lymphocyte repopulation and differentiation after bone marrow transplantation. Early shifts in the ratio between T4+ and T8+ T lymphocytes correlate with the occurrence of acute graft-versus-host disease. Blood. 1984 Jun;63(6):1416–1423. [PubMed] [Google Scholar]
- Griffiths P. D., Panjwani D. D., Stirk P. R., Ball M. G., Ganczakowski M., Blacklock H. A., Prentice H. G. Rapid diagnosis of cytomegalovirus infection in immunocompromised patients by detection of early antigen fluorescent foci. Lancet. 1984 Dec 1;2(8414):1242–1245. doi: 10.1016/s0140-6736(84)92797-1. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Maher P., O'Toole C. M., Wreghitt T. G., Spiegelhalter D. J., English T. A. Cytomegalovirus infection in cardiac transplant recipients associated with chronic T cell subset ratio inversion with expansion of a Leu-7+ TS-C+ subset. Clin Exp Immunol. 1985 Dec;62(3):515–524. [PMC free article] [PubMed] [Google Scholar]
- Millard P. H. Depression in old age. Br Med J (Clin Res Ed) 1983 Aug 6;287(6389):375–376. doi: 10.1136/bmj.287.6389.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolo G., Semenzato G., Chilosi M., Morittu L., Ambrosetti A., Warner N., Bofill M., Janossy G. Distribution and heterogeneity of cells detected by HNK-1 monoclonal antibody in blood and tissues in normal, reactive and neoplastic conditions. Clin Exp Immunol. 1984 Jul;57(1):195–206. [PMC free article] [PubMed] [Google Scholar]
- Prentice H. G., Blacklock H. A., Janossy G., Gilmore M. J., Price-Jones L., Tidman N., Trejdosiewicz L. K., Skeggs D. B., Panjwani D., Ball S. Depletion of T lymphocytes in donor marrow prevents significant graft-versus-host disease in matched allogeneic leukaemic marrow transplant recipients. Lancet. 1984 Mar 3;1(8375):472–476. doi: 10.1016/s0140-6736(84)92848-4. [DOI] [PubMed] [Google Scholar]
- Rodt H., Kolb H. J., Netzel B., Rieder I., Janka G., Belohradsky B., Haas R. J., Thierfelder S. GVHD suppression by incubation of bone marrow grafts with anti-T-cell globulin: effect in the canine model and application to clinical bone marrow transplantation. Transplant Proc. 1979 Mar;11(1):962–966. [PubMed] [Google Scholar]
- Schroff R. W., Gale R. P., Fahey J. L. Regeneration of T cell subpopulations after bone marrow transplantation: cytomegalovirus infection and lymphoid subset imbalance. J Immunol. 1982 Nov;129(5):1926–1930. [PubMed] [Google Scholar]
- Singer C. R., Tansey P. J., Burnett A. K. T lymphocyte reconstitution following autologous bone marrow transplantation. Clin Exp Immunol. 1983 Mar;51(3):455–460. [PMC free article] [PubMed] [Google Scholar]
- Thomas E. D., Storb R., Clift R. A., Fefer A., Johnson L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (second of two parts). N Engl J Med. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- Verdonck L. F., de Gast G. C. Is cytomegalovirus infection a major cause of T cell alterations after (autologous) bone-marrow transplantation? Lancet. 1984 Apr 28;1(8383):932–935. doi: 10.1016/s0140-6736(84)92391-2. [DOI] [PubMed] [Google Scholar]
- Wahren B., Gahrton G., Linde A., Ljungman P., Lönnqvist B., Ringdén O., Sundqvist V. A. Transfer and persistence of viral antibody-producing cells in bone marrow transplantation. J Infect Dis. 1984 Sep;150(3):358–365. doi: 10.1093/infdis/150.3.358. [DOI] [PubMed] [Google Scholar]
- Witherspoon R. P., Kopecky K., Storb R. F., Flournoy N., Sullivan K. M., Sosa R., Deeg H. J., Ochs H. D., Cheever M. A., Fefer A. Immunological recovery in 48 patients following syngeneic marrow transplantation or hematological malignancy. Transplantation. 1982 Feb;33(2):143–149. doi: 10.1097/00007890-198202000-00008. [DOI] [PubMed] [Google Scholar]
- Witherspoon R. P., Storb R., Ochs H. D., Fluornoy N., Kopecky K. J., Sullivan K. M., Deeg J. H., Sosa R., Noel D. R., Atkinson K. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981 Aug;58(2):360–368. [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin H. G., Astaldi A., Leupers T., van de Griend R. J., Dooren L. J., Schellekens P. T., Tanke H. J., Roos M., Vossen J. M. T lymphocyte characteristics in bone marrow-transplanted patients. II. Analysis with monoclonal antibodies. J Immunol. 1981 Jul;127(1):244–251. [PubMed] [Google Scholar]
- van Bekkum D. W., Balner H., Dicke K. A., van den Berg F. G., Prinsen G. H., Hollander C. F. The effect of pretreatment of allogeneic bone marrow graft recipients with antilymphocytic serum on the acute graft-versus-host reaction in monkeys. Transplantation. 1972 Apr;13(4):400–407. doi: 10.1097/00007890-197204000-00007. [DOI] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., Ploem J. S. Simultaneous detection of two cell populations by two-colour fluorescence and application to the recognition of B-cell determinants. Nature. 1976 Aug 26;262(5571):795–797. doi: 10.1038/262795a0. [DOI] [PubMed] [Google Scholar]


