Abstract
Ocular neovascularization is the leading cause of blindness in developed countries and often causes rapid loss of vision in age-related macular degeneration. Acute visual loss is most often due to hemorrhage from new vessels that have extended from the choroid into the subretinal space. Growth of abnormal vessels beneath the retina in this condition is known as subretinal neovascularization (SRN). Age-related animal models of macular degeneration and SRN have not been described. Current animal models of SRN depend on chemical or physical stimuli to initiate growth of subretinal vessels. The genes responsible for age-related human macular degeneration with SRN have not been firmly identified. We report an angiogenic phenotype in Bst/+ mice that is age-related, clinically evident, and resembles human SRN. This represents a spontaneous, genetically determined model of SRN. Bst/+ mice offer the possibility of exploring the molecular mechanisms of SRN without the need for exogenous agents.
Ocular neovascularization is responsible for most legal blindness in the United States (1). Proliferative diabetic retinopathy, retinopathy of prematurity, and neovascular age-related macular degeneration (ARMD) all give rise to blindness through inappropriate growth of new blood vessels (2). ARMD is the leading cause of blindness in the elderly in developed countries (1, 3). Avascular and neovascular forms exist and macular drusen are a frequent associated finding. The clinical phenotype varies over the spectrum from minor disturbance of the retinal pigment epithelium to advanced subretinal neovascularization (SRN) with submacular hemorrhage and fibrosis. In many cases of ARMD, the development of SRN is a poor prognostic sign for future visual function with 64% of patients losing six lines of visual acuity over a 2-yr span (4). SRN begins in the choroid with vascular invasion through Bruch's membrane into the subretinal space, with consequent photoreceptor loss. These abnormal vessels are often the source of subretinal hemorrhage, which may undergo fibrosis and cause additional retinal damage (1).
Although ARMD often occurs in families, it has not been possible to unequivocally identify specific genes responsible for the disease (5). Genetic studies are difficult because of the dissociation between visible and functional phenotypes: minor clinically apparent fundus changes may be associated with severe visual loss, whereas patients with moderate to severe changes may maintain good vision. These observations suggest that ARMD and SRN are likely complex genetic traits impacted by multiple genes. Despite the frequency of these diseases, the genes and molecular mechanisms that control the development and progression of ARMD and SRN (reviewed in ref. 6) remain unknown.
Although progress has been made in defining the molecular basis of some forms of retinal neovascular disease (7–9), understanding of ARMD is impeded by the absence of naturally occurring models of SRN related to aging, as happens in human patients. Currently available models of SRN are based on production of neovascularization by chemical (10) or physical (11) methods and in transgenic mice that overexpress vascular endothelial growth factor (VEGF) (12). As long as human genes causing SRN are undiscovered, animal models cannot be produced through techniques of genetic engineering. However, spontaneous mutations in mice are valuable for identifying molecular mechanisms of human disease as well as in the study of complex traits (13). For example, mice have been utilized to demonstrate that multiple genes influence developmental pathways (14) or can act together to alter clinical phenotypes (15). The conserved mammalian physiology and strong homologies between mouse and human genomes often make it possible to apply lessons learned in mice to the understanding of human genetic disease.
The spontaneous, autosomal, semidominant mouse mutation Belly spot and tail (Bst) arose in the C57BLKS inbred strain at The Jackson Laboratory, and the gene was mapped to mouse chromosome (Chr) 16, 1.9 +/− 1.1 cM from D16Mit168 (16, 17). Young mice demonstrate late closure of the optic fissure, delayed retinal differentiation, decreased numbers of retinal ganglion cells, and the presence of colobomas of the optic nerve and retina, associated with a highly variable phenotype (18, 19). These observations suggest that the Bst/+ phenotype is caused by developmental delay (19).
We surveyed a cohort of aging Bst/+ mice and observed the occurrence of focal, nonrhegmatogenous posterior pole retinal detachment temporal to the optic nerve in mice older than 7 mo of age. In 80% of affected mice with focal detachments, SRN arose from the choriocapillaris and extended into the outer portion of the sensory retina. Macular and peripapillary SRN beneath areas of detached retina have also been described in human cavitary defects of the optic nerve (20–24). It is likely, therefore, that Bst/+ mice are a model for SRN as well as for congenital optic nerve anomalies.
Materials and Methods
All mice were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Bst/± mice were housed in cages covered loosely with nonwoven polyester filters and containing white pine bedding. The environment was kept at 21°C with a 12:12 light-dark cycle. All mice were fed National Institutes of Health 4% fat chow, and their drinking water was acidified to pH 2.8–3.2. The colony was monitored for specific pathogens by The Jackson Laboratory's routine surveillance program (see http://jaxmice.jax.org/html/health/quality_control.shtml for details).
Clinical examinations were done on all mice older than 2 wk. The anterior segment of the eye was evaluated by using a Nikon or Haag-Streit slit lamp. After pupillary dilation with 1% cyclopentolate, the fundus was examined by using binocular indirect ophthalmoscopy. Mice older than 4 mo were examined every 3 mo. More than 100 normal littermates (+/+) and 64 Bst/+ mice were examined. Fluorescein angiograms were performed on 12 mice by injecting 0.01 ml/5 g body weight of 25% sodium fluorescein i.p., followed by serial fundus photographs taken as described (25).
Eyes from 64 Bst/+ mice ranging in age from 10 days to 22 mo of age were clinically evaluated. For histological examination, eyes were removed from all 64 mice immediately after carbon dioxide euthanasia and prepared as described (15). Six of the eyes were fixed in 4% paraformaldehyde for 1 h and stored in buffered 0.4% paraformaldehyde (26, 27) for embedding in plastic and preparation of step sections for detailed analysis (28). A total of 30 additional eyes that represented the full range of clinically observed abnormalities were selected for sectioning. The remaining eyes were not sectioned either because they appeared normal or had only minor changes on clinical examination.
Results
Examination of the +/+ mice revealed no retinal or optic nerve abnormalities. Of a total of 64 Bst/+ mice, only 8 were bilaterally normal; 31/64 demonstrated unilateral retinal or optic nerve abnormalities; 25/64 had bilateral involvement. No sexual difference in the incidence of nerve or retinal abnormalities was evident at any age. Retinal detachment, subretinal macrophages, and subretinal neovascularization were found only after 7 mo of age.
Clinical Examination.
Slit lamp examination before instillation of mydriatics demonstrated partially or totally dilated pupils in 40% of the mice (not illustrated). This finding usually was associated with the presence of optic nerve coloboma or retinal abnormalities. Mild nuclear or cortical cataracts were found in six eyes. There was considerable variation in the type and extent of retinal and optic nerve findings from eye to eye. Retinal folds and small patches of retinal thinning were common features. A total of 10 eyes developed a localized, posterior pole, nonrhegmatogenous retinal detachment that appeared as a round, white patch 2–4 disc diameters in size and usually located temporal to the optic nerve. In 8 of the 10 eyes with detachment, SRN was found on microscopic examination. Although other retinal abnormalities were found in these eyes, the association between focal detachment and SRN was a consistent phenotype. The optic nerve was often deeply excavated and surrounded by a ring of apparent depigmentation of the retinal pigment epithelium and choroid (Fig. 1a). Retinal arterioles were often small and pale, and free-floating vessels were frequently observed in the vitreous.
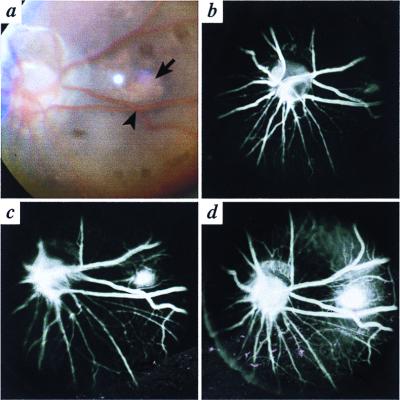
Figure 1.
Nine-month-old Bst/+ mouse. (a) Fundus photograph. The arrow indicates the edge of an area of focal retinal detachment with SRN temporal to the optic nerve. Two crossing vessels (arrowhead) mark the location of the retinal detachment and are useful landmarks for the angiograms. (b–d) There is progressive leakage of dye over a 90-s period, corresponding to the area indicated in a.
Fluorescein Angiography.
Early in the arterial cycle, faint staining of the area corresponding to the white spot in the fundus photograph (Fig. 1a) was identified (Fig. 1b). With the progression of time, the area of staining became larger and more prominent (Fig. 1 c and d), indicating leakage of dye from the focus of SRN. This mouse was subsequently euthanized and the presence of focal retinal detachment and SRN on microscopic examination confirmed the angiographic observations. All mice with the typical ophthalmoscopic findings demonstrated leakage on angiographic examination.
Histopathology.
Anterior and posterior subcapsular and cortical cataracts were evident in 19% of affected mice. The posterior hyaloid vascular system of mice usually undergoes involution in the first month of life. In Bst/+ mice, large hyaloid vessels were present up to 22 mo of age. Serial sections demonstrated that these vessels all came from the optic nerve and not from the sensory retina.
In affected Bst/+ mice, optic nerve colobomas were present and varied from a mild loss of nerve fibers on the surface of the optic cup to a complete absence of the nerve with severe abnormalities of the peripapillary neural retina, retinal pigment epithelium (RPE), and choroid (Fig. 2 a–d).
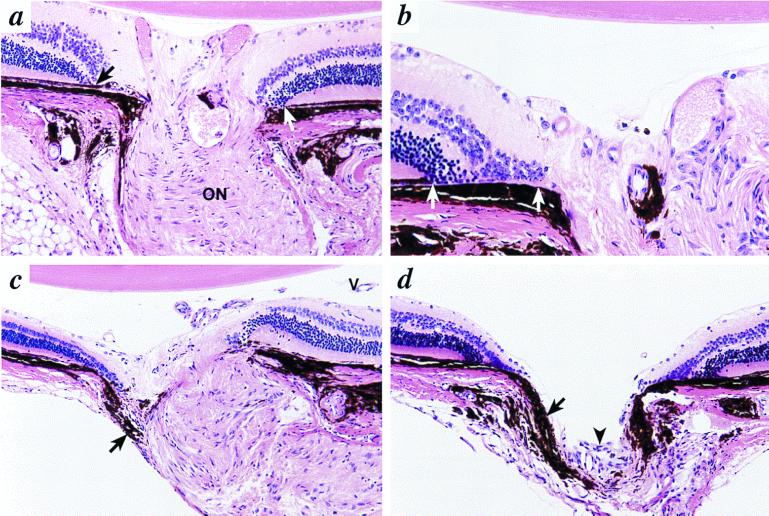
Figure 2.
Colobomas in 6-mo-old Bst/+ mice. (a) In a minimally involved optic nerve, the retina and RPE terminate prematurely before reaching the margin of the optic nerve (arrow). The RPE (white arrow) ends before the nerve on the opposite side. Nerve fibers entering the nerve are not clearly identified, although the optic nerve (ON) demonstrates nearly normal architecture. Original magnification, ×400. (b) At the edge of the optic disc, there is folding of the retina with direct contact between the choroid and inner and outer nuclear layers (white arrows). (c) In more severely affected mice, there is distortion of the peripapillary retina, RPE, and choroid. Misplaced choroidal melanocytes are present in the periphery of the optic nerve (arrow). Neither lamina cribrosa nor nerve fibers are identified. Persistent hyaloid vessels (V) are present in the posterior vitreous. (d) The optic nerve is not identifiable, other than by a tiny cluster of undifferentiated cells (arrowhead). Choroidal pigment (arrow) prolapses into the empty cup. Original magnification in b–d, ×200.
Small patches of retinal dysplasia were present close to the abnormal optic nerve or in the peripheral retina in 21% of affected mice, frequently adjacent to persistent hyaloid vessels (Fig. 3a). In three mice, there was focal absence of photoreceptor outer segments and aberrant location of cell nuclei (Fig. 3b). It should be noted that the retina adjacent to these abnormalities demonstrated normal anatomic features, including normal distribution of retinal ganglion cells and normal thickness of inner and outer nuclear layers.
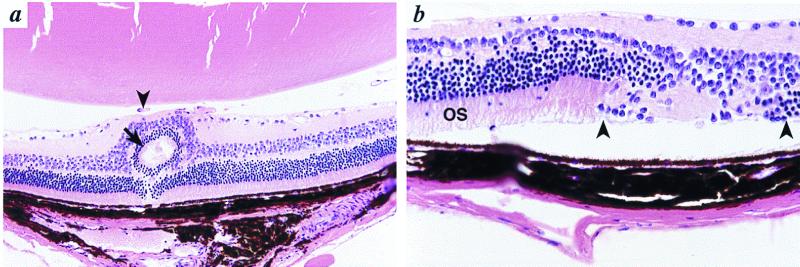
Figure 3.
Retinal abnormalities in Bst/+ mice. (a) A focus of retinal dysplasia is present (arrow). Persistent hyaloid vessels (arrowhead) separate the retina from the lens. These vessels originate from the optic nerve rather than the retina. Original magnification, ×200. (b) To the right of relatively normal retina with normal outer segments (OS), there is an area of total loss of photoreceptors with undifferentiated retina (between arrowheads). Original magnification, ×400.
In Bst/+ mice older than 7 mo, a total of 10 mice (19%) developed focal, nonrhegmatogenous, retinal detachments in the posterior pole of the globe adjacent to the optic nerve. Focal loss of photoreceptor outer segments and the presence of numerous pigment-filled cells in the subretinal space (Fig. 4a) characterized the detachments. In those eyes with posterior retinal detachments, cellular proliferation and SRN were present in 8/10 mice (80%) (Fig. 4 b and c). This typical plaque-like appearance bears a strong similarity to human SRN. A chorioretinal adhesion related to a choroidal vessel entering the retina in the area of detachment was demonstrated in three mice (Fig. 4d). A careful review of serial sections through such areas demonstrated continuity of the subretinal and retinal portions of the vascular channel with the choriocapillaris, discontinuity of Bruch's membrane and the retinal pigment epithelium, and perivascular pigment migration extending from the retinal pigment epithelium. Taken together, these findings support the choroidal origin of the SRN.
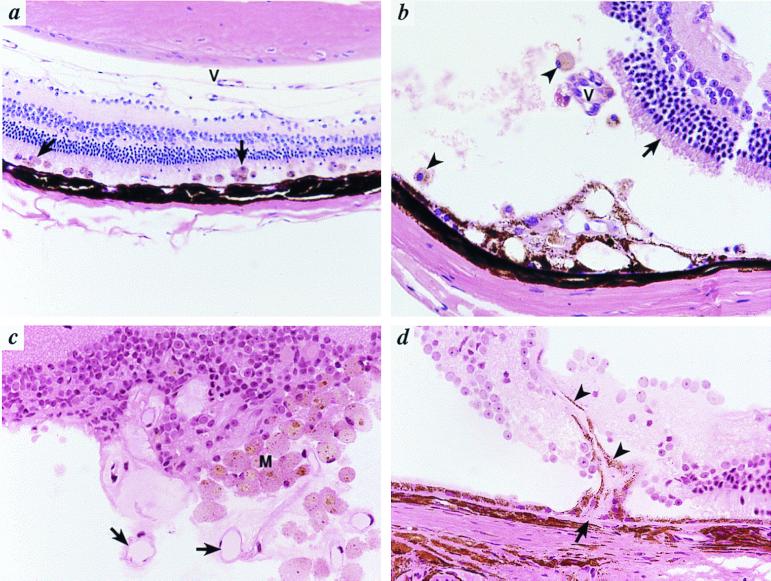
Figure 4.
Retinal detachment and SRN in Bst/+ mice. (a) There is a flat detachment of the retina containing many pigment-filled macrophages (arrows). Persistent hyaloid vessels (V) are present. Original magnification, ×200. (b) There is a more extensive retinal detachment with loss of photoreceptor outer segments (arrow). Pigmented macrophages are present (arrowheads). There is a focus of fibrovascular proliferation involving the RPE (*) that contains eosinophilic connective tissue and small vascular channels. Additional vascular channels (V) that appear to connect to this focus in other planes of section were also present. The tears in the retina are the result of artifacts of sectioning. (c) In an extensive retinal detachment, numerous proliferating vessels (arrows) and macrophages (M) are evident. (d) A new vessel arises from the choriocapillaris (arrow) and extends into the subretinal space and retina (arrowheads). Original magnification in b–d, ×400.
Discussion
The Bst mutation demonstrates a broad range of phenotypic expression that includes SRN, focal retinal detachment, retinal dysplasia, persistence of the hyaloid vascular system, patchy absence of retinal differentiation, and colobomas. Specific clinical and histological findings in Bst/+ mice varied and presented in different combinations in one or both eyes in 87% of mice examined. Similar phenotypic variability has been previously reported in human colobomas, with or without SRN (20, 29, 30). The coloboma phenotype in mice is produced by gene mutations at several loci on different chromosomes: Chr 2 (Cm) (31), Chr 4 (Tcm) (32), Chr 6 (Mitf) (33, 34), Chr 13 (Gli3) (35, 36), Chr 16 (Bst) (18, 19), and Chr 19 (Pax 2 and Krd) (37). SRN has not been reported in any other mouse coloboma mutation, but could have been missed if older mice were not evaluated, as was necessary for detection of SRN in Bst/+ mice.
The most important finding in aging Bst/+ mice is the occurrence of an angiogenic phenotype. The fact that SRN was never observed before 7 mo of age deserves emphasis because this confirms that the angiogenic phenotype is not developmental, but rather is an aging change. The development of SRN in aging Bst/+ mice is always associated with the presence of a focal retinal detachment, easily recognized with a binocular indirect ophthalmoscope. These clinical findings were confirmed by histopathology. Subretinal macrophages are a prominent feature and most likely represent mobilized phagocytic cells from the retinal pigment epithelium based on the large amount of ingested melanin pigment and their close proximity to the retinal pigment epithelium. In 80% of the eyes of Bst/+ mice with posterior retinal detachments, new vessels growing in the space between retina and retinal pigment epithelium were identified. Because vessels do not normally occur in this location, these findings are appropriately identified as vascular proliferation (SRN). Extension of vascularization into the retina was observed in 3 of 10 mice, a finding unusual in human SRN. Fluorescein angiography identified the presence of leaking subretinal vessels in the areas of detached retina. The angiographic interpretation was confirmed by microscopic evaluation.
SRN has been produced in rabbits by subretinal implants of basic fibroblast growth factor-containing microspheres (10) and in monkeys (11) and mice (38) in response to argon laser photocoagulation. Although these represent useful techniques for studying angiogenesis, it is not possible to determine whether chemically and traumatically induced neovascularization utilizes the same molecular mechanisms that occur in human ARMD and SRN. A form of SRN also has been described in transgenic mice that overexpress human VEGF. Because the vessels producing SRN in this experimental model originate from the deep retinal capillary bed rather than the choroid, (12) the role of VEGF in initiation of SRN remains unclear.
Aging Bst/+ mice are the first animal model in which SRN, similar to that observed in human disease, is a spontaneous occurrence. The clinical effects are presumably the result of a mutation at the Bst locus, for which high-resolution mapping or cloning has not been reported (16, 17). The onset of SRN in Bst/+ mice is age-related, a finding also typical of SRN occurring in human ARMD and optic nerve colobomas. Clearly, the presence of colobomas and SRN in Bst/+ mice suggests that these mice most closely resemble human patients with cavitary defects of the optic nerve that often develop SRN as they age (20, 29, 30). Because it is likely that the spontaneous occurrence of SRN has a specific mechanism, the presence of the optic nerve coloboma does not alter the importance of Bst/+ mice as a model that can be used to search for these mechanisms.
Because mice lack a macula, it is not appropriate to refer to the SRN that occurs in Bst/+ mice as a model of ARMD. Nevertheless, SRN is a central feature of some forms of ARMD. Because there are no spontaneous models of age-related SRN, Bst/+ mice provide a point of entry to study possible molecular mechanisms responsible for initiating SRN. Although the incidence of focal retinal detachment is only 19% in Bst/+ mice older than 7 mo, the ease of its detection by ophthalmoscopy allows rapid identification and enrichment of the population of mice with the angiogenic phenotype. The variable incidence of SRN suggests that chance effects and subtle environmental factors may interact in the expression of what is likely a complex trait.
It is known that the genetic background of a mouse strain may exert strong effects on phenotypic expression (39–42). By crossing the Bst mutation onto other genetic backgrounds, it may be possible to increase the incidence of SRN and make the model even more valuable. Interstrain crosses also may reveal interacting loci that cause SRN, as has been demonstrated in a mouse glaucoma model (15). Because SRN and ARMD are likely complex traits, these experiments may produce important insights into the mechanisms of these important human diseases.
The roles of the gene(s) responsible for the Bst/+ phenotype could include effects on axonal migration, rate of closure of the optic fissure, formation or involution of the hyaloid vascular system, and development of the vascular supply to the eye and optic nerve. Abnormal persistence of the hyaloid vascular system and the development of SRN in Bst/+ mice suggests the intriguing possibility that functional changes in these genes might also play a role in abnormal ocular neovascularization later in adult life. Cloning of the Bst gene and identification of the homologous human gene may be useful in testing these or other hypotheses.
Acknowledgments
We thank Drs. Leonard Shultz and Patsy Nishina for critical reading of the manuscript, and Jennifer Smith for assistance with graphics. This work is supported in part by The Foundation Fighting Blindness, PXE International, and by National Institutes of Health Cancer Center Core Grant CA 34196, and National Institutes of Health Grants RO1 EY07758 and RR01183. S.J. is an Assistant Investigator for the Howard Hughes Medical Institute.
Abbreviations
- SRN
subretinal neovascularization
- ARMD
age-related macular degeneration
- VEGF
vascular endothelial growth factor
- RPE
retinal pigment epithelium
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040531597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040531597
References
- 1.Klein R, Klein B, Moss S E, Cruickshanks K J. Arch Ophthalmol. 1994;112:1217–1228. doi: 10.1001/archopht.1994.01090210105023. [DOI] [PubMed] [Google Scholar]
- 2.Neely K A, Gardner T W. Am J Pathol. 1998;153:665–670. doi: 10.1016/S0002-9440(10)65607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Retinal Diseases Panel. Vision Research: A National Plan. U.S. Department of Health and Human Services; 1999. pp. 13–40. [Google Scholar]
- 4.Guyer D R, Fine S L, Maguire M G, Hawkins B S, Owens S L, Murphy R P. Arch Ophthalmol. 1986;104:702–705. doi: 10.1001/archopht.1986.01050170092029. [DOI] [PubMed] [Google Scholar]
- 5.Fishman G A, Stone E M, Grover S, Derlacki D J, Haines H L, Hockey R R. Arch Ophthalmol. 1999;117:504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 6.Hinton D R. Arch Ophthalmol. 1998;116:203–209. doi: 10.1001/archopht.116.2.203. [DOI] [PubMed] [Google Scholar]
- 7.Barinaga M. Science. 1995;267:452–453. [Google Scholar]
- 8.Friedlander M, Theesfeld C L, Sugita M, Fruttiger M, Thomas M A, Chang S, Cheresh D A. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salcedo R, Wasserman K, Young H A, Grimm M C, Howard O, Anver M R, Kleinman H K, Murphy W J, Oppenheim J J. Am J Pathol. 1999;154:1125–1135. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura H, Spee C, Sakamoto T, Hinton D R, Ogura Y, Tabata Y, Ikada Y, Ryan S J. Invest Ophthalmol Visual Sci. 1999;40:524–528. [PubMed] [Google Scholar]
- 11.Sakamoto T, Soriano D, Nassaralla J, Murphy T L, Oganesian A, Spee C, Hinton D R, Ryan S J. Arch Ophthalmol. 1995;113:222–226. doi: 10.1001/archopht.1995.01100020106040. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto N, Tobe T, Hackett S F, Ozaki H, Vinores M A, LaRochelle W, Zack D J, Campochiaro P A. Am J Pathol. 1997;151:281–291. [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel W N. Trends Genet. 1995;11:471–476. doi: 10.1016/s0168-9525(00)89155-6. [DOI] [PubMed] [Google Scholar]
- 14.Dunn N R, Winnier G E, Hargett L K, Schrick J J, Fogo A B, Hogan B L M. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- 15.Chang B, Smith R S, Hawes N L, Anderson M G, Zabaleta A, Savinova O, Roderick T H, Heckenlively J R, Davisson M T, John S W M. Nat Genet. 1999;21:405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- 16.Epstein R, Davisson M, Lehmann K, Akeson E C, Cohn M. Immunogenetics. 1986;23:78–83. doi: 10.1007/BF00377965. [DOI] [PubMed] [Google Scholar]
- 17.Rice D S, Williams R W, Ward-Bailey P, Johnson K R, Harris B S, Davisson M T, Goldowitz D. Mamm Genome. 1995;6:546–548. doi: 10.1007/BF00356174. [DOI] [PubMed] [Google Scholar]
- 18.Rice D S, Tang Q, Williams R W, Harris B S, Davisson M T, Goldowitz D. Invest Ophthalmol Visual Sci. 1997;38:2112–2124. [PubMed] [Google Scholar]
- 19.Tang Q, Rice D S, Goldowitz D. Dev Biol. 1999;207:239–255. doi: 10.1006/dbio.1998.9142. [DOI] [PubMed] [Google Scholar]
- 20.Slusher M M, Weaver R G, Greven C M, Mundorf T K, Cashwell L F. Ophthalmology. 1989;96:342–347. doi: 10.1016/s0161-6420(89)32886-7. [DOI] [PubMed] [Google Scholar]
- 21.Sobol W M, Blodi C F, Folk J C, Weingeist T A. Ophthalmology. 1990;97:1539–1542. doi: 10.1016/s0161-6420(90)32380-1. [DOI] [PubMed] [Google Scholar]
- 22.Dailey J R, Cantore W A, Gardner T W. Arch Ophthalmol. 1993;111:441–442. doi: 10.1001/archopht.1993.01090040031020. [DOI] [PubMed] [Google Scholar]
- 23.Theodossiadis G P, Damanakas A G, Theodissiadis P G. Am J Ophthalmol. 1995;120:798–800. doi: 10.1016/s0002-9394(14)72736-9. [DOI] [PubMed] [Google Scholar]
- 24.Theodossiadis G P. Arch Ophthalmol. 1996;114:493. doi: 10.1001/archopht.1996.01100130489029. [DOI] [PubMed] [Google Scholar]
- 25.Hawes N L, Smith R S, Chang B, Davisson M, Heckenlively J R, John S W M. Mol Vis. 1999;5:22. [PubMed] [Google Scholar]
- 26.Schlamp C L, Nickells R W. J Neurosci. 1996;16:2164–2171. doi: 10.1523/JNEUROSCI.16-07-02164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlamp C L, Williams D S. Exp Eye Res. 1996;63:613–619. doi: 10.1006/exer.1996.0155. [DOI] [PubMed] [Google Scholar]
- 28.John S W M J, Smith R S, Savinova O, Hawes N L, Chang B, Turnbull D, Davisson M T, Roderick T H. Invest Ophthalmol Visual Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 29.Frisen L, Holmegaard L. Br J Ophthalmol. 1978;62:7–15. doi: 10.1136/bjo.62.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi N, Valdes-Dapena M, Green W R. J Pediatr Ophthalmol Strab. 1998;35:100–106. doi: 10.3928/0191-3913-19980301-09. [DOI] [PubMed] [Google Scholar]
- 31.Hess E J, Collins K A, Copeland N G, Jenkins N A, Wilson M C. Genomics. 1994;21:257–261. doi: 10.1006/geno.1994.1254. [DOI] [PubMed] [Google Scholar]
- 32.Kratochvilova J, Favor J. Genet Res. 1988;52:125–134. doi: 10.1017/s001667230002749x. [DOI] [PubMed] [Google Scholar]
- 33.Hero I. Exp Eye Res. 1989;49:229–239. doi: 10.1016/0014-4835(89)90093-6. [DOI] [PubMed] [Google Scholar]
- 34.Hero I, Farjah M, Scholtz C L. Invest Ophthalmol Visual Sci. 1991;32:2622–2635. [PubMed] [Google Scholar]
- 35.Franz T, Besecke A. Anat Embryol. 1991;184:355–361. doi: 10.1007/BF00957897. [DOI] [PubMed] [Google Scholar]
- 36.Pohl T M, Mattei M, Ruther U. Development (Cambridge, UK) 1990;110:1153–1157. doi: 10.1242/dev.110.4.1153. [DOI] [PubMed] [Google Scholar]
- 37.Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatti B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, Schughart K. Proc Natl Acad Sci USA. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan H J, Leibole M A, Tezel T, Ferguson T A. Nat Med. 1999;5:292–297. doi: 10.1038/6509. [DOI] [PubMed] [Google Scholar]
- 39.Harata M, Shoji R, Semba R. Jpn J Genet. 1978;53:147–152. [Google Scholar]
- 40.Threadgill D W, Duglosz A A, Hansen L A, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, et al. Science. 1995;269:230–233. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 41.Wada E, Koyamo-ito H, Matsuzawa A. Exp Eye Res. 1991;52:501–506. doi: 10.1016/0014-4835(91)90049-k. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda S, Hawes N L, Chang B, Avery C S, Smith R S, Nishina P M. Invest Ophthalmol Visual Sci. 1999;40:1874–1878. [PubMed] [Google Scholar]