Abstract
Tumor angiogenesis is regulated by a dynamic cross-talk between tumor cells and the host microenvironment. Because membrane vesicles shed by tumor cells are known to mediate several tumor-host interactions, we determined whether vesicles might also stimulate angiogenesis. Vesicles shed by human ovarian carcinoma cell lines CABAI and A2780 stimulated the motility and invasiveness of endothelial cells in vitro. Enzyme-linked immunosorbent assay and Western blot analysis revealed relevant amounts of vascular endothelial growth factor (VEGF) and the two matrix metalloproteinases MMP-2 and MMP-9, but not fibroblast growth factor-2, contained in shed vesicles. An A2780 cell-derived clone transfected to overexpress VEGF shed the same amount of vesicles as did a control clone, but contained significantly more VEGF within the vesicles. Despite a greater amount of VEGF in vesicles of the over-expressing clone, vesicles of both clones stimulated endothelial cell motility to comparable levels, suggesting that VEGF was stored within the vesicle and was unavailable. Only following vesicle burst induced by acidic pH (a characteristic of the tumor microenvironment) was VEGF released, leading to significantly higher stimulation of cell motility. Thus, tumor-shed membrane vesicles carry VEGF and release it in a bioactive form in conditions typical of the tumor microenvironment.
Keywords: Shed membrane vesicles, VEGF, tumor angiogenesis, pH, ovarian cancer
Introduction
The development of new blood vessels is essential for the progression of solid tumors and the development of distant metastasis [1,2]. Angiogenesis is triggered by a shift in the normal balance between positive and negative effectors of angiogenesis, which favors activators [3]. Among angiogenic stimuli, vascular endothelial growth factor (VEGF) is a key mediator of physiological and pathological vessel development, particularly in tumors [4–6]. VEGF is a soluble factor that acts through its tyrosine kinase receptors, mainly VEGFR-2, to directly stimulate endothelial cell functions relevant to angiogenesis, including proliferation, survival, migration, and tube formation (reviewed in Refs. [4–6]). VEGF can also contribute to the formation of tumor neovessels by mobilizing endothelial progenitor cells from the bone marrow. Moreover, VEGF regulates vascular permeability, thus playing a role in the formation of malignant effusions. Aberrant production of VEGF has been reported in several tumor types. Inhibition of the VEGF pathway has shown promise as an approach to tumor therapy; indeed, VEGF inhibitors are among the few antiangiogenic compounds that have demonstrated efficacy in clinical trials [4,6].
Tumor progression is known to involve complex and dynamic interactions between cancer cells and the host microenvironment, with the host actually contributing to tumor malignancy. In this context, angiogenesis exemplifies the consequences of deregulated cross-talk between tumor cells and the local microenvironment to favor tumor progression through the generation of a functional blood vessel system. The interplay between tumor and host cells, especially with endothelial cells, is mediated by different mechanisms, including direct cell-cell contact and secretion of cytokines and bioactive molecules released as either soluble factors or components of shed membrane vesicles.
Shedding of membrane vesicles from the cell surface is widely observed in normal and tumor cells in vivo and in vitro [7]. In normal cells, the process appears to be tightly regulated because only a limited number of vesicles are shed from defined areas of the plasma membrane in response to specific stimuli [8]. In contrast, vesicle shedding by tumor cells is largely a deregulated process because numerous vesicles are constitutively shed from the entire cell surface [9,10].
Shed membrane vesicles carry bioactive molecules that modulate cell functions in an autocrine and a paracrine manner, ultimately affecting several aspects of tumor malignancy. Tumor-shed vesicles can affect host immune response [11–14], promote tumor invasiveness and metastasis [10,15–19], confer drug resistance [20], and, as recently described, carry angiogenic stimuli [21–23].
In the present study, we investigated the role and molecular mechanisms of tumor-shed membrane vesicles as mediators of tumor/endothelial cell cross-talk. Our findings demonstrate that tumor-shed vesicles transport VEGF and that the bioavailability of angiogenic factor depends on vesicle rupture induced by acidic pH in the microenvironment.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cord veins and grown on 1% gelatin-coated flasks in M199 supplemented with 10% fetal calf serum (FCS), 10% newborn calf serum, 20 mM HEPES, 6 U/ml heparin, 2 mM glutamine, 50 µg/ml endothelial cell growth factor (crude extract from bovine brain), penicillin, and streptomycin. HUVECs were routinely tested and found positive for both VEGFR-1 and VEGFR-2. Cells were used between the third and fifth passages.
The CABA I cell line was established from the ascitic fluid of an ovarian carcinoma patient not undergoing drug treatment [24]. Cells were grown as monolayers in RPMI 1640 (Euroclone, Devon, UK) with 5% FCS.
Human ovarian carcinoma A2780 cells were cultured in RPMI 10% FCS. The VEGF-overexpressing variant 1A9-VS-1 and the control 1A9-VAS-3 were generated from the A2780-derived cell line 1A9 by stable transfection with VEGF121 sense (1A9-VS-1) or antisense (1A9-VAS-3) cDNA. These cells did not express VEGFR-1 nor VEGFR-2, hence ruling out a possible autocrine activity of VEGF in these cells. [25]. In this study, the parental 1A9 and control 1A9-VAS-3 cells gave identical results; thus, only results obtained with 1A9-VAS-3 are shown.
Vesicles were isolated from subconfluent viable cell cultures in which the number of trypan blue-positive dead cells was negligible. We previously established that shedding of vesicles is optimal when cells are incubated for 6 to 16 hours in serum-containing medium [9]. Thus, ovarian carcinoma cells were rinsed once with serum-free medium and cultured for 6 to 16 hours in medium containing 5% FCS, and supernatants were collected and processed as described below. The remaining cells were trypsinized and counted. Vesicles isolated from at least two independent preparations of conditioned medium were analyzed for each experimental condition.
Isolation of Membrane Vesicles from Cell-Conditioned Medium
Vesicles were prepared as described [10]. Conditioned medium obtained as above was centrifuged at 600g for 15 minutes and at 1500g for 15 minutes to remove cells and large debris. Supernatants were then centrifuged at 100,000g for 1 hour at 4°C. Unless otherwise indicated, pelleted vesicles were resuspended in phosphate-buffered saline (PBS), pH 7.4. In some experiments, pellets were resuspended in water or in citrate-PBS at different pH values, as indicated (pH 5.6–6.0). Vesicles were quantified based on measurements of vesicle-associated protein levels, using the method of Bradford (Bio-Rad, Milan, Italy), with bovine serum albumin (BSA; Sigma, St. Louis, MO) as standard.
Electron Microscopy
Scanning electron microscopy (SEM) was carried out on subconfluent cells grown on coverslips and fixed with 2% glutaraldehyde in PBS for 30 minutes. Samples were critical point-dried, glued onto stubs, coated with gold in a SCD040 Balzer Sputterer, and observed using a Philips 505 SEM at 10 to 30 kV.
Ultrastructural morphology of isolated tumor-shed vesicles was analyzed by transmission electron microscopy (TEM). The ultracentrifugation pellet containing membrane vesicles was resuspended in PBS (unless otherwise indicated) and applied to collodion-coated grids. After washing, vesicles were negatively stained with 1% phosphotungstic acid, brought to pH 7.0 with NaOH, and examined by TEM as described [10].
Motility and Invasion Assays
Endothelial cell motility and invasiveness were assayed using modified Boyden chambers with polycarbonate PVP-free Nucleopore filters (pore size, 8 µm) [26]. Vesicles, added at the indicated concentrations to the lower compartment of the chamber, were used as attractants. Buffer alone or human recombinant VEGF (10 ng/ml; R&D Systems, Minneapolis, MN) were used as negative and positive reference controls, respectively. For motility (chemotaxis), filters were coated with 0.1% gelatin. For invasion, filters were coated with a thick layer of the reconstituted basement membrane Matrigel (0.5 mg/ml; Becton Dickinson, Bedford, MA), which cells must degrade to migrate through the filter. HUVECs were detached, washed in DMEM-0.1% BSA, resuspended in the same medium at a concentration of 5 x 105 ml-1, and added to the upper compartment of the chamber. After 4 hours (motility) or 6 hours (invasion), filters were stained with Diff-Quik (Baxter, Dudingen, Switzerland), and migrated cells in 10 high-power fields were counted. When experimental conditions required the use of different buffers, variations in endothelial cell motility in various media were taken into account by expressing the data as migration index: (cells that migrated to vesicles - cells that migrated to buffer alone) / (cells that migrated to VEGF - cells that migrated to buffer alone).
In some experiments, cells were incubated for 30 minutes with the VEGF receptor-2 inhibitor SU5416 [27] (provided by Sugen/Pfizer, San Francisco, CA), which was then left for the duration of the experiment. Before use, SU5416 was dissolved in DMSO (stock solution, 40 mM) followed by dilution with medium. Human recombinant TIMP-2 (R&D Systems), an inhibitor of matrix metalloproteinases, was added to the cells immediately before seeding in the Boyden chamber and left throughout the assays.
Enzyme-Linked Immunosorbent Assay (ELISA) for VEGF and Fibroblast Growth Factor-2 (FGF-2)
Human VEGF and FGF-2 were measured by ELISA (Quantikine Human VEGF Immunoassay; R&D Systems). ELISA for VEGF recognizes human VEGF121 and VEGF165. The sensitivity of the assays was 9.0 pg/ml for VEGF and 3 pg/ml for FGF-2. Vesicles were exposed to detergents to release potentially stored cytokines. Assays were conducted according to the manufacturer's directions. The amount of VEGF was normalized to protein content, and data are expressed as picograms per microgram.
Western Blot Analysis of VEGF
Vesicles (7 µg) from 1A9-VAS-3 and 1A9-VS-1 cells were resolved by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Nonspecific binding sites were blocked by overnight incubation with 10% nonfat dry milk in PBS containing 0.5% Tween-20. Blots were incubated with antibody against human VEGF (clone A-20, 1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour, followed by peroxidase-conjugated secondary antibody, in blocking buffer. After washing, reactive bands were visualized using a chemiluminescence detection kit (ECL; Amersham-Pharmacia, Uppsala, Sweden).
Zymography
Zymography was performed using SDS-(8%) polyacrylamide gels copolymerized with 1 mg/ml gelatin type B (Sigma). Vesicles (10 µg) were diluted in an SDS-PAGE sample buffer in nonreducing conditions without heating. After electrophoresis, gels were washed twice for 30 minutes in 2.5% Triton X-100 at room temperature and incubated overnight in collagenase buffer (50 mM Tris-HCI, pH 7.6,100 mM NaCl, 0.02% Brij 35, and 5 mM CaCl2) at 37°C. Gels were stained with Coomassie Blue R 250 (Bio-Rad) in 30% methanol and 10% acetic acid for 2 hours and destained in the same solution without dye. Gelatinase activity was visualized as white bands on a dark background, indicating proteolysis of the substrate. The supernatant of WM983A melanoma cells was used as a reference standard for MMP-2 and MMP-9.
Reverse Zymography
Vesicles were applied to a 14% polyacrylamide gel polymerized with 1 mg/ml gelatin and a 20% (vol/vol) 6x concentrated conditioned medium of HT-1080 cells, used as a source of gelatinases. After electrophoresis, gels were washed, incubated in collagenase buffer, and stained as described above for zymography. TIMP-1 and TIMP-2 appeared as dark bands at 28 and 21 kDa, respectively, corresponding to the areas where gelatin degradation by gelatinases added to the gel is prevented by inhibitors. The supernatant of WM983A cells was used as a reference standard for TIMPs.
Statistical Analysis
Data were analyzed using the Mann-Whitney U test (differences were considered statistically significant at P ≤ .05) or ANOVA, followed by Fisher exact text.
Results
Membrane Vesicles Shed by Ovarian Carcinoma Cells Stimulate Endothelial Cell Motility and Invasiveness
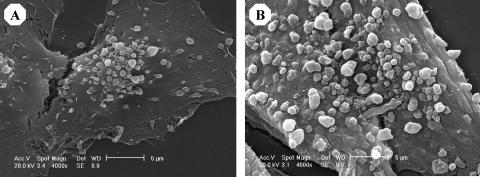
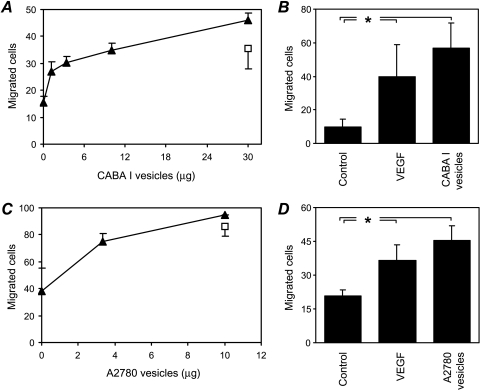
Scanning electron microscopic analysis of the human ovarian carcinoma cell lines CABA I and A2780 revealed the vesicle-shedding phenomenon in both cell lines (Figure 1). Vesicles were released after culture in a serum-containing medium (Figure 1) but not in a serum-free medium (not shown), consistent with previous results [10]. Vesicles appeared to bud from the cell plasma membrane. Their morphology and size (300–1200 nm diameter) were similar to vesicles shed by other tumor cells [9,16–18,21] and were clearly distinct from significantly smaller exosomes [28]. To assess whether tumor-shed vesicles might convey angiogenic stimuli, vesicles shed by CABA I and A2780 cells were isolated by centrifugation procedures that allow the recovery of intact vesicles and were tested for their ability to stimulate two endothelial cell functions relevant to angiogenesis, motility, and invasiveness. Vesicles derived from both cell lines stimulated HUVEC motility in a dose-dependent manner, with a significant induction observed at ≥3.3 µg (Figure 2, A and C). Vesicles (5 µg) were also able to stimulate HUVEC invasiveness (Figure 2, B and D), which requires motility as well as matrix degradation. In both the motility and invasiveness assays, the stimulatory effect of vesicles (≥3.3 µg) was significant (P < .05) and comparable to that of the reference angiogenic factor VEGF (Figure 2). Vesicles were also able to stimulate endothelial cell proliferation (not shown).
Figure 1.
Scanning electron micrograph of CABA I (A) and A2780 (B) cell vesicle shedding.
Figure 2.
Effect of tumor cell-shed vesicles on endothelial cell motility and invasiveness. HUVEC motility (A and C) was tested in the Boyden chamber using isolated vesicles shed by human ovarian carcinoma cells CABA I (A; triangles) and A2780 (C; triangles) used as attractants. VEGF (10 ng/ml; squares) was used as a reference stimulus. In the invasion assay (B and D), HUVECs were stimulated by vesicles (5 µg) shed by CABA I (B) or A2780 (D), or by VEGF (10 ng/ml) used as a reference stimulus. Data (mean and SD of triplicates) represent the number of cells that migrated in 10 high-power fields (representative of two to four experiments). *P ≤ .05.
These findings indicate that tumor-derived vesicles are able to stimulate endothelial cell functions that are crucial to the process of angiogenesis.
Molecular Characterization of Shed Vesicles
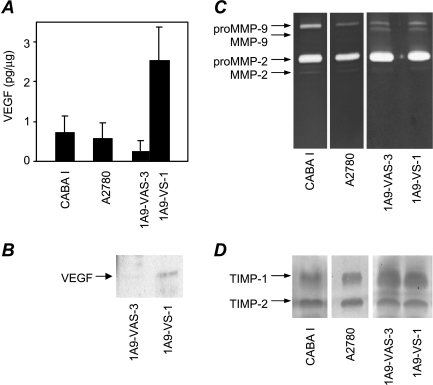
ELISA analysis to determine whether vesicles shed by CABA I and A2780 cells contained VEGF revealed measurable levels of this major angiogenic factor (Figure 3A). A comparison of vesicles derived from A2780-derived 1A9-VS-1 cells and transfected with VEGF121 cDNA and from control 1A9-VAS-3 cells [25] showed that the two cell variants shed essentially the same amount of vesicles (18.5 and 17.8 µg per 107 cells for 1A9-VAS-3 and 1A9-VS-1, respectively, in the presence of serum; 0.5 and 0.6 µg per 107 cells, respectively, in the absence of serum). However, vesicles isolated from the VEGF-overexpressing 1A9-VS-1 cells contained more VEGF than did their control cell-derived counterparts (P = .02; Figure 3A). Western blot analysis of the isolated vesicles using antibodies against human VEGF confirmed the presence of VEGF121 in the vesicles derived from 1A9-VS-1 cells, whereas VEGF was only barely detectable in control cell-shed vesicles (Figure 3B).
Figure 3.
Molecular characterization of vesicles shed by human ovarian carcinoma cell lines and transfected variants. (A) ELISA analysis of VEGF in vesicles. (B) Western blot analysis of vesicle-associated VEGF. (C) Zymographic analysis of vesicle-associated gelatinases. (D) Reverse zymographic analysis of vesicle-associated TIMP-1 and TIMP-2. Experiments were conducted as described in Materials and Methods.
ELISA analysis of FGF-2 in shed vesicles revealed no detectable levels of this angiogenic factor in either CABA I or A2780 cells (not shown).
Based on our previous findings that vesicles shed by normal and transformed cells contain matrix metalloproteinases, which modulate several cell functions including invasiveness, we analyzed vesicles isolated from CABA I and A2780, and the two 1A9 variants for the presence of the two gelatinases MMP-2 and MMP-9. Zymography revealed both the proenzyme and active forms of MMP-2 in all cell lines, whereas CABA I and A2780 contained only the MMP-9 proenzyme (Figure 3C). 1 A9-VAS-3-shed and 1A9-VS-1-shed vesicles showed a similar pattern of gelatinases. Vesicles also contained TIMP-1 and TIMP-2, endogenous inhibitors of matrix metalloproteinases, as assessed by reverse zymography (Figure 3D).
Functional Activity of Vesicle-Associated VEGF
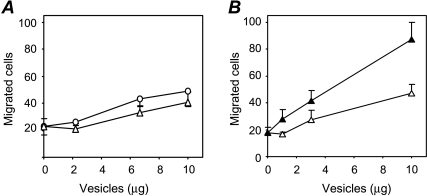
To evaluate the biologic relevance of vesicle-associated VEGF, we tested whether VEGF contained in shed vesicles stimulated endothelial cell motility. Vesicles from both cell variants stimulated endothelial cell motility at degrees comparable to the parental cell line A2780 (two-fold increase; Figures 2C and 4A). Notably, despite the greater VEGF content in 1A9-VS-1 vesicles (Figure 4A), the vesicles shed from the two variants had the same stimulatory activity, suggesting that VEGF present in the vesicles was not active/available for endothelial cells in these conditions.
Figure 4.
Stimulation of endothelial cell motility by shed vesicles. (A) Vesicles shed from 1A9-VAS-3 (circles) and 1A9-VS-1 (triangles) were isolated and tested for their ability to stimulate HUVEC motility (see Figure 2). (B) Vesicles isolated from 1A9-VS-1 cells were resuspended in either PBS (open triangles) or water (filled triangles), and, after correction of molarity, tested for motogenic activity. Data (mean and SD of triplicates) represent the number of cells that migrated in 10 high-power fields.
To test the hypothesis that breakage of vesicles was required to allow the release of stored bioactive VEGF, 1A9-VS-1-derived vesicles were exposed to hypotonic conditions (water) and tested for their ability to stimulate endothelial cell motility. Vesicles subjected to osmotic shock were indeed more active in inducing HUVEC motility (P = .02; Figure 4B), indicating that vesicle burst releases and makes stored motility factors available to cells.
pH-Dependent Vesicle Break Causes the Release of Bioactive VEGF
To identify the stimulus that might induce tumor vesicle rupture and the subsequent release of stimulatory factors in vivo, we focused on the known acidic extracellular pH in solid tumors [29,30]. After ultracentrifugation of 1A9-VAS-3-and 1A9-VS-1-conditioned media, isolated vesicles were resuspended in buffers at different pH values (7.4-5.6) and analyzed for morphology and functional activity (stimulation of HUVEC migration).
TEM and negative staining revealed rounded intact structures in vesicles treated with physiological buffer (pH 7.4; Figure 5A), whereas a reduction of pH caused a progressive break of vesicles. At pH 6, intact vesicles were present but rare, and linear membrane filaments, derived from broken vesicles, were evident (Figure 5B, arrows); analysis of vesicle pellets resuspended in pH 5.6 buffer revealed a complete disruption of rounded vesicular structures and only sporadic membrane fragments and shapeless masses of membrane remains (Figure 5C).
Figure 5.
Effect of pH on vesicle integrity and activity. Freshly isolated 1A9-VS-1-derived vesicle pellets were resuspended in buffers at the indicated pH and analyzed by TEM with negative staining for changes in morphology (A–C) and motogenic activity (D). (A) Pelleted vesicles resuspended in PBS (pH 7.4), showing intact rounded vesicle structures. (B) Pelleted vesicles resuspended in buffer at pH 6.0, showing both intact vesicles (short arrow) and small membrane portions derived from broken vesicles (arrows). (C) Pelleted vesicles resuspended in buffer at pH 5.6, revealing no intact vesicles and only membrane fragments (arrows), often aggregated in amorphous masses (inset). (D) Chemotactic activity for HUVECs by vesicles derived from 1A9-VS-1 cells and resuspended in buffers at the indicated pH (neutralized before the assay). Data (mean and SD of triplicates) are expressed as migration index (see Materials and Methods) (representative of three experiments). *P ≤ .05. Scale bars, 1 µm (A–C).
Analysis of vesicles exposed to buffers at different pH values for their ability to induce HUVEC motility showed that 1A9-VS-1-derived vesicles exposed to pH 5.6 and 6 were significantly more active (P < .05) than vesicles exposed to pH 7.4 in inducing endothelial cell motility (Figure 5D). Increased activity was also observed in vesicles derived from control 1A9-VAS-3 cells exposed to low pH (not shown), presumably due to endogenous VEGF. However, the pH-associated increase in activity was always higher in vesicles derived from the VEGF-overexpressing 1A9-VS-1 cells compared to the control cells (mean increase of 16.2 ± 9.5-fold vs 3.0 ± 0.6-fold, respectively, at pH 5.6), reflecting differences in VEGF content. These findings confirm that the break of vesicles induced by acidic pH results in the release of bioactive VEGF.
The specificity of the effect was tested using the small molecule inhibitor of the VEGF tyrosine kinase receptor SU5416 [27] and the physiological inhibitor of matrix metalloproteinases TIMP-2. Stimulation of endothelial cell motility by vesicles was dramatically reduced in the presence of SU5416 or, to a lesser extent, in the presence of TIMP-2 (Table 1). In contrast, SU5416 only partially reduced vesicle-stimulated endothelial cell invasion, whereas TIMP-2 blocked this function (Table 1). These findings indicate the role of VEGF and matrix metalloproteinases as major mediators of vesicle-induced endothelial cell motility and invasiveness, respectively.
Table 1.
Effect of Inhibitors on Vesicle-Induced Endothelial Cell Motility and Invasiveness.
| Inhibitor | Motility | Invasiveness |
| Control | 100 | 100 |
| SU5416 | 5.9 ± 9.4** | 39.3 ± 35.5 |
| TIMP-2 | 34.6 ± 7.6* | 13.1 ± 5.7** |
Endothelial cell motility and invasiveness in response to vesicles were assayed in the presence of SU5416 (10 µM) or TIMP-2 (1 µg/ml). Data are the percentages of control migration, after subtraction of baseline motility.
P < .05.
P < .02.
Discussion
The present study provides evidence that membrane vesicles shed by human ovarian carcinoma cells act as carriers of active angiogenic stimuli, specifically VEGF, which is able to promote endothelial cell functions relevant to angiogenesis. In addition, the data show that VEGF is released in a bioactive form by vesicle break induced by acidic pH.
Shedding of membrane vesicles by tumor cells is known to mediate tumor cell interaction with the environment and to contribute to tumor progression [12,18–20,31,32]. Recently, vesicles shed by tumor cells, platelets, and endothelial cells themselves have also been implicated in the process of angiogenesis. Vesicles have been described to stimulate proangiogenic activities of endothelial cells, including motility, invasiveness, capillary-like cord formation, and urokinase plasminogen activator production, to augment the synthesis of angiogenic factors in tumor cells and to stimulate angiogenesis in the chickchorioallantoic membrane [8,21–23]. Consistent with those studies, we found that vesicles shed by ovarian carcinoma cells contain proangiogenic activity, which is able to stimulate endothelial cell motility and invasiveness.
Different molecular mediators of the angiogenic activity of vesicles have been identified, including matrix metalloproteinases [8], FGF-2 [21], and sphingomyelin [22]. We also detected vesicle-associated matrix metalloproteinases but not vesicle-associated FGF-2, although additional studies are needed to completely exclude a role for this or other angiogenic factors in our system. With respect to sphingomyelin, our finding that heated vesicles lose most of their ability to stimulate endothelial cell motility (not shown) suggests that heat-resistant lipids, such as sphingomyelin [22], are not major players in our system. Instead, we have demonstrated that the angiogenic factor VEGF can be released by tumor cells as a component of membrane vesicles. The presence of VEGF in tumor-shed membrane vesicles was documented by ELISA and Western blot analysis, and by the finding that vesicles released by the VEGF-overexpressing 1A9-VS-1 cells contained more VEGF than did the control 1A9-VAS-3 cells.
Vesicles are known to carry a variety of active factors. Some of these molecules, such as MMP-2, MMP-9, uPA [18], HLA class I, integrin-β1 [9], FasL [33,34], and sphingomyelin [22], are associated with the vesicle membrane and therefore are readily available. The presence of these molecules might justify the observed chemotactic activity of intact vesicles. Other vesicle-associated molecules, mostly soluble factors such as FGF-2 [21], interleukin-1β [35], and TGF-β [12], are stored within the vesicle. Our study indicates that VEGF is also carried within the vesicles. Indeed, transmission electron immunogold analysis of intact vesicles revealed no positive staining for VEGF on the vesicle surface (not shown). Moreover, vesicle burst caused by either hypotonic shock or low pH increased the motogenic activity of VEGF-containing vesicles, further indicating the intravesicular location of this factor. VEGF is normally stored in cytoplasmic granules of tumor cells, and such granules might move inside membrane vesicles during vesicle formation.
The functional activity of VEGF in vesicles is exerted only after vesicle burst. Indeed, VEGF in intact vesicles is not available because high-VEGF vesicles from 1A9-VS-1 cells had the same chemotactic activity as low-VEGF vesicles from control 1A9-VAS-3 cells. Only when vesicles were broken did VEGF become bioavailable and did chemotactic activity increase, with the highest activity in vesicles from 1A9-VS-1 cells. This behavior is similar to that reported for microvesicle-associated interleukin-1β [35] and vesicle-associated secreted FasL, which were isolated from ovarian cancer cells and were functional only when the integrity of the vesicle membrane was disrupted [33].
How vesicle disruption occurs in vivo is presently unclear, although different mechanisms have been proposed. Vesicles are unstable structures and, at physiological temperatures (37°C), degrade in a few hours [21]. Fusion with target cells has also been hypothesized as a mechanism of release of vesicle-associated factors [21]. Our present study provides evidence for a mechanism by which a low-pH environment induces loss of vesicle integrity (as observed by TEM) and release of their contents, including active VEGF. In fact, the tumor microenvironment is characterized by interstitial acidity, often associated with hypoxia [29,30]. Acidification is the consequence of tumor cell-elevated glycolysis, initially driven by hypoxia and then becomes constitutive [30]. Normal cells cannot survive in an acidic environment, whereas tumor cells are fully adapted to growth in low-pH conditions. Low tumor pH has been reported to promote malignant progression by altering the response to radiation and chemotherapy, promoting mutagenesis, and stimulating invasive and metastatic potentials [30]. An acidic environment has also been reported to stimulate the production of proangiogenic cytokines [36]. Because hypoxia is known to increase the synthesis of VEGF though HIF1-α activation [37], it seems possible that the high levels of VEGF released in hypoxic-acidic regions reflect both an increased synthesis of the angiogenic factor and a release from burst vesicles.
The mechanism of pH-dependent vesicle break is still unclear. Whereas a direct effect of pH on the structure of the lipid bilayer appears unlikely, acidity might act on pH-sensitive enzymatic complexes present on the vesicle surface. The exact mechanism of vesicle burst awaits further study.
In conclusion, this study shows that tumor-shed membrane vesicles act as carriers of VEGF, which contributes to the cross-talk between tumor and endothelial cells essential for tumor angiogenesis. Moreover, the present data are the first to demonstrate that tumor-promoting activities of membrane vesicles are modulated by the extracellular microenvironment and are activated in conditions of acidic pH typical of tumor settings.
Footnotes
This work was supported by grants from the Italian Ministry of University and Scientific and Technological Research to A.P. and V.D.; from FIRB to A.P. and G.T.; from AIRC to R.G. and G.T.; and from the European Union FP6 (LSHC-CT-2003-503297) to G.T.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am J Pathol. 2003;162:1747–1757. doi: 10.1016/s0002-9440(10)64309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DD, Black PH. Shedding of plasma membrane fragments. In: Steinberg M, editor. Developmental Biology. New York: Plenum Press; 1986. pp. 33–57. [DOI] [PubMed] [Google Scholar]
- 8.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolo V, Adobati E, Canevari S, Picone MA, Vittorelli ML. Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clin Exp Metastasis. 1995;13:277–286. doi: 10.1007/BF00133483. [DOI] [PubMed] [Google Scholar]
- 10.Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML. Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. J Submicrosc Cytol Pathol. 1994;26:173–180. [PubMed] [Google Scholar]
- 11.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, Dainiak N. Biologically active Fas antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood. 1998;91:3862–3874. [PubMed] [Google Scholar]
- 12.Dolo V, Pizzurro P, Ginestra A, Vittorelli ML. Inhibitory effects of vesicles shed by human breast carcinoma cells on lymphocyte 3H-thymidine incorporation, are neutralised by anti TGF-beta antibodies. J Submicrosc Cytol Pathol. 1995;27:535–541. [PubMed] [Google Scholar]
- 13.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 14.Zitvogel L, Fernandez N, Lozier A, Wolfers J, Regnault A, Raposo G, Amigorena S. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur J Cancer. 1999;35(Suppl 3):S36–S38. doi: 10.1016/s0959-8049(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 15.Dolo V, D'Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17:131–140. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- 16.Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, Nagase H, Canevari S, Pavan A, Vittorelli ML. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58:4468–4474. [PubMed] [Google Scholar]
- 17.Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli ML. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J Biol Chem. 1997;272:17216–17222. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 18.Angelucci A, D'Ascenzo S, Festuccia C, Gravina GL, Bologna M, Dolo V, Pavan A. Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin Exp Metastasis. 2000;18:163–170. doi: 10.1023/a:1006778000173. [DOI] [PubMed] [Google Scholar]
- 19.Poste G, Nicolson GL. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci USA. 1980;77:399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 21.Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, Saladino F, Dolo V, Dell'Era P, Pavan A, et al. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J Biol Chem. 2003;278:51911–51919. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- 22.Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 23.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 24.Dolo V, Ginestra A, Violini S, Miotti S, Festuccia C, Miceli D, Migliavacca M, Rinaudo C, Romano FM, Brisdelli F, et al. Ultrastructural and phenotypic characterization of CABA I, a new human ovarian cancer cell line. Oncol Res. 1997;9:129–138. [PubMed] [Google Scholar]
- 25.Manenti L, Riccardi E, Marchini S, Naumova E, Floriani I, Garofalo A, Dossi R, Marazzo E, Ribatti D, Scanziani E, et al. Circulating plasma vascular endothelial growth factor in mice bearing human ovarian carcinoma xenograft correlates with tumor progression and response to therapy. Mol Cancer Ther. 2005;4:715–725. doi: 10.1158/1535-7163.MCT-04-0305. [DOI] [PubMed] [Google Scholar]
- 26.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendel DB, Schreck RE, West DC, Li G, Strawn LM, Tanciongco SS, Vasile S, Shawver LK, Cherrington JM. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin Cancer Res. 2000;6:4848–4858. [PubMed] [Google Scholar]
- 28.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 29.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 30.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 31.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 32.Zucker S, Wieman JM, Lysik RM, Wilkie DP, Ramamurthy N, Lane B. Metastatic mouse melanoma cells release collagen-gelatin degrading metalloproteinases as components of shed membrane vesicles. Biochim Biophys Acta. 1987;924:225–237. doi: 10.1016/0304-4165(87)90091-2. [DOI] [PubMed] [Google Scholar]
- 33.Abrahams VM, Straszewski SL, Kamsteeg M, Hanczaruk B, Schwartz PE, Rutherford TJ, Mor G. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 2003;63:5573–5581. [PubMed] [Google Scholar]
- 34.Martinez-Lorenzo MJ, Anel A, Alava MA, Pineiro A, Naval J, Lasierra P, Larrad L. The human melanoma cell line MelJuSo secretes bioactive FasL and APO2L/TRAIL on the surface of microvesicles. Possible contribution to tumor counterattack. Exp Cell Res. 2004;295:315–329. doi: 10.1016/j.yexcr.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 35.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 2000;60:4610–4616. [PubMed] [Google Scholar]
- 37.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]