Abstract
Malaria and other haemosporin parasites must undergo a round of sexual reproduction in their insect vector in order to produce stages that can be transmitted to vertebrate hosts. Consequently, it is crucial that parasites produce the sex ratio (proportion of male sexual stages) that will maximize the number of fertilizations and thus, transmission to new vertebrate hosts. There is some evidence to show that, consistent with evolutionary theory, the sex ratios of malaria parasites are negatively correlated to their inbreeding rate. However, recent theory has shown that when fertilization success is compromised, parasites should respond by increasing their investment in sexual stages or by producing a less female biased sex ratio than predicted by their inbreeding rate alone. Here, we show that two species of rodent malaria, Plasmodium chabaudi and Plasmodium vinckei petteri, adopt different strategies in response to host anaemia, a factor thought to compromise transmission success: P. chabaudi increases investment in sexual stages, whereas P. vinckei produces a less female biased sex ratio. We suggest that these different transmission strategies may be due to marked species differences in host cell preference.
Keywords: anaemia, sex ratio, erythropoietin, Plasmodium, gametocyte, fertility insurance, reticulocyte
1. Introduction
Sex allocation theory predicts how organisms should alter the proportion of their offspring which are male (termed the sex ratio) in response to environmental conditions (Charnov 1982; Godfray & Werren 1996). This theory has been successfully applied to a broad range of taxa and provides some of the clearest examples of adaptation (Charnov 1982; West & Sheldon 2002). Malaria and related protozoan blood parasites produce sexual stages, termed gametocytes, which undergo gametogenesis when taken up in a vector's blood meal. In the blood meal, male gametes locate female gametes and fertilization occurs. Recently, there has been much interest in applying sex ratio theory to malaria and related protozoan parasites (Read et al. 1995, 2002; West et al. 2000, 2001, 2002). In this case, theory predicts that the sexual stage (gametocyte) sex ratio has been shaped by the inbreeding rate, with the evolutionary stable (ES) sex ratio (r*) given by r*=(1−F)/2, where F is Wright's coefficient of inbreeding (Read et al. 1992; Dye & Godfray 1993; Nee et al. 2002). Although this theory has provided a general understanding of protozoan sex ratios, there are many discrepancies that are yet to be explained, such as why sex ratios show considerable variation during infections (Schall 1989; Shutler et al. 1995; Robert et al. 1996; Shutler & Read 1998; West et al. 2001; Paul et al. 2002b; Read et al. 2002).
Recently, Paul et al. (2000) have shown that in both rodent and avian malaria parasites (Plasmodium vinckei and P. gallinaceum, respectively) the sex ratio changes during the course of an infection, from extremely female biased to approximately equal numbers of male and female gametocytes. This change appeared to be a response to host anaemia. When hosts are anaemic, the kidney and liver cells are stimulated to secrete erythropoietin (Epo) that initiates the differentiation of young red blood cells (reticulocytes) in a process termed erythropoiesis (Jelkmann & Hellwig-Burgel 2001). Three to four days after stimulation by Epo, reticulocytes appear in the circulation where they mature into erythrocytes. Although reticulocytes are a known cue for gametocyte production in many Plasmodium species (Trager & Gill 1992; Gautret et al. 1996b, 1997; Trager et al. 1999), Paul et al.'s experiments demonstrate that parasites used Epo levels as a cue for sex ratio shifts, rather than changes in reticulocyte or erythrocyte density. Why should parasites increase their sex ratio in response to a rise in their host's Epo level? Paul et al. suggested that Epo levels in natural infections may correlate with the appearance of host immune factors that decrease the likelihood of male gametes encountering female gametes (Paul et al. 2000, 2002a, 2003; Reece & Read 2000). In this case, producing a less female biased sex ratio is a form of ‘fertility insurance’ to increase the probability that all female gametes in a blood meal are fertilized (West et al. 2001, 2002; Gardner et al. 2003). In natural infections, if an increase in Epo correlates with the appearance of factors that can block fertilization in the vector, parasites that responded to Epo would be favoured (Paul et al. 2003). Although fertility insurance offers the potential to explain why sex ratios of Plasmodium species show considerable variation during the course of an infection, the hypothesis has not been tested empirically.
A number of studies have demonstrated that a variety of host immune factors impair fertilization in the mosquito, but it is not known if any of these factors correlate with host anaemia or circulating Epo levels. For instance, host antibodies, especially immunoglobulin G, can immobilize and agglutinate motile male gametes in the blood meal (Gwadz 1976; Aikawa et al. 1981; Mendis & Targett 1981; Ranawaka et al. 1994). Host nitric oxide (NO) can block transmission, possibly by preventing the DNA replication required by male gametocytes to undergo gametogenesis (Luckhart et al. 1998; Balmer et al. 2000). In addition, slight anaemia facilitates vector feeding, but severe anaemia could reduce transmission success (Shieh & Rossignol 1992). Vectors feeding on very anaemic hosts obtain blood meals containing few red blood cells and this could result in too few male gametes being present in the blood meal to fertilize all of the female gametes (Shutler & Read 1998; West et al. 2001).
Here, we aim to verify Paul et al.'s finding that P. vinckei undergoes a sex ratio shift in response to Epo and test whether another rodent malaria parasite, P. chabaudi, responds in the same way. We also explore our data in the context of fertility insurance and the life history traits of these species. We conducted three experiments to investigate the effect of manipulating host Epo, and the roles NO, anaemia and reticulocyte density play in shaping both the investment in gametocytes and the sex ratios of these species. In P. chabaudi, we artificially increased Epo at the start of infection (experiment 1a) and further into infection, during the second wave of gametocyte production (experiment 1b). In P. chabaudi the gametocyte density is low at the start of infection so experiment 1b overcame the possible problem of weak statistical power due to low gametocyte densities. We also manipulated Epo at the start of P. vinckei infections (experiment 2). The Epo manipulations early in infection were consistent with Paul et al., and avoided confounding our artificial Epo with the endogenous Epo produced as hosts become anaemic.
2. Materials and methods
(a) Parasites and hosts
In experiments 1a and 1b, we used C57 black mice and in experiment 2 we used NIH mice. All mice were 10–12 weeks old (Harlan-Olac, UK) and received an intra-peritoneal inoculation of 106 parasitized red blood cells in a 0.1 ml dose consisting of 47.5% Ringer's (27 mM KCl, 27 mM CaCl2, 0.15 M NaCl), 50% heat inactivated calf serum and 2.5% heparin (200 units ml−1). Mice in experiments 1a and 1b were infected with P. chabaudi, clone ER, and mice in experiment 2 with P. vinckei petteri, clone BS (WHO Registry of Standard Malaria Parasites, University of Edinburgh, UK). We housed all mice randomly in groups of five at 20 °C with a 12 h light/12 h dark cycle, and provided food (41B, Harlan-Teklad, UK) and water with 0.05% PABA (to enhance parasite growth) ad libitum.
(b) Experimental design
For experiments 1a and 2 we followed Paul et al. (2000) and administered Epo when parasites first became detectable in the blood (patency). By administering Epo before extensive parasite growth and the resulting host anaemia, we avoided confounding the effects of our artificial Epo with varying levels of naturally produced Epo. In experiment 1a we used a total of 15 C57 mice, eight in the Epo‐treated group and seven in the control group; in experiment 2 we used 20 MF1 mice, 10 in each group. Mice in the Epo‐treated groups received 5×0.1 ml intra-peritoneal injections of 100 U l−1 of mouse recombinant Epo dissolved in distilled water (Roche Biochemicals, UK) for five days from patency (approximately days 3–7 post-infection; PI); control mice received placebo injections of distilled water on the same days. Paul et al. observed sex ratio shifts in P. vinckei using Epo doses of 70 and 700 U l−1, so we chose an intermediate dose of 100 U l−1. We sampled all mice in experiments 1a and 2 daily, from day 3 PI to day 17 PI.
In a third experiment (1b) to investigate the effects of Epo on infections when gametocyte density is high, we administered Epo prior to and during the second gametocyte peak of P. chabaudi infections (which occurs on days 14 and 15 PI). We used a total of 15 female C57 mice for this experiment, eight in the Epo‐treated group and seven in the control group. Treatment mice received 5×0.1 ml intra-peritoneal injections of 100 U l−1 of mouse recombinant Epo dissolved in distilled water from days 11 to 15 PI, and control mice received placebo injections of distilled water. In this experiment, we also assayed the NO concentration in the blood of all mice from days 12 to 17 PI as NO is a possible transmission blocking factor (Luckhart et al. 1998). This assay (Oxford Biochemicals; USA), based on the Greiss reagent, is suitable for samples with high protein content and low concentrations of NO and is therefore appropriate for blood samples of 10 μl (Luckhart et al. 1998). We sampled all of the mice in experiment 1b daily, from day 11 PI to day 18 PI.
(c) Data collection
On each daily sampling point we took thin blood smears from the tail vein of each mouse to determine the proportion of red blood cells infected with gametocytes (gametocytaemia), asexual parasites (parasitaemia) and the proportion of red blood cells that were immature (reticulocytes). We stained smears using 10% Giemsa buffered in 90% phosphate solution for 15 min. Giemsa staining does not provide detailed information on the developmental stage of red blood cells, therefore we only differentiated red blood cells as either immature (reticulocytes) or mature cells. We calculated daily parasite, reticulocyte and gametocyte densities (per ml−1) from daily red blood cell densities (Coulter Electronics).
We sexed P. chabaudi gametocytes using a methodology that we have recently developed (Reece et al. 2003). We collected a sample of blood from the tail vein, and allowed it to cool in a humid environment for 15 mins. This procedure allowed the gametocytes to begin to differentiate into gametes, which facilitates the accurate sexing of gametocytes when made into thin smears (Reece et al. 2003). For P. vinckei, we sexed gametocytes from standard thin smears as, in this species, female gametocytes cannot be easily be distinguished from large trophozoites when using the new method suitable for P. chabaudi.
(d) Analysis
To investigate the effects of the Epo treatment in all three experiments, we used generalized linear modelling techniques in S-Plus (Insightful Corporation). We used F-tests to assess the effect of each term in a nested GLM to avoid pseudo-replication problems associated with sampling each mouse every day (Crawley 1993, 2002). To retain maximum power when analysing sex ratio (proportion) data we assumed binomial errors and a logit link function in an analysis of deviance, as proportion data often have non-normally distributed error variance and unequal sample sizes (Crawley 1993, 2002; Pickering et al. 2000). To test whether our Epo treatment was successful we calculated total reticulocyte production by calculating the area under the curve for each host and compared the Epo and control groups using a one tailed two sample t-test. In experiment 1a only, we calculated gametocyte production using the area under the curve for each infection to investigate gametocyte production in more detail. We split the total gametocyte density per infection into gametocytes produced before and after the anaemia crisis (on day 10 PI), and compared the Epo and control groups using two sample t-tests.
3. Results
(a) Epo manipulations
The total reticulocyte density of Epo‐treated hosts was significantly higher than control hosts in all three experiments, indicating that the experimental manipulation was successful (experiment 1a: t=2.167, p=0.024, n=15; experiment 1b: t=3.197, p=0.003, n=15; experiment 2: t=3.251, p=0.002, n=20; table 1).
Table 1.
The effect of Epo on the total reticulocyte density (×106 ml−1) produced by hosts in all three experiments. (Experiments 1a and 1b, P. chabaudi with Epo administered at the beginning of infection and after crisis, respectively; experiment 2, P. vinckei with Epo administered at the start of infection. Group means and standard errors are presented (test results in text).)
| experiment and species | Epo mean±s.e. | control mean±s.e. |
|---|---|---|
| 1a: P. chabaudi | 18.09±0.66 | 15.45±0.78 |
| 1b: P. chabaudi | 15.70±1.07 | 12.90±0.88 |
| 2: P. vinckei | 78.20±3.20 | 51.60±6.02 |
(b) Effects of Epo on P. chabaudi infections
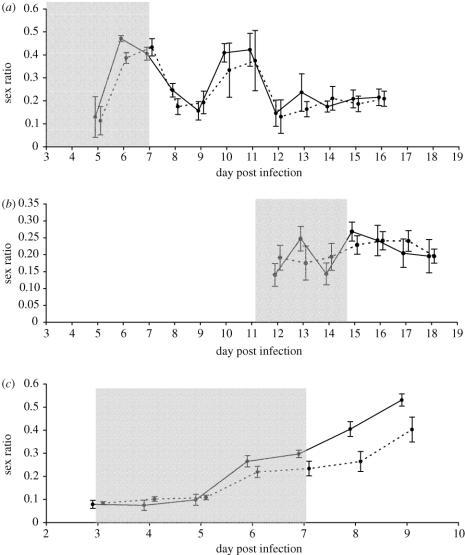
When administered at the start of P. chabaudi infections (experiment 1a) Epo did not have a significant effect on the sex ratio (F1,13=1.17, p=0.30; figure 1a). Epo significantly increased total gametocyte density before the anaemia crisis, which occurs around day 10 PI (figure 2a; Epo mean: 42.05±7.41×106 ml−1 and control mean: 17.18±4.28×106 ml−1, t=3.44, p=0.004), although not after the asexual peak (Epo mean: 20.68±2.05×106 ml−1 and control mean: 28.45±4.20×106 ml−1, t=1.71, p=0.110). Epo did not significantly alter the density of asexual stages (F1,13=1.2, p=0.28) or mature red blood cells (F1,13=0.67, p=0.43).
Figure 1.
Sex ratios (proportion of male gametocytes) observed throughout the course of infection: (a) experiment 1a, when P. chabaudi hosts were treated with Epo at the start of infections; (b) experiment 1b, when hosts were treated with Epo during the second wave of gametocyte production; (c) experiment 2, when P. vinckei hosts were treated with Epo at the start of infections. Dashed line represents untreated controls and error bars are shifted to the right; solid line represents Epo‐treated hosts and error bars are shifted to the left. Plotted points are mean±s.e. Shaded area represents period of Epo administration.
Figure 2.
Gametocyte densities (×106 ml−1) observed throughout the course of infection: (a) experiment 1a, when P. chabaudi hosts were treated with Epo at the start of infections; (b) experiment 1b, when hosts were treated with Epo during the second wave of gametocyte production; (c) experiment 2, when P. vinckei hosts were treated with Epo at the start of infections. Dashed line represents untreated controls and error bars are shifted to the right; solid line represents Epo‐treated hosts and error bars are shifted to the left. Plotted points are mean±s.e. Shaded area represents period of Epo administration.
When administered during the second wave of gametocyte production in P. chabaudi infections (days 11–15 PI; experiment 1b), Epo did not significantly affect sex ratios (F1,13=0.01, p=0.92; figure 1b), or daily densities of gametocytes (F1,13=1.00, p=0.34; figure 2b). Epo treatment did not significantly alter host NO levels (F1,13=2.01, p=0.18) or the density of asexual stages (F1,13=0.05, p=0.83). There was a border-line trend for higher mature red blood densities in the Epo group (F1,13=4.75, p=0.048).
(c) Effects of Epo on P. vinckei infections
When administered at the start of P. vinckei infections (experiment 2), the Epo group had significantly higher sex ratios throughout the sampling period than the control group (F1,18=13.85, p=0.002; figure 1c). However, Epo did not have a significant effect on daily densities of gametocytes (F1,18=1.64, p=0.22; figure 2c). Epo did not significantly alter the density of asexual stages (F1,18=3.80, p=0.07), but Epo significantly increased the density of mature red blood cells (F1,13=5.64, p=0.03).
4. Discussion
Our results show that, in P. vinckei, Epo induces a facultative increase in the gametocyte sex ratio, but has no influence on gametocyte density, consistent with the results of Paul et al. (2000). In contrast, Epo has no effect on the gametocyte sex ratio in P. chabaudi, but when administered at the start of infections, does increase gametocyte density. Epo administered during the second wave of gametocyte production did not affect gametocyte density. This could be due to two factors: (i) at this stage in all infections, parasites were already committed to maximum gametocyte conversion so could not increase this further; (ii) hosts are still recovering from their anaemia crisis during this period so high levels of endogenous Epo could have confounded our Epo treatment.
(a) Different strategies for ensuring transmission?
Recent ‘fertility insurance’ theory has shown that when the probability of fertilizing all the female gametes in a blood meal is reduced, selection favours parasites that respond by either increasing their gametocyte sex ratio (proportion male) or increasing their investment in gametocytes (West et al. 2002; Gardner et al. 2003). Fertilization success could be reduced by host anaemia, the variety of immune factors that appear in the host's circulation or a combination of these factors (Paul et al. 2000, 2002a,2003; Reece & Read 2000; West et al. 2001, 2002) . If a reduction in fertilization success is not consistent throughout infections, then the importance of a ‘fertility insurance strategy’ will vary throughout infections. Our results show that P. vinckei parasites increase their sex ratio and P. chabaudi parasites increase their gametocyte density in response to Epo, a factor that correlates with host anaemia. This suggests that these two species have different strategies for ensuring fertilization when host anaemia, or factors that correlate with host anaemia, compromise transmission success. An alternative explanation is that the increase in the sex ratio of P. vinckei gametocytes is due to sex specific sequestration or differential mortality, with female gametocytes being cleared more quickly from the circulation than males (Fallis & Desser 1974; Schall 1989; Reece et al. 2003). However, it is unlikely that Epo would influence sequestration or antigen recognition in this way. The increase in investment in P. chabaudi gametocytes could also be explained if hosts in control infections produced a stronger gametocyte specific immune response than in the Epo infections. However, gametocyte densities are thought to be relatively unaffected by host immunity and, again, it is unlikely that Epo could affect this (Carter & Graves 1988).
(b) Mechanisms for fertility insurance strategies
Why should different species have different strategies to cope with the problem of fertility insurance? As hosts become anaemic, uninfected mature red blood cells become scarce and the availability of reticulocytes (immature red blood cells) increases. In order to prolong the duration of an infection to enable future transmission, parasites must maintain as large a population of asexual stages as possible (Paul et al. 2003). When red blood cells are scarce there may be a trade off between using the available resources for asexuals and future transmission, or gametocytes for current transmission. In this situation, the ability to invade reticulocytes may determine a parasite's fertility insurance strategy. P. vinckei cannot use reticulocytes (Veins et al. 1971), so when hosts become anaemic and fertility insurance is expected to become important, mature red blood cells become a limited resource. This may leave few suitable cells for both P. vinckei asexuals and gametocytes to invade. Therefore, increasing the sex ratio of those gametocytes that can be produced would be the best way for P. vinckei to maintain transmission. In contrast, P. chabaudi can invade reticulocytes so host cells are not limited when fertility insurance becomes important. Consequently, P. chabaudi parasites can respond by increasing their investment in gametocytes without compromising the resources available for asexuals. The influence of phenylhydrazine induced reticulocyte production on gametocyte density has been investigated in several species of rodent malaria. Consistent with our results, phenylhydrazine does not result in increased gametocyte production in P. vinckei but does in P. chabaudi (Gautret et al. 1996b,1997). Consistent with our hypothesis, phenylhydrazine also increases gametocyte production in P. berghei, a species that preferentially invades reticulocytes (Gautret et al. 1997). In both cases, it is unclear if reticulocytes or Epo are a cue for gametocyte production in these instances.
Our data also show that differences between control and Epo infections in their sex ratios (P. vinckei; figure 1c) and gametocyte densities (P. chabaudi; figure 2a) appeared approximately three days after first Epo administration. These two observations indicate that parasites may be responding to the reticulocyte density resulting from increases in Epo levels rather than Epo itself. In rodent malarias, gametocytes are thought to require 24–36 h to mature after host cell invasion (Carter & Walliker 1975; Landau & Boulard 1978; Gautret et al. 1996a), so a direct response to Epo should have been apparent when sampling 24 or 48 h after first administration. Mechanisms by which parasites could respond to reticulocytes include the following: (i) in species like P. chabaudi, parasites that invade reticulocytes could have a higher probability of producing gametocytes than parasites in mature cells (Ohnishi & Nishimura 2001); (ii) in species like P. vinckei, the sex of gametocytes could be determined by how long they spend in the circulation before locating a mature cell to invade. Invasion could be less efficient when hosts are anaemic, as gametocytes may have to spend longer in the blood before encountering a suitable mature cell, and host immune factors could impair invasion further.
(c) Conclusions and future directions
Our results show that Epo and/or reticulocytes can affect sex allocation and gametocyte density, suggesting that host anaemia may play a role in shaping these transmission strategies. Our data raise several interesting areas for future research into transmission strategies. (i) Experimental manipulations to address whether parasites respond to fluctuations in Epo and/or reticulocytes are challenging, but crucial to understanding sex determination and fertility insurance. (ii) If parasites can detect and assay host Epo levels, they may also respond to other environmental cues such as host antibodies or other immune factors. Understanding how and why they do this will improve medical interventions. It is well known that P. chabaudi and P. falciparum increase their investment in gametocytes in response to sub-curative drug therapy (Buckling et al. 1999a,b). Whether P. vinckei increases its sex ratio under these circumstances remains to be tested. (iii) Given the different responses observed in the two species studied, it may not be appropriate to generalize across species when considering transmission strategies. Recent data show that the sex ratio of the human malaria, P. falciparum, is positively correlated with host anaemia (Robert et al. 2003), but increasing reticulocyte density results in increased gametocyte densities (Gautret et al. 1997). Thus, it is of crucial importance to establish whether the rodent malarias that are used as model systems respond to their host environment in the way that human malaria parasites do.
Acknowledgments
We thank A. Graham for help with the experimental work; the staff at the March Animal House for husbandry; R. Paul and C. Janse for discussion; comments from two anonymous reviewers, A. Mullie and D. Shutler greatly improved the manuscript; and The NERC, BBSRC, Royal Society, and Wellcome Trust for financial support.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Aikawa M, Rener J, Carter R, Miller L.H. An electron microscopical study of the interaction of monoclonal antibodies with gametes of the malaria parasite Plasmodium gallinaceum. J. Protozool. 1981;28:383–388. doi: 10.1111/j.1550-7408.1981.tb02871.x. [DOI] [PubMed] [Google Scholar]
- Balmer P, Phillips H.M, Maestre A.E, McMonagle F, Phillips R.S. The effect of nitric oxide on the growth of Plasmodium falciparum, P. chabaudi and P. berghei in vitro. Parasite Immunol. 2000;22:97–106. doi: 10.1046/j.1365-3024.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Buckling A, Crooks L, Read A. Plasmodium chabaudi: effect of antimalarial drugs on gametocytogensis. Exp. Parasitol. 1999;93:45–54. doi: 10.1006/expr.1999.4429. [DOI] [PubMed] [Google Scholar]
- Buckling A.G.J, Ranford-Cartwright L, Miles A, Read A.F. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118:339–346. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- Carter R, Graves P.M. Churchill Livingstone; London: 1988. Gametocytes in malaria. Principles and practice of malariology. [Google Scholar]
- Carter R, Walliker D. New observations on the malaria parasites of rodents of the Central African Republic—Plasmodium vinkeii petteri subsp. nov. and Plasmodium chabaudi, Landau 1965. Ann. Trop. Med. Parasitol. 1975;69:187–196. doi: 10.1080/00034983.1975.11687000. [DOI] [PubMed] [Google Scholar]
- Charnov E.L. Princeton University Press; 1982. The theory of sex allocation. [PubMed] [Google Scholar]
- Crawley M. Blackwell Scientific; Oxford: 1993. GLIM for ecologists. [Google Scholar]
- Crawley M. Statistical computing. Wiley; Chichester: 2002. [Google Scholar]
- Dye C, Godfray H.C.J. On sex ratio and inbreeding in malaria parasite populations. J. Theor. Biol. 1993;161:131–134. doi: 10.1006/jtbi.1993.1045. [DOI] [PubMed] [Google Scholar]
- Fallis A.M, Desser S.S. On species of Leucocytozoan. Adv. Parasitol. 1974;12:1–67. doi: 10.1016/s0065-308x(08)60386-3. [DOI] [PubMed] [Google Scholar]
- Gardner A, Reece S.E, West S.A. Even more extreme fertility insurance and the sex ratios of protozoan blood parasites. J. Theor. Biol. 2003;223:515–521. doi: 10.1016/s0022-5193(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Gautret P, Gantier J.-C, Baccam D, Miltgen F, Saulai M, Chabaud A.G, Landau I. The gametocytes of Plasmodium vinckei petteri, their morphological stages, periodicity and infectivity. Int. J. Parasitol. 1996;26:1095–1101. [PubMed] [Google Scholar]
- Gautret P, Miltgen F, Gantier J.C, Chabaud A.G, Landau I. Enhanced gametocyte formation by Plasmodium chabaudi in immature erythrocytes: patterns of production and infectivity to mosquitoes. J. Parasitol. 1996;82:900–906. [PubMed] [Google Scholar]
- Gautret P, Coquelin F, Chabaud A.G, Landau I. The production of gametocytes by rodent Plasmodium species in mice during phenylhydrazine induced reticulocytosis. Acta Parasitol. 1997;42(2):65–67. [Google Scholar]
- Godfray H.C.J, Werren J.H. Recent developments in sex ratio studies. Trends Ecol. Evol. 1996;11:59–63. doi: 10.1016/0169-5347(96)81043-3. [DOI] [PubMed] [Google Scholar]
- Gwadz R.W. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Hellwig-Burgel T. Biology of erythropoietin. Adv. Exp. Med. Biol. 2001;502:169–187. doi: 10.1007/978-1-4757-3401-0_12. [DOI] [PubMed] [Google Scholar]
- Landau I, Boulard Y. Life cycles and morphology. In: Killick-Kendrick R, Peters W, editors. Rodent malaria. Academic Press; London: 1978. pp. 53–84. [Google Scholar]
- Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl Acad. Sci. USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis C, Targett G.A.T. Immunisation to produce a transmission blocking immunity in Plasmodium yoelii malaria infections. Trans. R. Soc. Trop. Med. Hyg. 1981;75:158–159. doi: 10.1016/0035-9203(81)90053-5. [DOI] [PubMed] [Google Scholar]
- Nee S, West S.A, Read A.F. Inbreeding and parasite sex ratios. Proc. R. Soc. B. 2002;269:755–760. doi: 10.1098/rspb.2001.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y, Nishimura K. Role of reticulocytes on gametocytogenesis in chickens infected with Leucocytozoon caulleryi. J. Med. Vet. Sci. 2001;63(7):797–800. doi: 10.1292/jvms.63.797. [DOI] [PubMed] [Google Scholar]
- Paul R.E.L, Coulson T.N, Raibaud A, Brey P.T. Sex determination in malaria parasites. Science. 2000;287:128–131. doi: 10.1126/science.287.5450.128. [DOI] [PubMed] [Google Scholar]
- Paul R.E.L, Brey P.T, Robert V. Plasmodium sex determination and transmission to mosquitoes. Trends Parasitol. 2002;18:32–38. doi: 10.1016/s1471-4922(01)02122-5. [DOI] [PubMed] [Google Scholar]
- Paul R.E.L, Nu V.A.T, Krettli A.U, Brey P.T. Interspecific competition during transmission of two sympatric species to the mosquito vector. Proc. R. Soc. B. 2002;269:2551–2557. doi: 10.1098/rspb.2002.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R.E.L, Ariey F, Robert V. The evolutionary ecology of Plasmodium. Ecol. Lett. 2003;6:866–880. [Google Scholar]
- Pickering J, Read A.F, Guerrero S, West S.A. Sex ratio and virulence in two species of lizard malaria parasites. Evol. Ecol. Res. 2000;2:171–184. [Google Scholar]
- Ranawaka M.B.R, Fleck S.L, Alejo-Blanco R, Sinden R.E. Characterisation of the effector mechanisms of a transmission blocking antibody upon differentiation of Plasmodium berghei gametocytes into ookinetes in vitro. Parasitology. 1994;109:11–17. doi: 10.1017/s0031182000077702. [DOI] [PubMed] [Google Scholar]
- Read A.F, Narara A, Nee S, Keymer A.E, Day K.P. Gametocyte sex ratios as indirect measures of outcrossing rates in malaria. Parasitology. 1992;104:387–395. doi: 10.1017/s0031182000063630. [DOI] [PubMed] [Google Scholar]
- Read A.F, Anwar M, Shutler D, Nee S. Sex allocation and population structure in malaria and related parasitic protozoa. Proc. R. Soc. B. 1995;260:359–363. doi: 10.1098/rspb.1995.0105. [DOI] [PubMed] [Google Scholar]
- Read A.F, Smith T.G, Nee S, West S.A. Sex ratios of malaria parasites and related protozoa. In: Hardy I.C.W, editor. Sex ratio handbook. Cambridge University Press; 2002. pp. 314–332. [Google Scholar]
- Reece S.E, Read A.F. Malaria sex ratios. Trends Ecol. Evol. 2000;15:259–260. doi: 10.1016/s0169-5347(00)01893-0. [DOI] [PubMed] [Google Scholar]
- Reece S.E, Duncan A.B, West S.A, Read A.F. Sex ratios in the rodent malaria parasite Plasmodium chabaudi. Parasitology. 2003;127:419–425. doi: 10.1017/s0031182003004013. [DOI] [PubMed] [Google Scholar]
- Robert V, Read A.F, Essong J, Tchuinkam T, Mulder B, Verhave J.-P, Carnevale P. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 1996;90:621–624. doi: 10.1016/s0035-9203(96)90408-3. [DOI] [PubMed] [Google Scholar]
- Robert V, Sokhna C.S, Rogier C, Ariey F, Trape J.F. Sex ratios of Plasmodium falciparum gametocytes in inhabitants of Dielmo, Senegal. Parasitology. 2003;127:1–8. doi: 10.1017/s0031182003003299. [DOI] [PubMed] [Google Scholar]
- Schall J.J. The sex ratio of Plasmodium gametocytes. Parasitology. 1989;98:343–350. doi: 10.1017/s0031182000061412. [DOI] [PubMed] [Google Scholar]
- Shieh J.N, Rossignol P.A. Opposite influences of host anaemia on blood feeding rate and fecundity of mosquitoes. Parasitology. 1992;105:159–163. doi: 10.1017/s0031182000074060. [DOI] [PubMed] [Google Scholar]
- Shutler D, Read A.F. Local mate competition, extraordinary and ordinary blood parasite sex ratios. Oikos. 1998;82:417–424. [Google Scholar]
- Shutler D, Bennett G.F, Mullie A. Sex proportions of Haemoproteus blood parasites and local mate competition. Proc. Natl Acad. Sci. USA. 1995;92:6748–6752. doi: 10.1073/pnas.92.15.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Gill G.S. Enhanced gametocyte formation in young erythrocytes by Plasmodium falciparum in vitro. J. Protozool. 1992;39:429–432. doi: 10.1111/j.1550-7408.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Trager W, Gill G.S, Lawerence C, Nagel R.L. Plasmodium falciparum: enhanced gametocyte formation in vitro in reticulocyte rich blood. Exp. Parasitol. 1999;91:115–118. doi: 10.1006/expr.1998.4347. [DOI] [PubMed] [Google Scholar]
- Veins P, Chevalier J.L, Sonea S, Yoeli M. The effect of reticulocytosis on Plasmodium vinckei infection in white mice. Action of phenylhydrazine and of repeated bleedings. Can. J. Microbiol. 1971;17:257–261. doi: 10.1139/m71-043. [DOI] [PubMed] [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- West S.A, Smith T.G, Read A.F. Sex allocation and population structure in apicomplexan (protozoa) parasites. Proc. R. Soc. B. 2000;267:257–263. doi: 10.1098/rspb.2000.0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A, Reece S.E, Read A.F. The evolution of gametocyte sex ratios in malaria and related apicomplexan (protozoan) parasites. Trends Parasitol. 2001;17:525–531. doi: 10.1016/s1471-4922(01)02058-x. [DOI] [PubMed] [Google Scholar]
- West S.A, Smith T.G, Read A.F. Fertility insurance and the sex ratios of malaria and related hemospororin blood parasites. J. Parasitol. 2002;88:258–263. doi: 10.1645/0022-3395(2002)088[0258:FIATSR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]