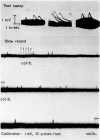
Abstract
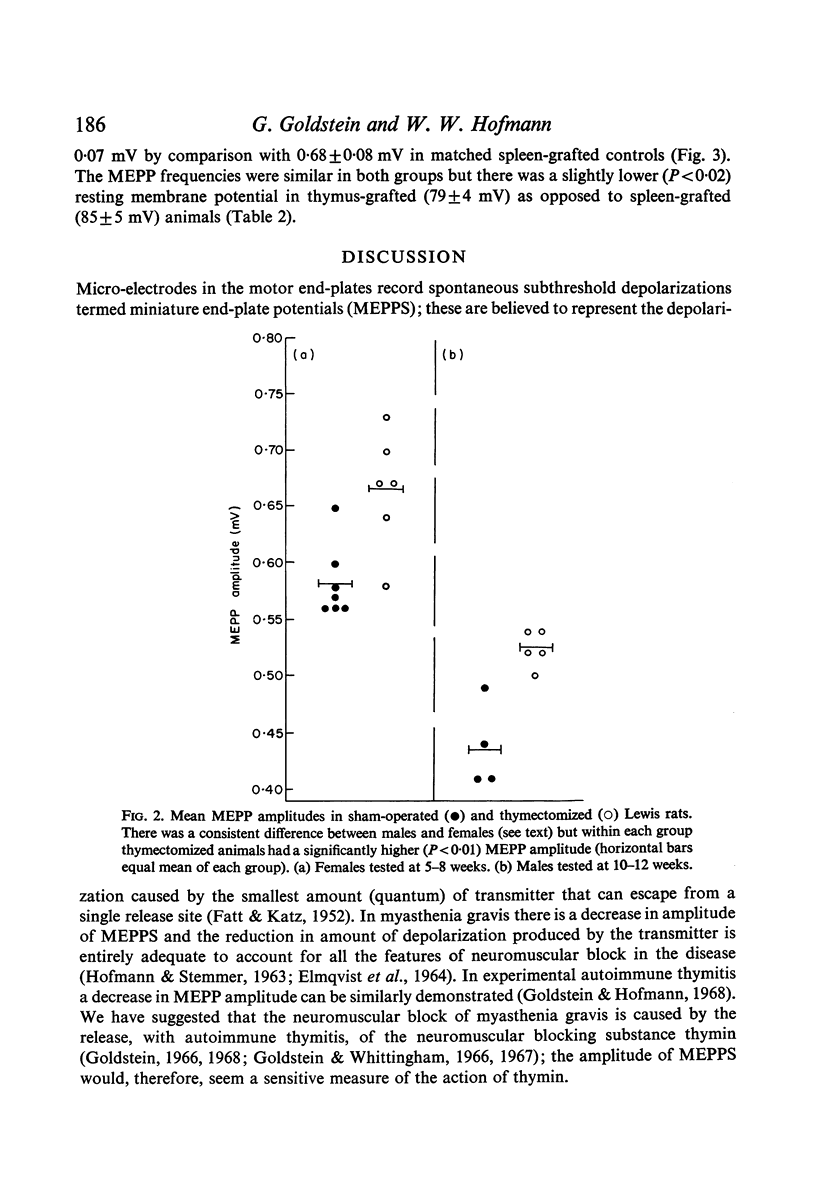
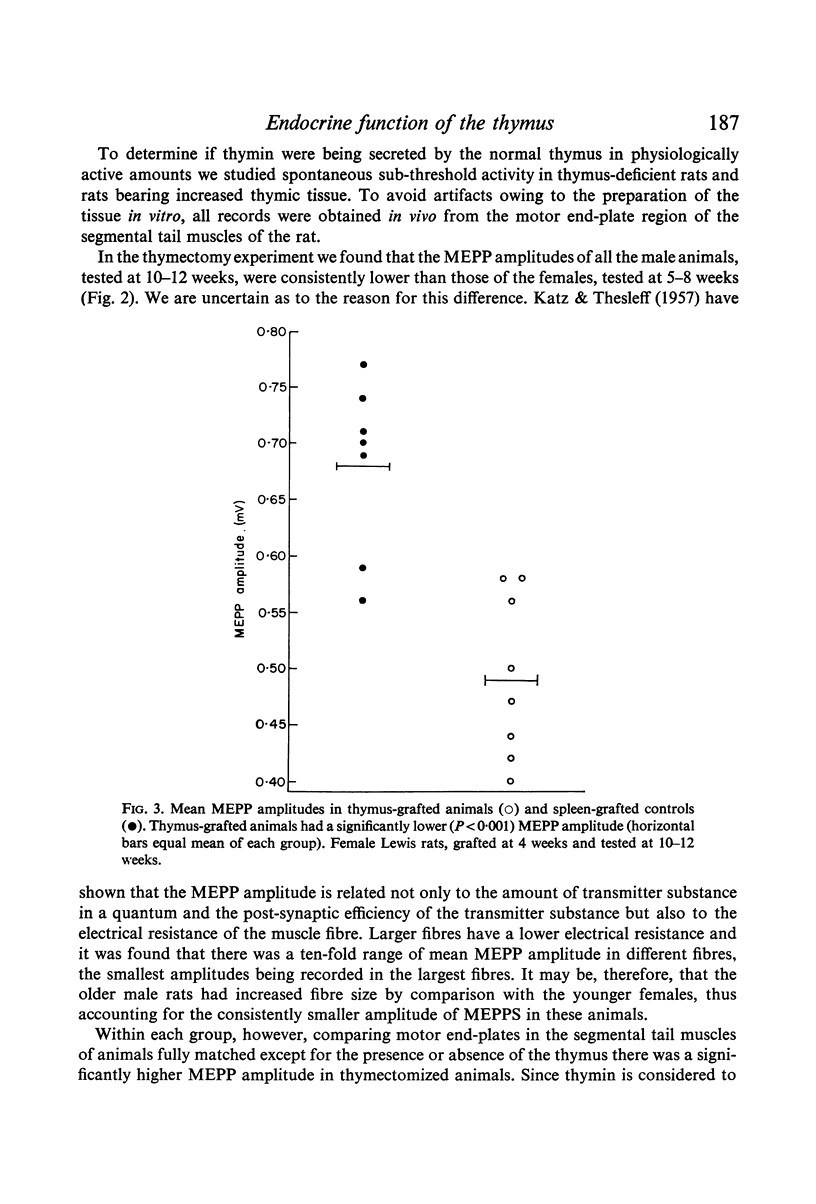
Neuromuscular transmission was studied with micro-electrodes in thymus-deficient Lewis rats thymectomized within 3 days of birth and in Lewis rats bearing increased thymic tissue in the form of multiple isogeneic thymic grafts. The amplitude of miniature end-plate potentials (MEPPS) was increased in thymus-deficient animals and decreased in animals with increased thymic tissue.
These data suggest that a substance we have previously termed thymin is being secreted by the normal thymus in physiologically active amounts which inhibit transmission at the neuromyal synapse. The proposed substance thus appears to act like a hormone. It is suggested that in myasthenia gravis autoimmune thymitis causes the release of toxic amounts of thymin which produce the characteristic myasthenic neuromuscular block.
Full text
PDF

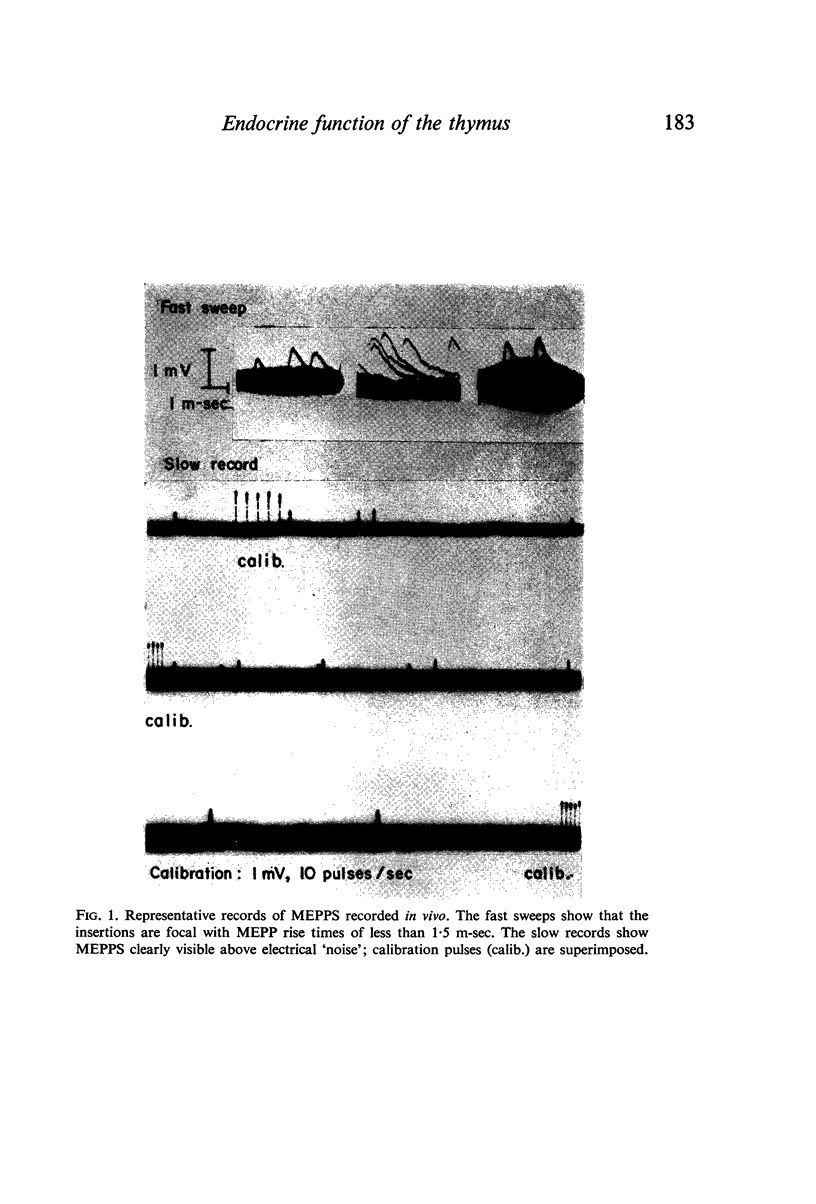
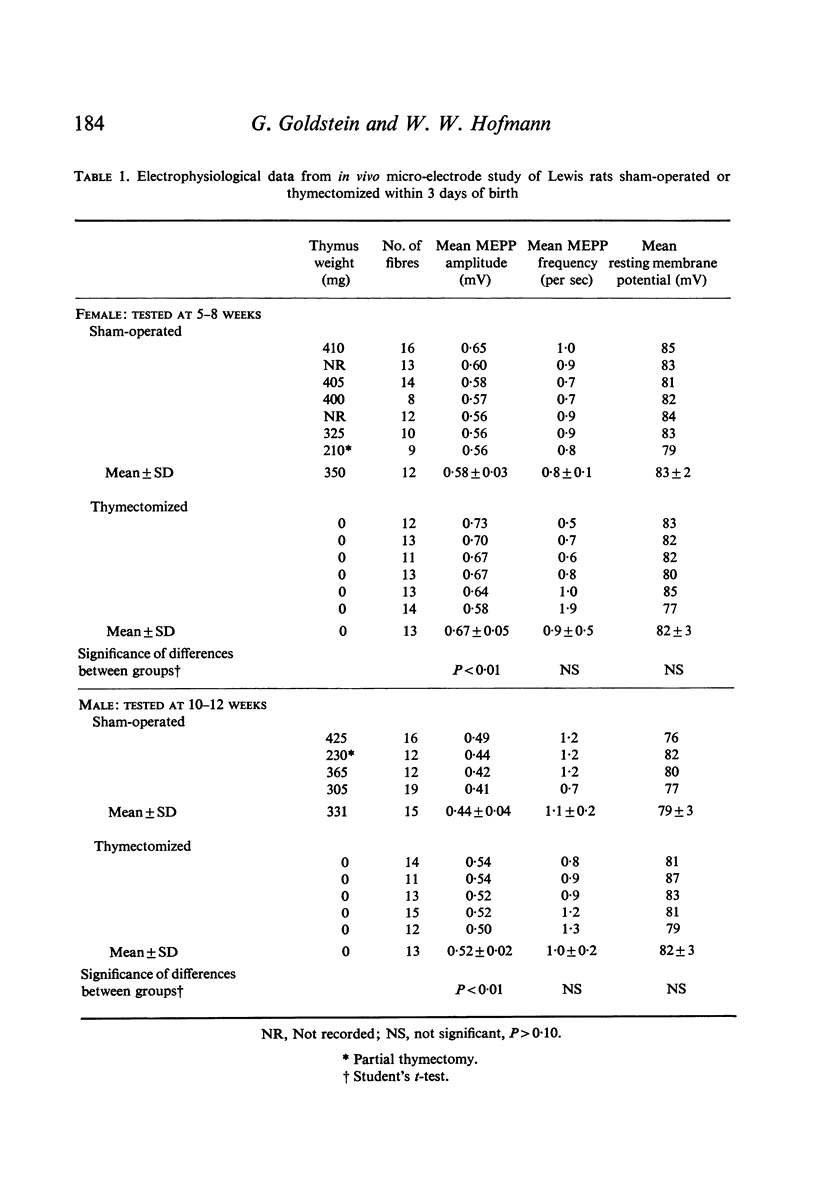
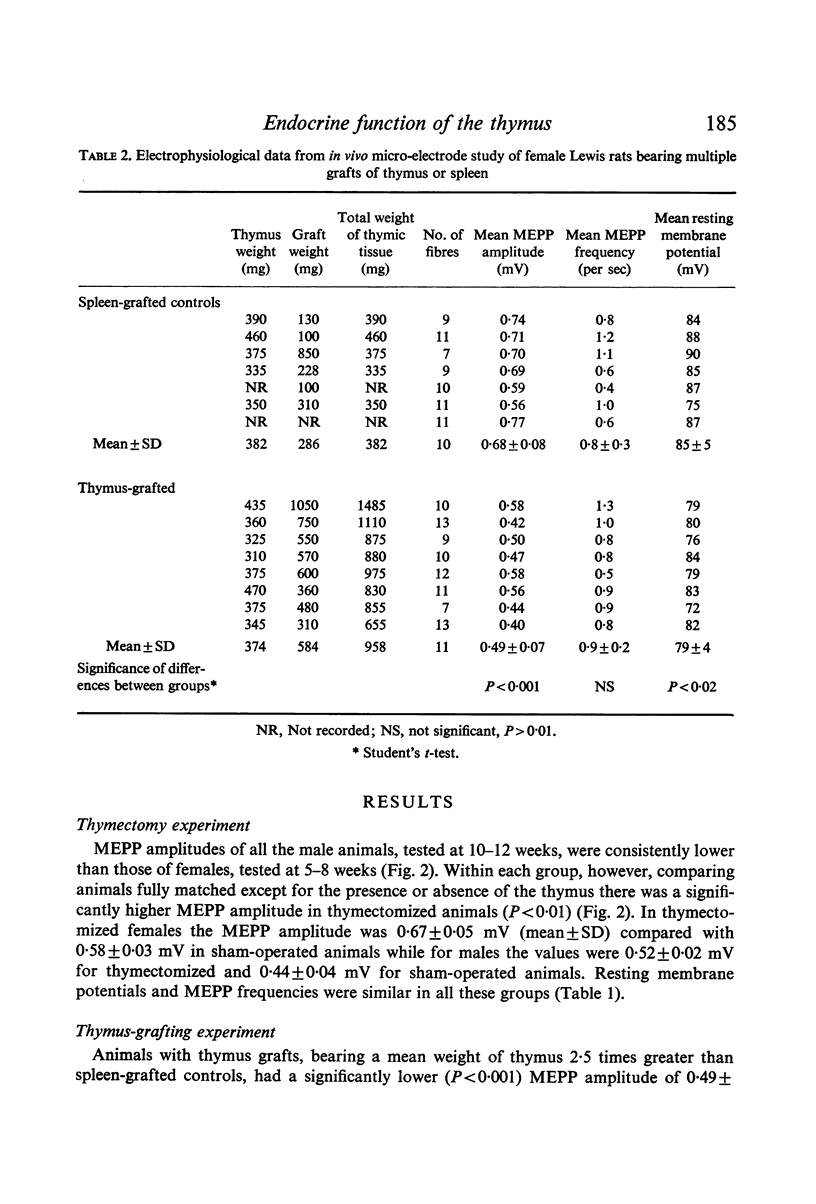


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELMQVIST D., HOFMANN W. W., KUGELBERG J., QUASTEL D. M. AN ELECTROPHYSIOLOGICAL INVESTIGATION OF NEUROMUSCULAR TRANSMISSION IN MYASTHENIA GRAVIS. J Physiol. 1964 Nov;174:417–434. doi: 10.1113/jphysiol.1964.sp007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FELTKAMP T. E., van der GELD, KRUYFF K., OOSTERHUIS H. J. Antinuclear factor in myasthenia gravis. Lancet. 1963 Mar 23;1(7282):667–667. doi: 10.1016/s0140-6736(63)91302-3. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Hofmann W. W. Electrophysiological changes similar to those of myasthenia gravis in rats with experimental autoimmune thymitis. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):453–459. doi: 10.1136/jnnp.31.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. The thymus and neuromuscular function. A substance in thymus which causes myositis and myasthenic neuromuscular block in guineapigs. Lancet. 1968 Jul 20;2(7560):119–122. doi: 10.1016/s0140-6736(68)90414-5. [DOI] [PubMed] [Google Scholar]
- Goldstein G. Thymic germinal centres in myasthenia gravis: a correlative study. Clin Exp Immunol. 1967 Jan;2(1):103–107. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. Thymitis and myasthenia gravis. Lancet. 1966 Nov 26;2(7474):1164–1167. doi: 10.1016/s0140-6736(66)90479-x. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Whittingham S. Experimental autoimmune thymitis. An animal model of human myasthenia gravis. Lancet. 1966 Aug 6;2(7458):315–318. doi: 10.1016/s0140-6736(66)92599-2. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Whittingham S. Histological and serological features of experimental autoimmune thymitis in guinea-pigs. Clin Exp Immunol. 1967 May;2(3):257–268. [PMC free article] [PubMed] [Google Scholar]
- HOFMANN W. W., STEMMER E. A. Subthreshold activity at normal and myasthenic end plates. Neurology. 1963 Mar;13:227–236. doi: 10.1212/wnl.13.3.227. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVEY R. H., TRAININ N., LAW L. W. EVIDENCE FOR FUNCTION OF THYMIC TISSUE IN DIFFUSION CHAMBERS IMPLANTED IN NEONATALLY THYMECTOMIZED MICE. PRELIMINARY REPORT. J Natl Cancer Inst. 1963 Aug;31:199–217. [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- MILLER J. F. Studies on mouse leukaemia. The role of the thymus in leukaemogenesis by cell-free leukaemic filtrates. Br J Cancer. 1960 Mar;14:93–98. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., Engel W. K., Strauss A. J. Does myasthenic serum bind to the neuromuscular junction? Ann N Y Acad Sci. 1966 Jan 26;135(1):656–663. doi: 10.1111/j.1749-6632.1966.tb45512.x. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Osoba D. Current concepts of the immunological function of the thymus. Physiol Rev. 1967 Jul;47(3):437–520. doi: 10.1152/physrev.1967.47.3.437. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- OSOBA D., MILLER J. F. EVIDENCE FOR A HUMORAL THYMUS FACTOR RESPONSIBLE FOR THE MATURATION OF IMMUNOLOGICAL FACULTY. Nature. 1963 Aug 17;199:653–654. doi: 10.1038/199653a0. [DOI] [PubMed] [Google Scholar]
- Roberts D. V., Thesleff S. Neuromuscular transmission in vivo and the actions of decamethonium: a micro-electrode study. Acta Anaesthesiol Scand. 1965;9(3):165–172. doi: 10.1111/j.1399-6576.1965.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Trainin N., Linker-Israeli M. Restoration of immunologic reactivity of thymectomized mice by calf thymus extracts. Cancer Res. 1967 Feb;27(2):309–313. [PubMed] [Google Scholar]
- WHITE R. G., MARSHALL A. H. The autoimmune response in myasthenia gravis. Lancet. 1962 Jul 21;2(7247):120–123. doi: 10.1016/s0140-6736(62)90005-3. [DOI] [PubMed] [Google Scholar]