Abstract
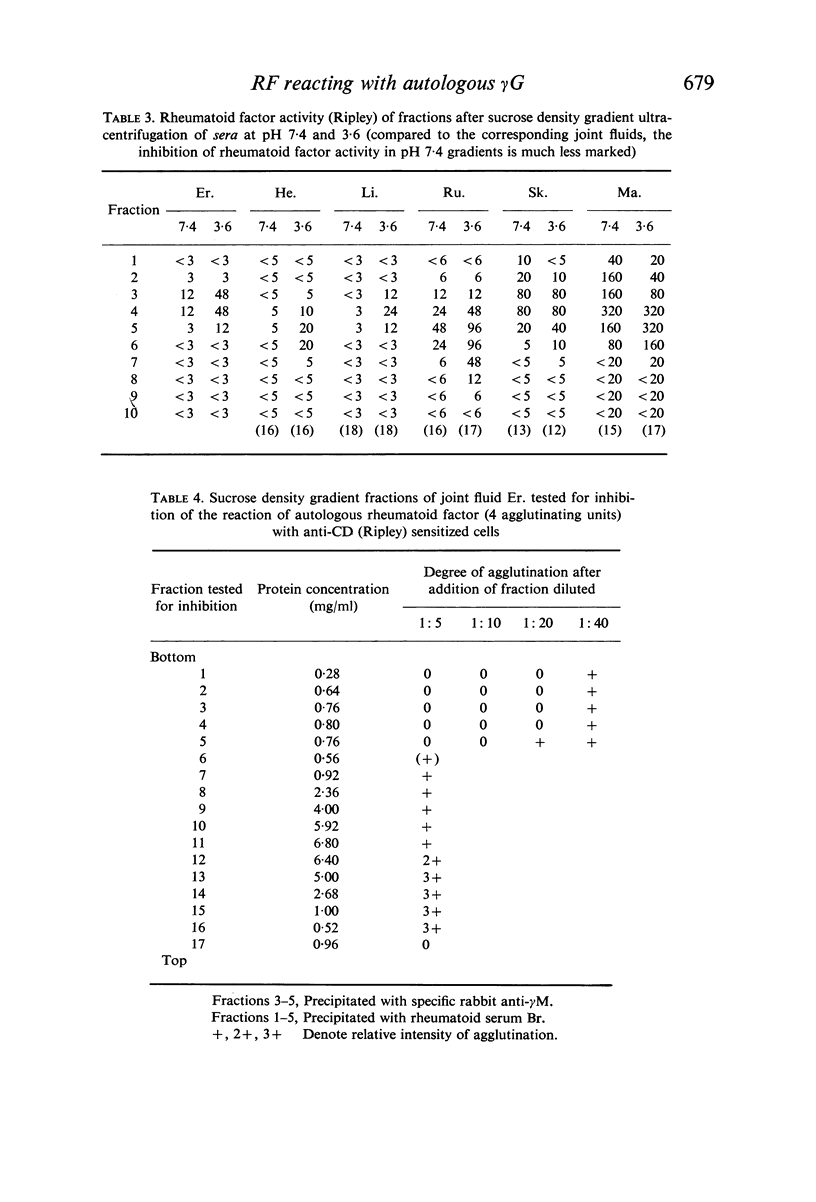
Inflammatory joint fluids from patients with definite or classical rheumatoid arthritis (RA), selected because they precipitated strongly with rheumatoid factors (RF), were examined by sucrose density gradient ultracentrifugation for sedimentation of γG-globulin (γG) and behaviour of RF activity. All contained some γG that sedimented to the γM globulin (γM) zone at pH 7·4; at pH 3·6, γG disappeared from the γM zone. With four joint fluids, no RF activity was detected by cells sensitized with anti-CD Ripley at pH 7·4, but RF activity appeared in the γM zone at pH 3·6. This demonstration of inhibition of RF activity by autologous γG aggregates depended upon the relative amount of RF and γG aggregates in the joint fluid. Two joint fluids which contained γG aggregates and high titre RF activity precipitated with γM RF isolated in high concentration from the corresponding sera.
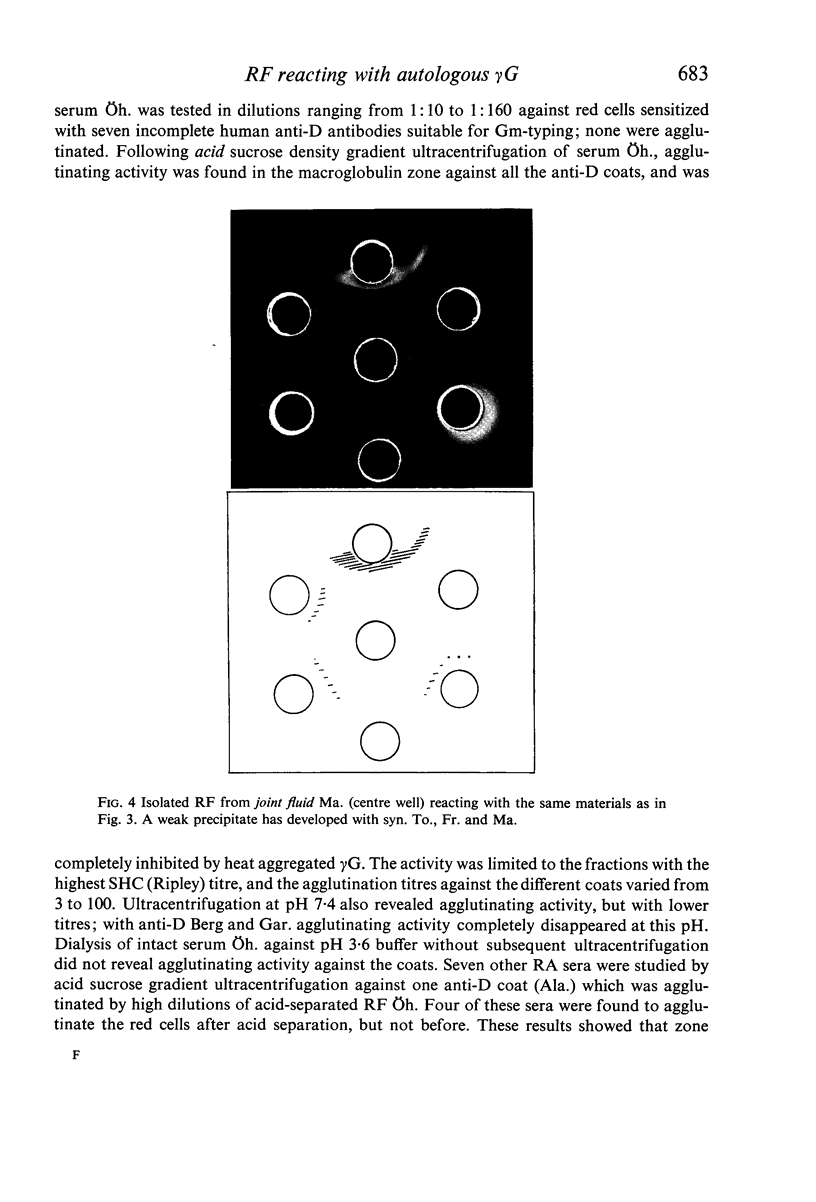
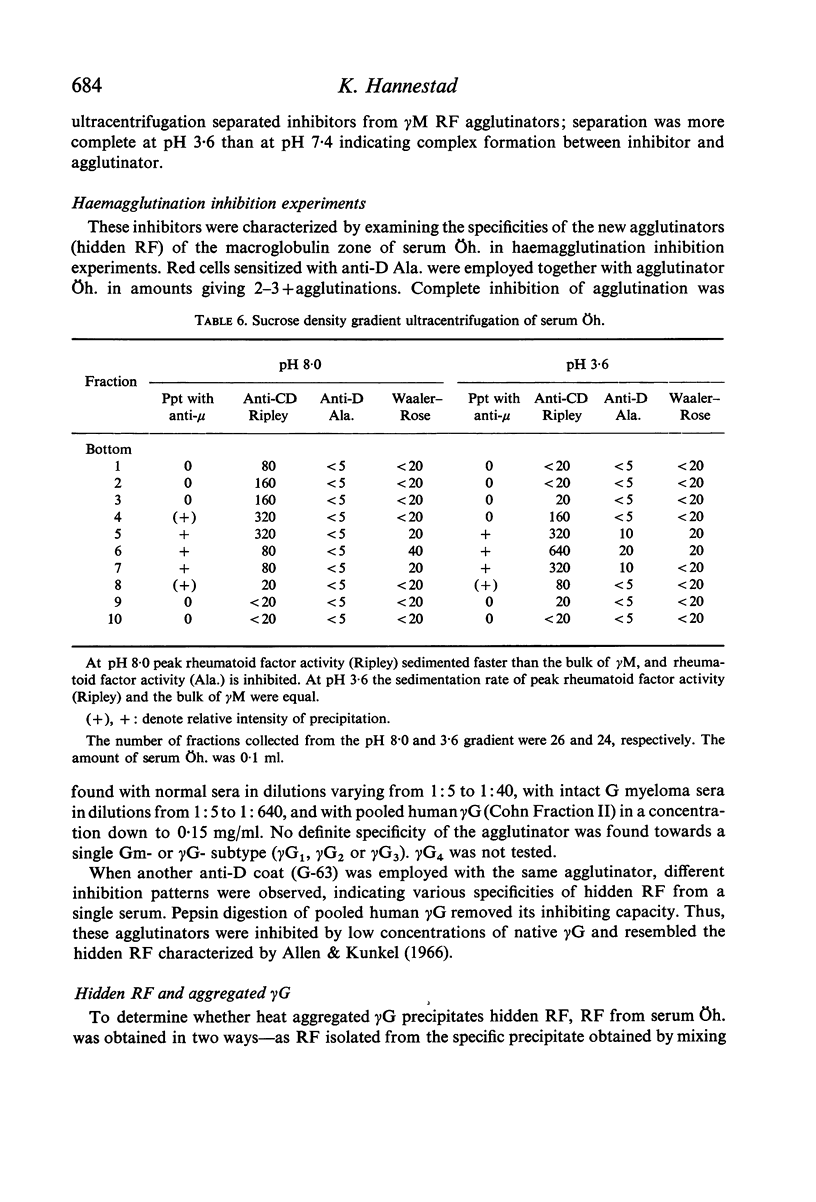
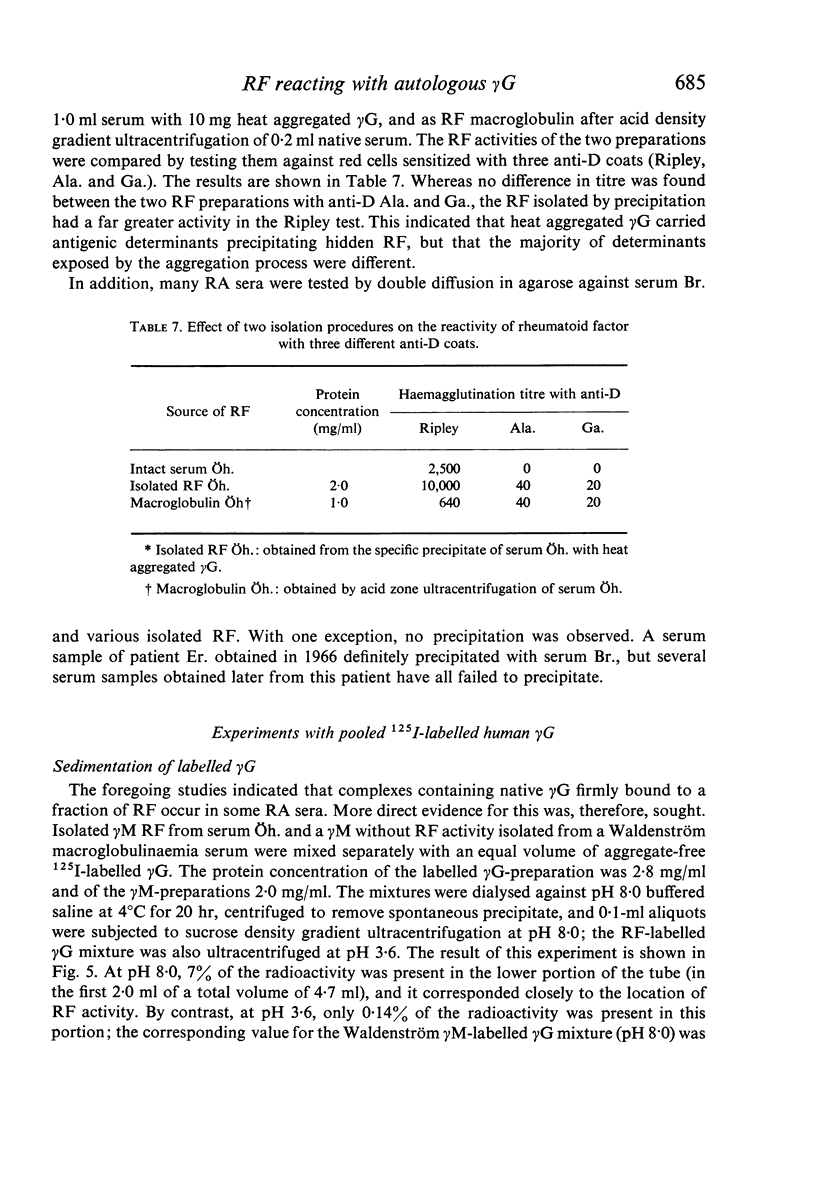
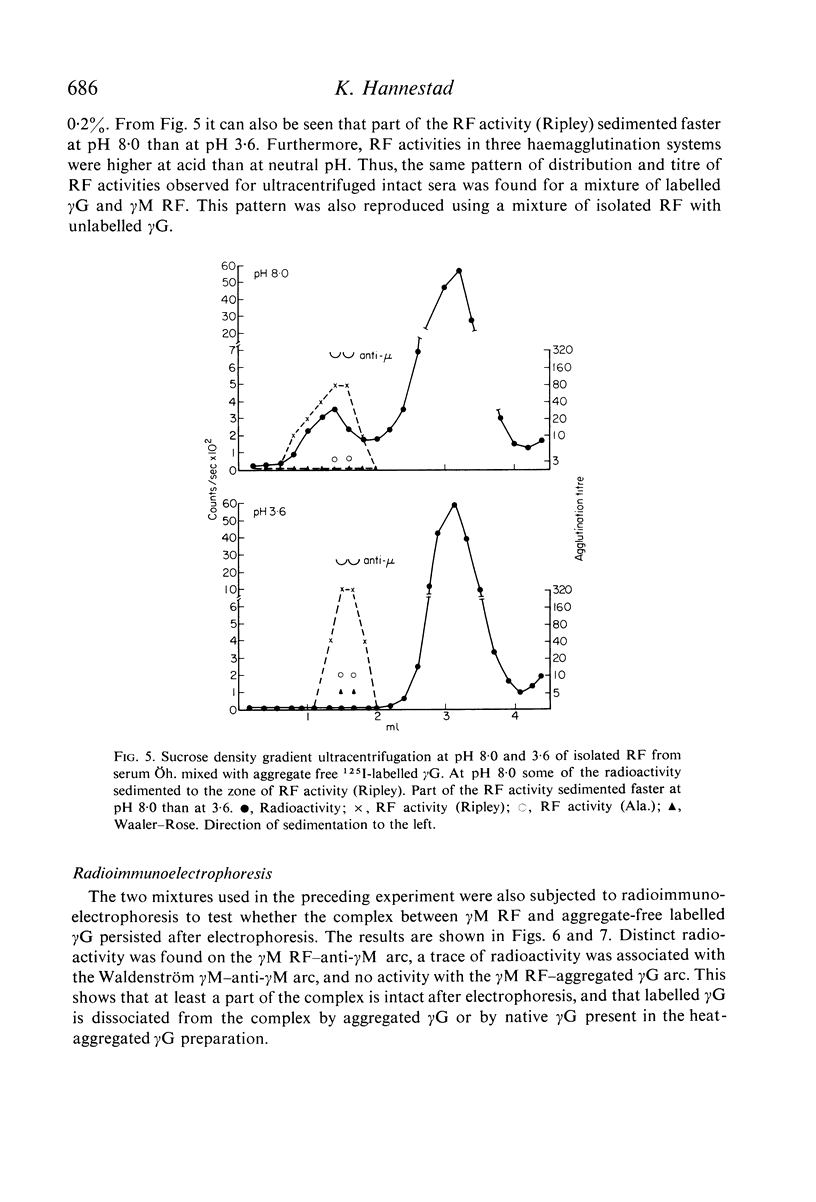
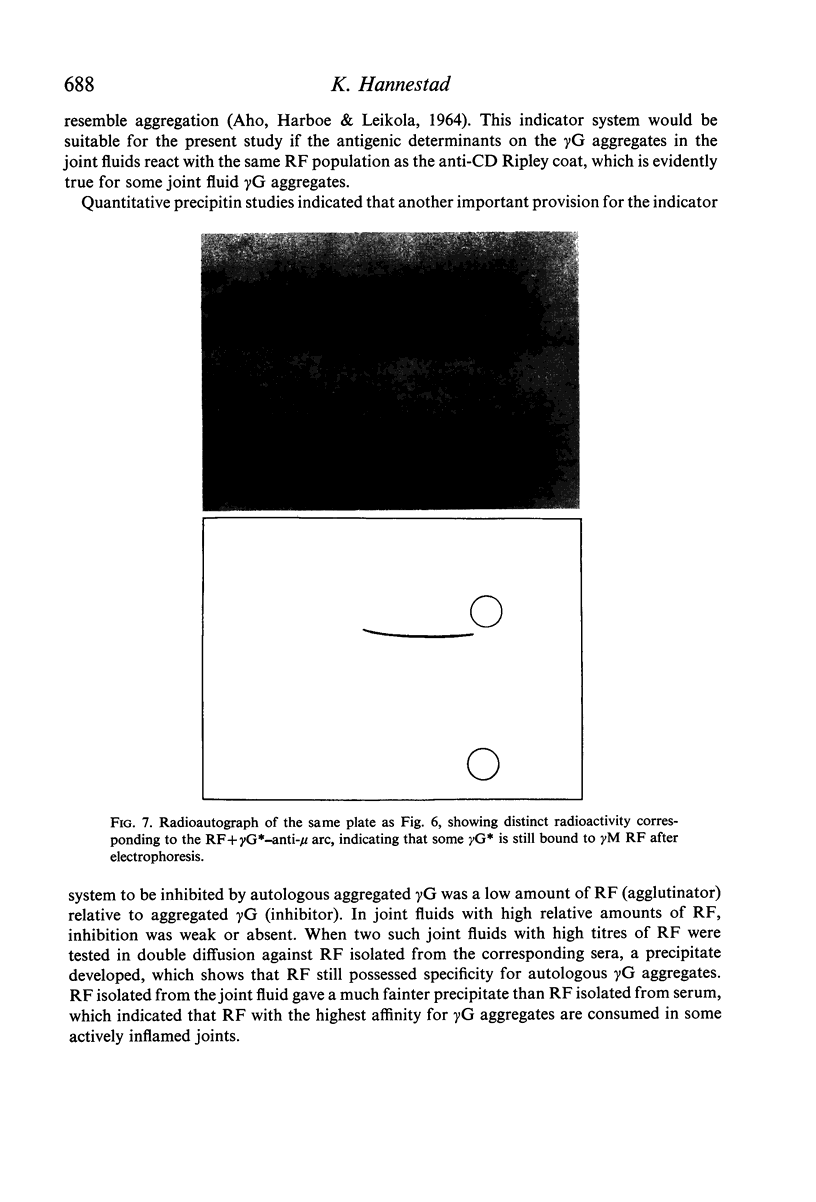
In sera, RF activity often sedimented faster at pH 7·4 than at pH 3·6, and inhibitors of RF activity against red cells sensitized with various anti-D sera were found in the macroglobulin zone at pH 7·4. These inhibitors were characterized as γG. When aggregate-free 125I-labelled pooled human γG was mixed with isolated γM RF, 7% of the radioactivity sedimented with RF activity by zone ultracentrifugation at pH 8·0; when mixed with a Waldenström type γM globulin (γM), only 0·2% of the radioactivity was found in the γM zone. Radioimmunoelectrophoresis incidated that 125I-labelled γG was still bound to γM RF following electrophoresis. These results suggest that native γG is firmly bound to a fraction of RF in some sera. The relationship between these complexes and the 22S complexes of certain rheumatoid sera is discussed.
Full text
PDF













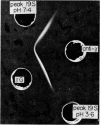
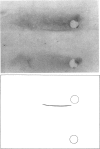
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHO K., HARBOE M., LEIKOLA J. STUDIES OF THE ANTIBODY NATURE OF THE RHEUMATOID FACTOR. REACTION OF THE RHEUMATOID FACTOR WITH SHEEP ERYTHROCYTES SENSITIZED WITH HUMAN ANTI-SHEEP CELL ANTIBODIES AND WITH O RH POSITIVE CELLS SENSITIZED WITH INCOMPLETE ANTI-RH ANTIBODIES. Immunology. 1964 Jul;7:403–418. [PMC free article] [PubMed] [Google Scholar]
- AHO K., HARBOE M., SIEVERS K. RHEUMATOID FACTOR--AUTOSPECIFICITY OR ISOSPECIFICITY? Vox Sang. 1965 Mar-Apr;10:225–227. doi: 10.1111/j.1423-0410.1965.tb04344.x. [DOI] [PubMed] [Google Scholar]
- Allen J. C., Kunkel H. G. Hidden rheumatoid factors with specificity for native gamma globulins. Arthritis Rheum. 1966 Dec;9(6):758–768. doi: 10.1002/art.1780090603. [DOI] [PubMed] [Google Scholar]
- Boyer J. T. Complement and cold agglutinins. II. Interactions of the components of complement and antibody within the haemolytic complex. Clin Exp Immunol. 1967 Mar;2(2):241–252. [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C., HOLMAN H. R., MULLER-EBERHARD H. J., KUNKEL H. G. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J Exp Med. 1957 May 1;105(5):425–438. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUDENBERG H. H., KUNKEL H. G. Specificity of the reaction between rheumatoid factors and gamma globulin. J Exp Med. 1961 Aug 1;114:257–278. doi: 10.1084/jem.114.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMERMAN D., SCHUBERT M. Diarthrodial joints, an essay. Am J Med. 1962 Oct;33:555–590. doi: 10.1016/0002-9343(62)90266-8. [DOI] [PubMed] [Google Scholar]
- HEIMER R., NOSENZO C. J. PSEUDOGLOBULIN RHEUMATOID FACTORS. J Immunol. 1965 Apr;94:502–509. [PubMed] [Google Scholar]
- Hannestad K., Mellbye O. J. Rheumatoid factor in synovial effusions: local production and consumption. Clin Exp Immunol. 1967 Jul;2(4):501–509. [PMC free article] [PubMed] [Google Scholar]
- Hannestad K. Presence of aggregated gamma-G-globulin in certain rheumatoid synovial effusions. Clin Exp Immunol. 1967 Jul;2(4):511–529. [PMC free article] [PubMed] [Google Scholar]
- KAMMERER W. H., FREIBERGER R. H., RIVELIS A. L. Peptic ulcer in rheumatoid patients on corticosteroid therapy; a clinical, experimental and radiologic study. Arthritis Rheum. 1958 Apr;1(2):122–141. doi: 10.1002/art.1780010205. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Ultracentrifugal characteristics and carbohydrate content of macromolecular gamma-globulins. Clin Chim Acta. 1959 Mar;4(2):252–258. doi: 10.1016/0009-8981(59)90138-x. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Normansell D. E., Stanworth D. R. Ultracentrifugal studies of the reactions of rheumatoid factor with native human gamma-G-globulin. Immunology. 1966 Jun;10(6):527–533. [PMC free article] [PubMed] [Google Scholar]
- Schrohenloher R. E. Characterization of the gamma-globulin complexes present in certain sera having high titers of anti-gamma-globulin activity. J Clin Invest. 1966 Apr;45(4):501–512. doi: 10.1172/JCI105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Deutsch H. F. Occurrence and properties of a macroglobulin dimer in some hyperglobulinemic sera. J Exp Med. 1966 Nov 1;124(5):819–832. doi: 10.1084/jem.124.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönder O., Natvig J. B., Milgrom F. Reactions of rheumatoid factor with natural human antibodies. Arthritis Rheum. 1966 Oct;9(5):703–712. doi: 10.1002/art.1780090507. [DOI] [PubMed] [Google Scholar]