Abstract
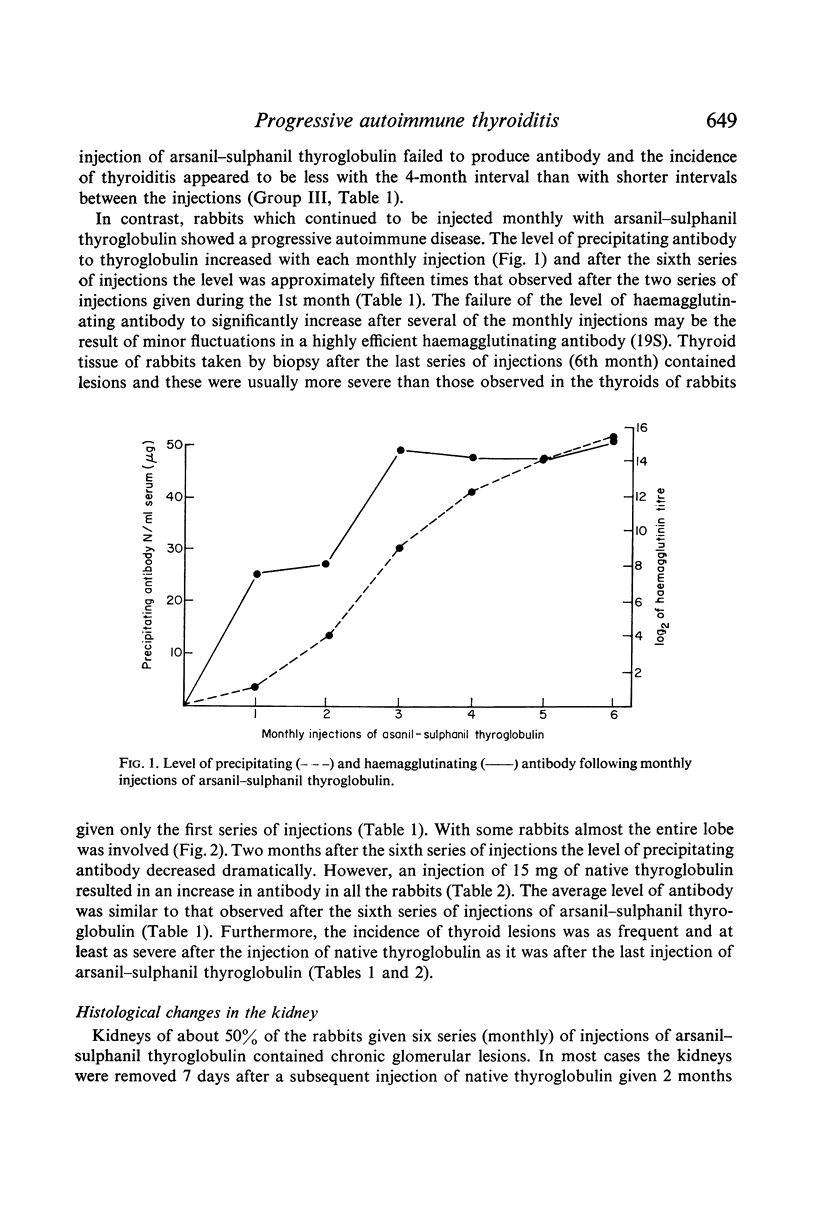
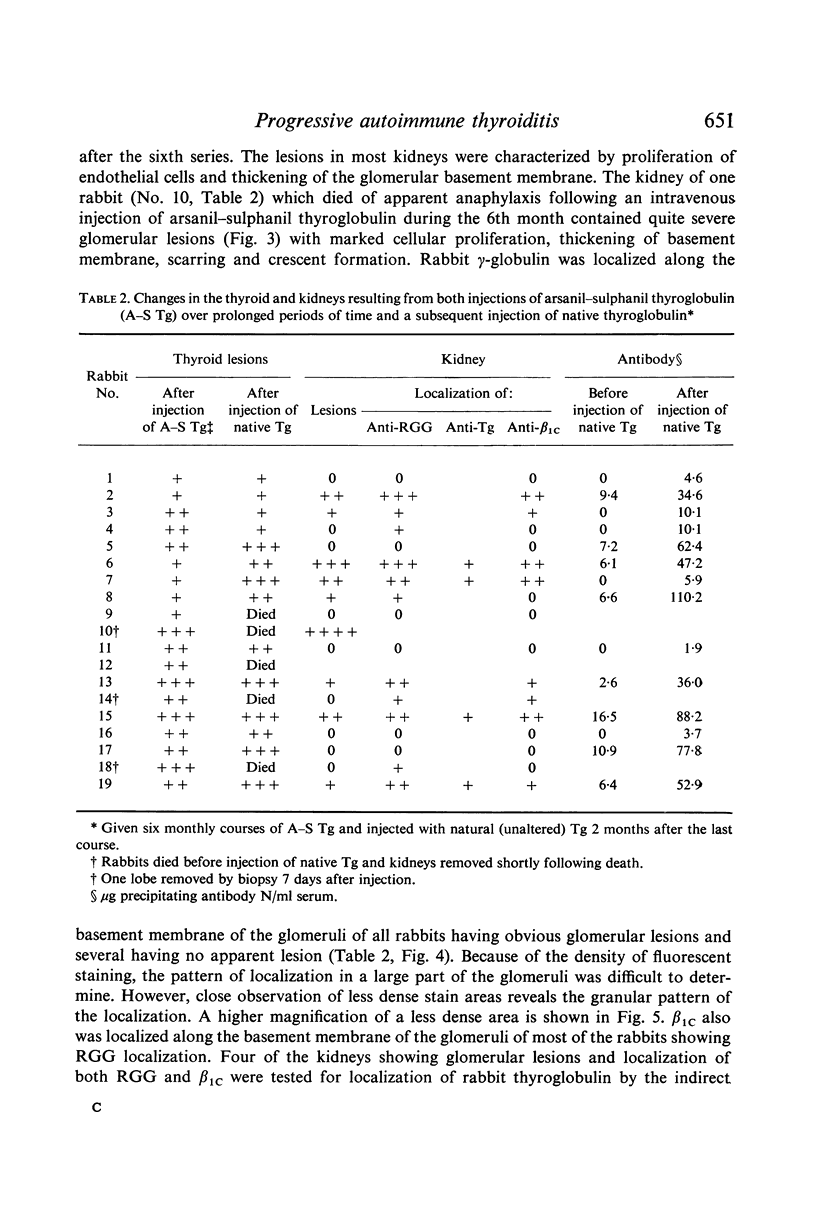
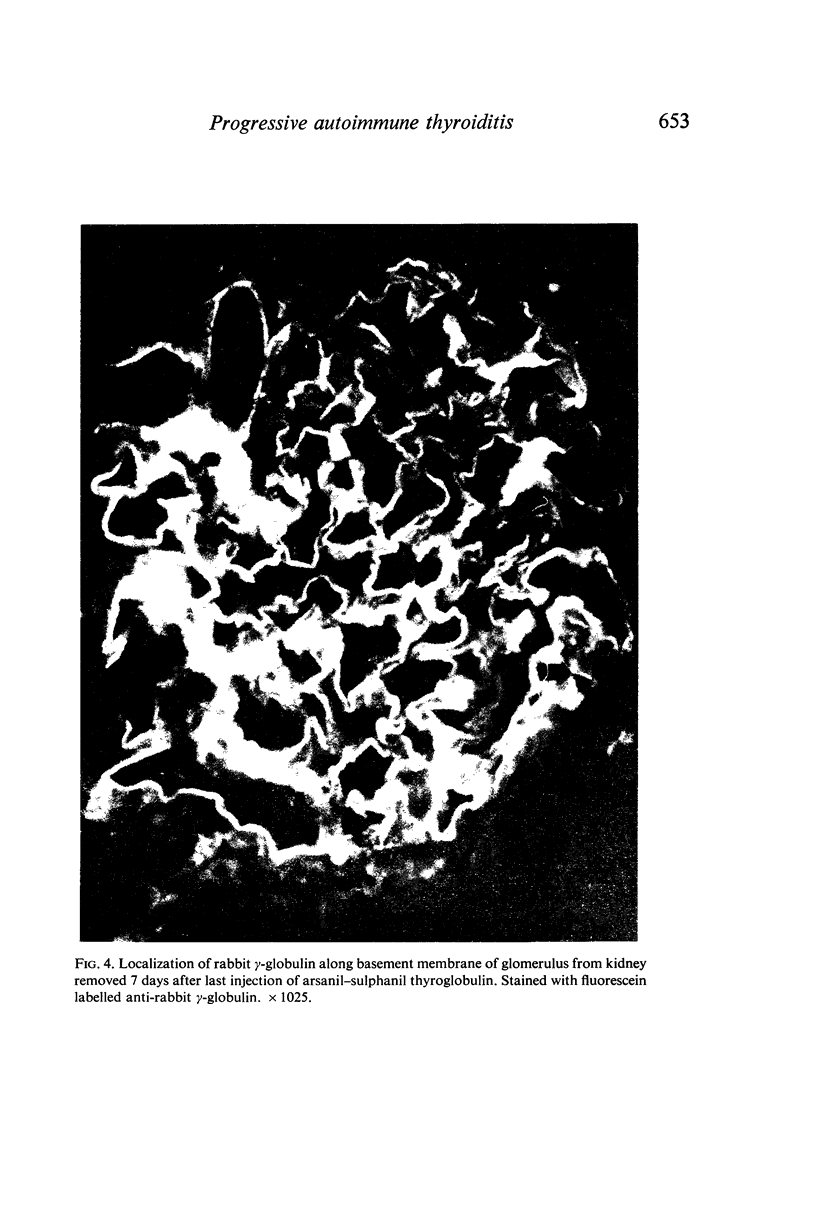
Periodic injections of aqueous preparations of thyroglobulin coupled to the diazonium derivatives of arsanilic and sulphanilic acids (arsanil–sulphanil thyroglobulin) resulted in perpetuation of both the synthesis of circulating antibody to native thyroglobulin and thyroiditis. Two months after the last injection, the rabbits made a strong immune response to a subsequent injection of native thyroglobulin. Without the periodic injections both circulating antibody and thyroid lesions disappeared and the ability to respond to native thyroglobulin was lost. The level of circulating antibody and the incidence and severity of lesions were considerably greater in rabbits receiving periodic injections over a 6-month period of time than in rabbits given a series of injections during a period of 1 month. The relation of these findings to the progressive nature of some autoimmune diseases is discussed. Complex-induced renal injury, secondary to the immune response to arsanil–sulphanil thyroglobulin, was observed in the rabbits given the periodic injections over a 6-month period of time. Chronic glomerulonephritis apparently resulted from deposition of thyroglobulin–anti-thyroglobulin complexes and complement along the glomerular basement membranes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELHOCH H. The properties of thyroglobulin. I. The effects of alkali. J Biol Chem. 1960 May;235:1326–1334. [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune-complex pathogenesis of experimental allergic glomerulonephritis. Science. 1967 Mar 17;155(3768):1432–1434. doi: 10.1126/science.155.3768.1432. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G., MULLER-EBERHARD H. J., FUDENBERG H. H., TOMASI T. B. Gamma globulin complexes in rheumatoid arthritis and certain other conditions. J Clin Invest. 1961 Jan;40:117–129. doi: 10.1172/JCI104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARDINEY M. R., Jr, MUELLER-EBERHARD H. J. MOUSE BETA-1C-GLOBULIN: PRODUCTION OF ANTISERUM AND CHARACTERIZATION IN THE COMPLEMENT REACTION. J Immunol. 1965 Jun;94:877–882. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Passive transfer of experimental autoimmune thyroiditis from donor rabbits injected with soluble thyroglobulin without adjuvant. Int Arch Allergy Appl Immunol. 1967;32(5):506–520. doi: 10.1159/000229962. [DOI] [PubMed] [Google Scholar]
- ROSE N. R., WITEBSKY E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956 Jun;76(6):417–427. [PubMed] [Google Scholar]
- TALMAGE D. W., MAURER P. H. I131-Labelled antigen precipitation as a measure of quantity and quality of antibody. J Infect Dis. 1953 May-Jun;92(3):288–300. doi: 10.1093/infdis/92.3.288. [DOI] [PubMed] [Google Scholar]
- UNANUE E., DIXON F. J. EXPERIMENTAL GLOMERULONEPHRITIS. IV. PARTICIPATION OF COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1964 Jan 1;119:965–982. doi: 10.1084/jem.119.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle W. O., High G. J. The behavior of autologous thyroglobulin in the circulation of rabbits immunized with either heterologous or altered homologous thyroglobulin. J Immunol. 1967 Jun;98(6):1105–1114. [PubMed] [Google Scholar]
- Weigle W. O., Nakamura R. M. The development of autoimmune thyroiditis in rabbits following injection of aqueous preparations of heterologous thyroglobulins. J Immunol. 1967 Jul;99(1):223–231. [PubMed] [Google Scholar]
- Weigle W. O. The production of thyroiditis and antibody following injection of unaltered thyroglobulin without adjuvant into rabbits previously stimulated with altered thyroglobulin. J Exp Med. 1965 Dec 1;122(6):1049–1062. doi: 10.1084/jem.122.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]