Abstract
The oxygen isotope composition of atmospheric CO2 is an important signal that helps distinguish between ecosystem photosynthetic and respiratory processes. In C4 plants the carbonic anhydrase (CA)-catalyzed interconversion of CO2 and bicarbonate (HCO3−) is an essential first reaction for C4 photosynthesis but also plays an important role in the CO2-H2O exchange of oxygen as it enhances the rate of isotopic equilibrium between CO2 and water. The C4 dicot Flaveria bidentis containing genetically reduced levels of leaf CA (CAleaf) has been used to test whether changing leaf CA activity influences online measurements of C18OO discrimination (Δ18O) and the proportion of CO2 in isotopic equilibrium with leaf water at the site of oxygen exchange (θ). The Δ18O in wild-type F. bidentis, which contains high levels of CA relative to the rates of net CO2 assimilation, was less than predicted by models of Δ18O. Additionally, Δ18O was sensitive to small decreases in CAleaf. However, reduced CA activity in F. bidentis had little effect on net CO2 assimilation, transpiration rates (E), and stomatal conductance (gs) until CA levels were less than 20% of wild type. The values of θ determined from measurements of Δ18O and the 18O isotopic composition of leaf water at the site of evaporation (δe) were low in the wild-type F. bidentis and decreased in transgenic plants with reduced levels of CA activity. Measured values of θ were always significantly lower than the values of θ predicted from in vitro CA activity and gas exchange. The data presented here indicates that CA content in a C4 leaf may not represent the CA activity associated with the CO2-H2O oxygen exchange and therefore may not be a good predictor of θ during C4 photosynthesis. Furthermore, uncertainties in the isotopic composition of water at the site of exchange may also limit the ability to accurately predict θ in C4 plants.
The oxygen isotope composition (δ18O) of atmospheric CO2 is an important tool for monitoring variations in the global exchange of CO2 (Farquhar et al., 1989, 1993; Farquhar and Lloyd, 1993; Yakir and Wang, 1996; Flanagan and Ehleringer, 1998; Yakir and Sternberg, 2000). The use of isotopes in this context relies on the fact that photosynthesis and ecosystem respiration generally have different effects on the isotope composition of atmospheric CO2 and the δ18O of CO2 in the atmosphere has been used to distinguish between the photosynthetic CO2 uptake and CO2 release during respiration (Farquhar et al., 1993; Gillon and Yakir, 2001). Dissolved CO2 exchanges oxygen molecules with water allowing the CO2 to take on the isotopic signature of the water that is in a much higher molar concentration. The water in leaves is generally highly enriched in 18O relative to ground water due to the preferential evaporation of H216O during leaf transpiration (Yakir and Wang, 1996; Yakir and Sternberg, 2000). Furthermore the exchange of 18O between CO2 and water is facilitated in leaves by the presence of carbonic anhydrase (CA), which catalyzes the interconversion of CO2 and bicarbonate (HCO3−). Thus, the retrodiffusion of CO2 out of a leaf during photosynthesis drives the 18O enrichment of atmospheric CO2. Root respiration and decomposition of organic material by soil microbes work in the opposite direction, releasing CO2 depleted in 18O because it is in equilibrium with unenriched water (Flanagan and Ehleringer, 1998; Yakir and Sternberg, 2000).
To accurately interpret the δ18O of atmospheric CO2 requires an understanding of the isotopic fractionation steps associated with specific processes during leaf gas exchange (Yakir and Sternberg, 2000). The δ18O of the water at the site CO2-H2O oxygen exchange within a leaf (δex) is the primary factor influencing the δ18O of CO2 diffusing out of a leaf (Farquhar et al., 1993). Additionally, the proportion of CO2 in isotopic equilibrium with the water at the site of oxygen exchange (θ) will also influence the δ18O of the CO2. The value of θ is determined by the balance between the gross flux of CO2 into the leaf and the activity of CA at the site of CO2-H2O oxygen isotope exchange as this influences the residence time of CO2 within a leaf and thus the number of hydration reactions per CO2 molecule (Gillon and Yakir, 2000a, 2000b).
The CA-catalyzed hydration of CO2 to HCO3− is the first enzymatic step of the C4 photosynthetic pathway, a biochemical CO2 concentrating mechanism that concentrates CO2 around Rubisco in bundle sheath cells (BSC; Hatch, 1987; Kanai and Edwards, 1999). The HCO3− is subsequently fixed via phosphoenol pyruvate carboxylase into a four-carbon acid that diffuses to the BSC for decarboxylation (Kanai and Edwards, 1999). The majority of CA in a C4 plant occurs within the mesophyll cytoplasm and the CA-catalyzed oxygen exchange between CO2-H2O occurs throughout the cytoplasmic space (Ku and Edwards, 1975; Burnell and Hatch, 1988; Hatch and Burnell, 1990). This is in contrast to C3 plants where the chloroplasts, which contain the majority of the leaf's CA, are appressed against the cell walls adjacent to the intercellular air space and the sites of water evaporation during transpiration. In C3 plants the majority of leaf CA activity is generally thought to be in close proximity to the water at the site of evaporation (Williams et al., 1996; Gillon and Yakir, 2000a). Under such conditions the isotopic signature of water at the site of evaporation (δe) is likely similar to the site of CO2-H2O oxygen exchange δex; however, this may not be the case in C4 species.
Online measurements of C18OO discrimination (Δ18O) during gas exchange of leaves have found Δ18O to be much lower in two C4 monocots (Zea mays and Sorghum bicolor) than what is commonly observed for C3 species (Gillon and Yakir, 2000a, 2000b). This lower discrimination can be explained by a combination of lower stomatal conductances and low CA activities assuming that the CO2-H2O oxygen exchange takes place in water with the same isotopic signature as the water at the site of evaporation (Gillon and Yakir, 2000b). In a survey of leaf CA activity of species belonging to different functional types it was found that C4 dicots contain more CA than their C4 monocot counterparts (Gillon and Yakir, 2001). The large amount of CA relative to net rates of CO2 assimilation in C4 dicots suggests that Δ18O ought to be higher in these plants, but this has not yet been tested (Gillon and Yakir, 2001). In this article we examine the influence changing CA activity has on Δ18O and the proportion of CO2 in isotopic equilibrium with leaf water at the site of oxygen exchange (θ) during C4 photosynthesis in a C4 plant with high levels of CA activity.
The efficient genetic transformation of the C4 dicot Flaveria bidentis provides the opportunity to manipulate various aspects of the C4 photosynthetic pathway, allowing this plant to be used as a model system for understanding the controls and limitation of C4 photosynthesis and isotope exchange (Chitty et al., 1994; Furbank et al., 1997; von Caemmerer et al., 1997a; Ludwig et al., 1998; Cousins et al., 2006). Additionally, under steady-state conditions F. bidentis has higher stomatal conductance than most other C4 plants allowing fractionation factors other than stomatal limited diffusion to influence Δ18O. These attributes make F. bidentis an excellent model system to study how changes in C4 photosynthesis and CA activity influence the isotopic exchange of CO2. Here we present data from online measurements of Δ18O, leaf CA activity and the isotope composition of transpired water in F. bidentis with genetically modified levels of leaf CA. The antisense suppression of CA activity provides us with the unique opportunity to asses the role of CA in determining Δ18O without modifying environmental conditions. We show that Δ18O in F. bidentis leaves, which contain high levels of CA activity, is nevertheless sensitive to small changes in CA activity even when stomatal conductances and CO2 assimilation rates are unaffected. These results are discussed in relationship to the isotopic signature of water at the site of exchange and the internal conductance of CO2 diffusion from the intercellular airspaces to the site of phosphoenol pyruvate carboxylase reaction.
ISOTOPE THEORY
The δ18O of water at the sites of evaporation within a leaf (δe) can be estimated from the Craig and Gordon model of evaporative enrichment (Craig and Gordon, 1965; Farquhar and Lloyd, 1993)
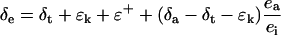 |
(1) |
where ea and ei are the vapor pressures in the atmosphere and the leaf intercellular spaces. δa and δt are the isotopic composition of water vapor in the air and transpired by the leaf, respectively. The kinetic fractionation during diffusion of water from leaf intercellular air spaces to the atmosphere (ɛk) can be calculated as (Cernusak et al., 2004)
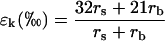 |
(2) |
where rs and rb are the stomatal and boundary layer resistance and 32 and 21 are the fractionation factors in parts per mil. The equilibrium fractionation between liquid water and water vapor (ɛ+) is calculated as (Cernusak et al., 2004)
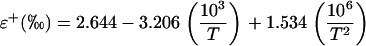 |
(3) |
where T is leaf temperature in degrees Kelvin. Under steady-state conditions the value of δt is equal to the isotopic composition of source water (δs), the water taken up by the plant (Harwood et al., 1998). A summary of the symbols used in the text are listed in Table I.
Table I.
Symbols used in the text
| Symbol | Description |
|---|---|
| A | Net CO2 assimilation |
| a | Fractionation during diffusion of CO2 from the mesophyll to the atmosphere (7.7‰) |
| α | Fractionation factor for 18O between water and CO2 at 25°C (41.1‰) |
| Δea | 18O enrichment of CO2 at the site of exchange compared to the atmosphere when the CO2 is in full isotopic equilibrium with the water |
| Δca | 18O enrichment of CO2 at the site of exchange compared to the atmosphere |
| Δ13C | 13CO2 isotope discrimination |
| Δ18O | C18OO isotope discrimination |
| δ18O | Isotopic ratio of oxygen (‰ relative to VSMOW) |
| δa | 18O isotopic composition of water vapor in the air (‰ relative to VSMOW) |
| δc | The dilution corrected value of δ18O |
| δe | 18O isotopic composition of water at the site of evaporation in the leaf (‰ relative to VSMOW) |
| δex | 18O isotopic composition of water at the site of CO2-H2O oxygen exchange (‰ relative to VSMOW) |
| δg | δ18O value of the equilibrating CO2 |
| δm | Measured δ18O value |
| δt | 18O isotopic composition of water transpired by the leaf (‰ relative to VSMOW) |
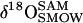 |
18O isotopic composition of water sample (‰ relative to VSMOW) |
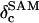 |
Dilution corrected 18O isotopic composition of the water sample |
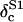 |
Dilution corrected 18O isotopic composition of reference water no. 1 |
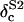 |
Dilution corrected 18O isotopic composition of reference water no. 2 |
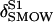 |
The 18O isotopic composition of reference water no. 1 relative to VSMOW (−6.44‰) |
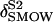 |
The 18O isotopic composition of reference water no. 2 relative to VSMOW (−22.83‰) |
| E | Transpiration rate |
| ea | Vapor pressure in the atmosphere |
| ei | Vapor pressure in the leaf intercellular air spaces |
| ɛ | pm/(pa − pm) |
| ɛk | Kinetic fractionation during diffusion of water from leaf intercellular air spaces to the atmosphere |
| ɛw | The equilibrium 18O fractionation between CO2 and water (40.17‰ at 30°C) |
| ɛ+ | The equilibrium 18O fractionation between liquid and vapor water (8.77‰ at 30°C) |
| Fin | Gross influx of CO2 into a leaf |
| gs | Stomatal conductance |
| gw | The internal conductance to the diffusion of CO2 between the intercellular air space and the site of carboxylation in the mesophyll cytoplasm |
| k | The ratio of oxygen atoms in the water to the oxygen atoms in CO2 |
| kCA | The hydration rate constant of CA |
| kt | The number of hydration reactions per CO2 molecule |
| pCO2 | Partial pressure of CO2 |
| pa | pCO2 of dry air in the atmosphere |
| pe | pCO2 of dry air entering the leaf chamber |
| pm | pCO2 of the mesophyll cytoplasm and site of CO2-H2O oxygen exchange |
| po | pCO2 of dry air leaving the leaf chamber |
| Re | 13C/12C of the air entering the leaf chamber |
| Ro | 13C/12C of the air leaving the leaf chamber |
| rb | Boundary layer resistance to water vapor diffusion (m2 s mol−1) |
| rs | Stomatal resistance to water vapor diffusion (m2 s mol−1) |
| θ | The proportion of CO2 in isotopic equilibrium with water at the site of oxygen exchange |
| ξ | pe/(pe − po) |
| τ | Residence time of CO2 in the leaf cytosol |
Discrimination against C18OO (Δ18O) when water at the site of exchange and CO2 are at full isotopic equilibrium (θ = 1) can be predicted (Farquhar and Lloyd, 1993) as
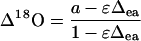 |
(4) |
where a is the diffusional discrimination (7.7‰) and ɛ is calculated as pm/(pa − pm) where pa is the CO2 partial pressure (pCO2) in air and pm is the pCO2 at the site of leaf CO2-H2O oxygen exchange in the mesophyll cytosol. The 18O enrichment of CO2 compared to the atmosphere at the site of exchange in full oxygen isotope equilibrium with the water was calculated as (Cernusak et al., 2004)
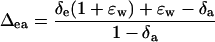 |
(5) |
where the equilibrium fractionation between water and CO2 (ɛw) can be calculated as (Cernusak et al., 2004)
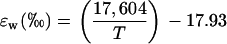 |
(6) |
where T is leaf temperature in degrees Kelvin. The proportion of CO2 in isotopic equilibrium with water at the site of oxygen exchange can be calculated from (Gillon and Yakir, 2000a)
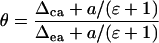 |
(7) |
where Δca is the oxygen isotope composition of CO2 at the site of exchange during photosynthesis determined by
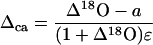 |
(8) |
where Δ18O is the discrimination against C18OO as defined above.
There has been much interest in the role CA plays in enhancing isotopic equilibrium (θ) because it has a significant influence on the atmospheric CO2 isotope signature. It has been suggested that the extent of θ in a leaf can be determined by in vitro CA assays coupled with the unidirectional flux of CO2 into the leaf (Gillon and Yakir, 2000a, 2000b, 2001) from the equation initially developed by Mills and Urey (1940)
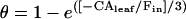 |
(9) |
where CAleaf/Fin represents the mean number of hydration reactions for each CO2 molecule inside the leaf (Gillon and Yakir, 2000a). Leaf CA activity (CAleaf) is determined as the product of the CA hydration rate constant (kCA, μmol m−2 s−1 Pa−1) and the mesophyll pCO2 (pm). The rate constant kCA is calculated from in vitro measurements of CA activity in leaf extracts (see “Materials and Methods”). The gross influx of CO2 into a leaf (Fin = gt pa) as well as pm determine the residence time (τ = pm/Fin) of CO2 within the leaf. The parameter gt is defined as the total conductance of CO2 from the atmosphere to the site of CO2-H2O oxygen exchange (Gillon and Yakir, 2000a). The relationship of CAleaf/Fin indicates that conditions that influence pa, pm, gt, or kCA can alter the value of θ.
RESULTS
Light Response and Isotopic Equilibrium
The online Δ18O values were determined by directly coupling a mass spectrometer to the outlet of a LI-6400 gas exchange system via a gas permeable silicone membrane (Cousins et al., 2006). This allowed the measurements of the C18OO/C16OO ratio of the CO2 in the air stream without prior purification of CO2. We obtained a range of Δ18O values and rates of net CO2 assimilation by manipulating the light conditions that F. bidentis and tobacco (Nicotiana tabacum) leaves were exposed to during the gas exchange measurements (Table II). Leaf CA activity (CAleaf), determined as the product of the rate constant (kCA, μmol m−2 s−1 Pa−1) and the mesophyll pCO2 (pm), increased as rates of net CO2 assimilation decreased under limiting light conditions because pm increased (Table II). The kCA was determined from leaf extracts using mass spectrometry to measure the rates of 18O2 exchange from labeled 13C18O2 to H216O (von Caemmerer et al., 2004; Cousins et al., 2006). The CAleaf activity for tobacco was similar to earlier published values (Williams et al., 1996; Gillon and Yakir, 2000a), whereas CAleaf in Z. mays was higher than previously reported by Gillon and Yakir (2001) but similar to a more recent publication (Affek et al., 2006). F. bidentis plants with reduced levels of Rubisco (caused by antisense RNA constructs targeted to the nuclear-encoded gene for the small subunit of Rubisco [SSU; anti-SSU plants]) had lower photosynthetic rates than wild-type plants and higher CAleaf activity at similar light levels because of higher values of pm (Table II).
Table II.
Gas exchange, CA activity, Δ18O, and isotopic equilibrium measurements
Net CO2 assimilation rate (A), the pCO2 at the site of CO2-H2O oxygen exchange in the mesophyll cytosol (pm), the ratio of mesophyll cytosolic to ambient CO2 partial pressure (pm/pa), the residence time of CO2 in the aqueous phase within the leaf (τ), stomatal conductance (gs), leaf CA activity (CAleaf), online Δ18O discrimination, and proportion of chloroplast CO2 in isotopic equilibrium with chloroplast water (θ) determined from online Δ18O measurements (Eq. 7) and predicted from in vitro CA assays and gas exchange (Eq. 9) in F. bidentis and tobacco measured at various irradiances (μmol quanta m−2 s−1) and the F. bidentis with reduced levels of Rubisco (anti-SSU) and wild-type Z. mays measured at 2,000 (μmol quanta m−2 s−1). Other conditions are as described in the legend to Figure 1. gw = 10 mol m−2 s−1 Pa−1 for all plants except tobacco where gw = 5 mol m−2 s−1 Pa−1. n = 3 to 5.
| Plant | Irradiance | A | gs | CAleaf | Δ18O | Measured | Predicted | |||
|---|---|---|---|---|---|---|---|---|---|---|
| μmol m−2 s−1 | μmol m−2 s−1 | pm | pm/pa | τ | mol m−2 s−1 | μmol m−2 s−1 | ‰ | θ | θ | |
| F. bidentis wild type | 150 | 6.2 ± 0.2 | 410 ± 19 | 0.77 ± 0.03 | 11.5 ± 1.6 | 0.12 ± 0.03 | 2,855 ± 602 | 182 ± 38 | 1.06 ± 0.04 | 1.00 ± 0.01 |
| 300 | 12.2 ± 0.2 | 363 ± 32 | 0.70 ± 0.06 | 7 ± 1 | 0.21 ± 0.07 | 2,558 ± 652 | 103 ± 29 | 0.89 ± 0.02 | 1.00 ± 0.01 | |
| 800 | 25.9 ± 0.9 | 283 ± 28 | 0.59 ± 0.06 | 3.8 ± 0.4 | 0.33 ± 0.09 | 2,000 ± 532 | 43 ± 10 | 0.65 ± 0.04 | 0.99 ± 0.01 | |
| 1,400 | 33.7 ± 1.3 | 252 ± 25 | 0.56 ± 0.05 | 2.8 ± 0.2 | 0.41 ± 0.07 | 1,844 ± 262 | 31 ± 5 | 0.54 ± 0.04 | 0.99 ± 0.01 | |
| 2,000 | 37.6 ± 1.2 | 234 ± 15 | 0.53 ± 0.03 | 2.6 ± 0.1 | 0.42 ± 0.04 | 1,868 ± 180 | 23 ± 2 | 0.45 ± 0.04 | 0.99 ± 0.01 | |
| Tobacco | 300 | 15.9 ± 0.9 | 161 ± 14 | 0.45 ± 0.04 | 5.0 ± 0.3 | 0.18 ± 0.03 | 1,197 ± 194 | 37 ± 4 | 0.89 ± 0.08 | 0.99 ± 0.01 |
| 500 | 21.1 ± 1.5 | 142 ± 13 | 0.41 ± 0.04 | 3.6 ± 0.2 | 0.25 ± 0.04 | 1,060 ± 182 | 29 ± 2 | 0.81 ± 0.10 | 0.99 ± 0.01 | |
| 800 | 24.7 ± 1.9 | 130 ± 11 | 0.39 ± 0.04 | 2.9 ± 0.2 | 0.30 ± 0.06 | 976 ± 183 | 23 ± 1 | 0.67 ± 0.04 | 0.99 ± 0.01 | |
| F. bidentis anti-SSu | 2,000 | 23.6 ± 3.1 | 323 ± 5 | 0.68 ± 0.02 | 4.0 ± 0.7 | 0.40 ± 0.09 | 2,285 ± 231 | 51 ± 3 | 0.63 ± 0.02 | 0.99 ± 0.01 |
| Z. mays | 2,000 | 33.9 ± 1.5 | 86 ± 8 | 0.19 ± 0.02 | 1.9 ± 0.1 | 0.18 ± 0.01 | 296 ± 32 | 13 ± 1 | 0.62 ± 0.10 | 0.88 ± 0.03 |
In both wild-type F. bidentis and tobacco the predicted isotopic equilibrium (θ) determined from the in vitro CA assays (Eq. 9) did not vary with irradiance, whereas θ determined from the measured Δ18O (Eq. 7) decreased dramatically with increasing irradiance (Table II). The residence time (τ = pm/Fin) of CO2 within the leaf increased in both F. bidentis and tobacco at the low irradiances (Table II). This was due to an increase in pm as well as a decrease in Fin caused by a reduction in gs (Table II). In both species, the values of θ predicted from in vitro CA assays (Eq. 9) were substantially higher than the values measured from Δ18O (Eq. 7), except at the low light levels where the predicted and measured values of θ started to converge. In F. bidentis we used the low light measurement, when isotopic equilibrium is expected to be complete (i.e. θ = 1), to estimate an internal conductance to the diffusion of CO2 between the intercellular air space and the site of CO2-H2O oxygen exchange (gw) of 10 mol m−2 s−1 Pa−1 (method described by Gillon and Yakir, 2000a). The value of gw for tobacco (5 mol m−2 s−1 Pa−1) was determined from Δ13C measurements as described by von Caemmerer and Evans (1991). Both the predicted θ values from in vitro CA assays (Eq. 9) and the measured values determined from Δ18O (Eq. 7) in Z. mays were lower than in wild-type F. bidentis plants at similar light levels (Table II). The F. bidentis anti-SSU plants with reduced levels of Rubisco had higher online determined values of θ and τ than wild-type F. bidentis at similar light conditions because of higher values of pm (Table II).
Gas Exchange and CA Activity
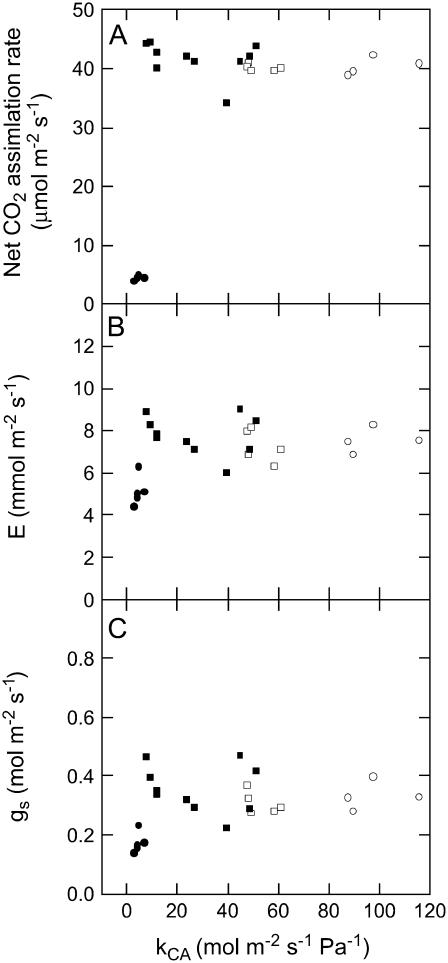
We obtained plants with a range of CA activity from the progeny of F. bidentis plants containing the antisense RNA constructs targeted toward the putative cytosolic CA (anti-CA plants, see Fig. 1). As previously reported, decreases in CA activity in F. bidentis had little effect on net CO2 assimilation until CA levels were less than 20% of wild type (Fig. 1A; see also von Caemmerer et al., 2004; Cousins et al., 2006). The transpiration rate (E) and stomatal conductance (gs) recorded after approximately 1.5 h in the leaf chamber under steady-state conditions were similar in plants with a large range of leaf CA activities (Fig. 1, B and C). However, both E and gs were lower (5.1 versus 7.5 for E and 0.36 versus 0.17 for gs) in the anti-CA plants with dramatically reduced CA activity and net CO2 assimilation rates compared to wild-type plants (Fig. 1).
Figure 1.
Net CO2 assimilation rate, transpiration rate (E), and stomatal conductance (gs) as a function of the rate constant of leaf CA (kCA mol m−2 s−1 Pa−1). Each point represents a measurement made on a different plant grown in a glass house at ambient CO2 or in a growth cabinet at 0.96 kPa CO2: wild-type plants grown at ambient CO2 (□); anti-CA plants grown at ambient CO2 (▪); wild type grown at 0.96 kPa CO2 (○); and anti-CA plants grown at 0.96 kPa CO2 (•). Gas exchange measurements were made at an irradiance of 2,000 μmol quanta m−2 s−1, leaf temperature of 30°C and an inlet CO2 concentration of 52 Pa in 90.5 kPa of N2 and 4.8 kPa of O2 gas mixture.
δ18O of Water at the Site of Evaporation
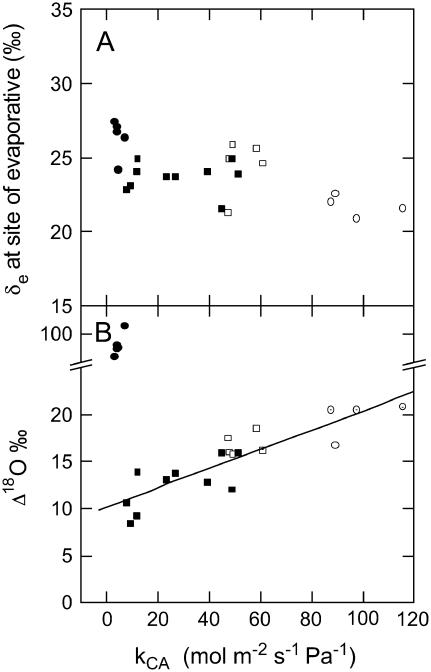
The oxygen isotope composition of water at the evaporative site in the leaves (δe) determined from the model of Craig and Gordon (1965) and further developed by Farquhar and Lloyd (1993) ranged between 18‰ and 27‰ (Eq. 1). We waited a minimum of 1.5 h under steady conditions to measure Δ18O, and subsequently collected transpired water to calculate δe. Under steady-state conditions the isotopic composition of transpired water vapor (δt) should be equal to the isotopic composition of source water (δs, the water take up by the root system). The values of δt and δs were similar for plants grown under 1% CO2, −6.2 ± 0.3‰ and −5.1 ± 0.4‰, respectively. However, δt and δs in the glass house-grown plants were −2.6 + 0.5‰ and −5.3 ± 0.3‰, respectively. Values of δe, calculated from Equation 1 using values of δt, were similar between anti-CA and wild-type plants grown under ambient CO2 in the glass house (Fig. 2). However, δe in wild-type plants grown in 1% CO2 tended to be more depleted in 18O, whereas δe in the anti-CA plants grown under similar conditions was more enriched in 18O (Fig. 2). The difference in δe between the high CO2-grown wild-type and low anti-CA plants is attributed to the differences in E and gs, which caused ɛk (Eq. 2) to increase from 30 ± 0.1‰ to 31 ± 0.1‰ and the ratio of ea/ei (used in Eq. 1) to decrease from 0.45 ± 0.01‰ to 0.32 ± 0.02‰. These changes in E and gs as well as the shift in pm/pa contribute to the large shift in Δ18O in the low anti-CA plants (see below).
Figure 2.
The isotopic composition (‰) of the water at the site of evaporation (δe) and the oxygen isotope discrimination (Δ18O) as a function of the rate constant of leaf CA (kCA mol m−2 s−1 Pa−1). Values of δe were calculated as described in the text using Equation 1 and source water (δs) was −5.3 ± 0.3‰. The line represents a linear regression (R2 = 0.87) for all data except the plants with high Δ18O values. Symbols and gas exchange conditions are as in Figure 1 and δ values are expressed in reference to VSMOW.
Measured C18OO Discrimination and Isotopic Equilibrium
Although there was no detectable change in the net CO2 assimilation rate in anti-CA plants with greater than 20% of wild-type CA activity, Δ18O decreased with reduced CA activity (Fig. 2B). There was a strong relationship between Δ18O and CA activity, where Δ18O decreased with CA activity until low levels of CA dramatically altered the rates of net CO2 assimilation. Plants with low photosynthetic rates had Δ18O values markedly higher than the other anti-CA plants corresponding to the high pm/pa in these plants (Figs. 2B and 3).
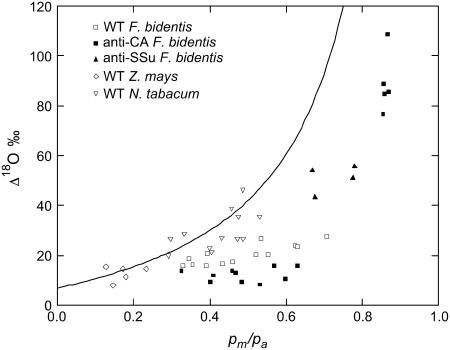
Figure 3.
Oxygen isotope discrimination (Δ18O) as a function of the ratio of mesophyll cytosolic to ambient CO2 partial pressure (pm/pa). Where pm was calculated with gw = 10 mol m−2 s−1 Pa−1 for the C4 plants and 5 mol m−2 s−1 Pa−1 for tobacco. The line represents the theoretical relationship of Δ18O and pm/pa at full isotopic equilibrium where a = 7.7‰ and Δea = 33.7‰ (Eq. 4) and the CO2 supplied to the leaf had a δ18O of 24‰ relative to VSMOW. Each point represents a measurement made on a different plant grown under ambient conditions in a glass house or at 0.96 kPa CO2 in growth chambers. Measurement conditions are as in Table II and Figure 1. Each point represents a measurement made on a different plant or different light level: wild-type F. bidentis (□); anti-CA F. bidentis (▪); anti-SSU F. bidentis (▴); wild-type Z. mays (⋄); and wild-type tobacco (▵). Plants grown in ambient and 0.96 kPa CO2 were grouped together in this figure.
The values of Δ18O increased with pm/pa as predicted from Equation 4, but the measured values for F. bidentis were always less than those predicted at full isotopic equilibrium with an assumed constant Δea of 33.7‰ (Fig. 3). The value of Δea was taken as the average value determined from all wild-type plants at high irradiance (2,000 μmol quanta m−2 s−1). At similar pm/pa values the anti-CA plants, with wild-type rates of net CO2 assimilation, had lower Δ18O and were thus further away from the predicted values of Δ18O. The values of Δ18O and pm/pa were high in the anti-CA plants with extremely low CA activity compared to wild-type plants due to the lack of net CO2 assimilation (Fig. 3). However, the measured values of Δ18O were even further away than wild type from the predicted Δ18O values. F. bidentis plants with reduced amounts of Rubisco (anti-SSU plants) have a high pm/pa, due to the low rates of net CO2 assimilation, but wild-type levels of extractible CA activity. Their measured values of Δ18O are closer to the predicted values of Δ18O, compared to wild-type plants (Fig. 3). The values of Δ18O for both Z. mays and tobacco correspond to lower pm/pa and were closer to the predicted values of Δ18O compared to wild-type F. bidentis (Fig. 3).
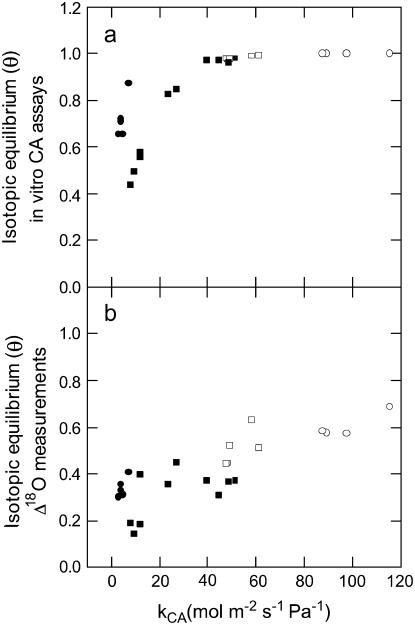
Using Equation 9 and in vitro CA assays to predict θ indicated that wild-type plants should be at complete isotopic equilibrium with water at the site of oxygen exchange (Fig. 4A). This is in contradiction to the relatively low θ values determined from measurements of Δ18O and Equation 7 (Fig. 4B), highlighting the discrepancy in θ determined from either Equation 7 or 9. The values of θ predicted from in vitro CA assays (Eq. 9) were substantially higher in both the wild-type and anti-CA F. bidentis plants than the values measured from Δ18O (Eq. 7), as shown in Figure 4 and Table II. Both methods of determining θ indicated a decrease in θ as the amount of CA decreased in the leaves of anti-CA plants (Fig. 4), although values estimated from in vitro CA assays were more sensitive to changes in CA (Fig. 4A).
Figure 4.
The extent of isotopic equilibrium (θ) as a function of the rate constant of leaf CA (kCA mol m−2 s−1 Pa−1) in wild-type and anti-CA F. bidentis plants. The predicted values of θ were determined from in vitro CA assays using Equation 9 (A) and the measured values of θ were determined from Δ18O using Equation 7 (B). Symbols and gas exchange conditions are as in Figure 1. Calculations were made with gw = 10 mol m−2 s−1 Pa−1.
The Water at the Site of Exchange, Internal CO2 Conductance, and the Extent of Isotopic Equilibrium
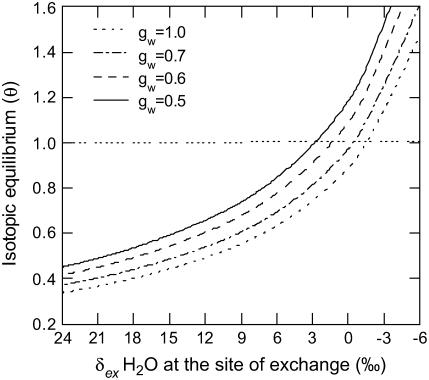
The value of θ calculated from the online isotope measurements (Eq. 7) is influenced not only by Δ18O but also by the partial pressure of CO2 and the isotopic signature of leaf water at the site of CO2-H2O oxygen exchange, pm and δex, respectively. The Craig-Gordon model describes the water enrichment at the site of evaporation (δe), but does not provide information on the isotopic gradient of water within a leaf. It is generally assumed that δe is equal to δex, but theoretically δex can vary between δe (δe = 18‰ to 25‰) and source water (δs= −5.3 ± 0.3‰). Figure 5 shows that modeled changes in δex shifts θ from around 0.4 to 1.4, demonstrating that δex is crucial for calculating θ when using Equation 7. The model is also used to vary gw, which is used to estimate ɛ in Equation 4, to see how gw influences the value of θ estimated from Δ18O measurements (Fig. 5).
Figure 5.
The predicted isotopic equilibrium (θ) determined with various isotopic compositions of water at the site of CO2-H2O oxygen exchange (δex). Calculations were made with gw values ranging from 10 to 5 mol m−2 s−1 Pa−1. θ was calculated using Equation 7 where Δea was determined using Equation 5 and substituting δe with various values of δex. Δca was calculated from parameters taken from the high light wild-type F. bidentis measurements in Table II. δex values are presented relative to VSMOW.
DISCUSSION
The Influence of CA on C18OO Discrimination during C4 Photosynthesis
We measured Δ18O concurrently with gas exchange in transgenic F. bidentis with a range of leaf CA activity due to an antisense construct targeted to a putative cytosolic CA to explore the role CA plays in Δ18O in a C4 species. These plants have been previously described by von Caemmerer et al. (2004) and Cousins et al. (2006). Our measurements show that even small reductions in CA activity resulted in decreased Δ18O (Fig. 2) and reductions in the extent of isotopic equilibrium, θ, estimated from Δ18O (Fig. 4). However, these plants showed no differences in the rate of net CO2 assimilation or stomatal conductance (Fig. 1). It has been reported that low levels of leaf CA, particularly in C4 monocots, limits the extent of θ (Gillon and Yakir, 2000a, 2000b, 2001). The limited amount of CA activity in these leaves is thought to be insufficient to fully equilibrate the exchange of oxygen between CO2 and water. However, the C4 dicot F. bidentis has high levels of leaf CA, compared to these C4 monocots, with levels comparable to many C3 plants (Table II; Gillon and Yakir, 2000a, 2000b, 2001; von Caemmerer et al., 2004; Cousins et al., 2006). It was therefore intriguing to measure low Δ18O and θ values in wild-type F. bidentis and observe the responsiveness of Δ18O to small reductions in CA activity.
The value of θ is related to the mean number of hydration reactions a CO2 molecule experiences inside a leaf. This in turn is the product of residence time (τ = pm/Fin) and the hydration constant of leaf CA, kCA (as described in the theory section). We therefore also experimentally thought to increase θ by using transgenic F. bidentis with reduced Rubisco activity. This increased τ, Δ18O, and the measured θ values because reduced assimilation rates are not matched by reductions in stomatal conductance, thus leading to an increased pm and pm/pa (Table II; Fig. 3). This further suggests that CA activity is not saturating for isotopic equilibrium in wild-type F. bidentis plants.
CA Activity as a Predictor of Isotopic Equilibrium
In both Z. mays and F. bidentis the value of θ determined from Δ18O measurements was considerably less than that estimated from in vitro measurements of CA activity that predicted θ values close to one for all species (Table II; Fig. 4). As previously reported and shown in Table II, θ in the C3 plant tobacco determined from Δ18O measurements was higher than in C4 plants (Williams et al., 1996; Gillon and Yakir, 2000a, 2000b). As noted above, the extent of θ is in part determined by the CA activity associated with the CO2-H2O oxygen exchange, but knowing the isotopic signature of the water at the site of exchange (δex), as well as knowing the pCO2 at the site of exchange, are also both important factors (see below).
The discrepancy between our estimates of θ in F. bidentis from online measurements and the in vitro CA activity (Fig. 4) could be due to the fact that measurements of total leaf CA activity are not representative of the CA activity associated with the CO2-H2O oxygen exchange that influences Δ18O. Multiple forms of CA have been reported from leaf tissue of both C3 and C4 species (Badger and Price, 1994; Badger, 2003). In C4 plants the majority of CA is located within the mesophyll cytoplasm (Ku and Edwards, 1975; Burnell and Hatch, 1988; Hatch and Burnell, 1990) and in F. bidentis approximately only 5% of the total leaf CA activity is specifically located within the BSC cells (Ludwig et al., 1998). In C3 plants the chloroplasts, which contain the majority of the leaf's CA, are appressed against the cell walls adjacent to the intercellular air space, thus positioning CA in close proximity to the water at the site of evaporation. Under such conditions it is likely that the isotopic signature of water at the site of evaporation (δe) is similar to δex and this may explain why in tobacco we found quite close agreement between θ estimated from Δ18O and in vitro CA activity (Gillon and Yakir, 2000a, 2000b). However, in C4 plants, because the CA activity is distributed throughout the mesophyll cytoplasm, the majority of CA may not be positioned adjacent to the intercellular air spaces and the site of evaporation. This suggests that whole leaf in vitro CA assays likely overestimate the amount of CA important for determining Δ18O in C4 plants. The different localization of CA activity in C3 and C4 species may be in part responsible for the different relationship between in vitro CA activity and Δ18O in F. bidentis and tobacco.
The Water Isotopic Signature and the Internal Conductance to CO2
The Craig-Gordon model (Eq. 1) is used for predicting the isotopic composition of water at the site of evaporation, δe, but there are a number of studies showing that there can be a large isotopic gradient within a leaf between the water supplied via the vascular tissue and water at the site of evaporation (Farquhar and Gan, 2003; Barbour and Farquhar, 2004; Barbour et al., 2004). As shown in Figure 5, hypothetically varying the isotopic signature of the water at the site of exchange (δex) has a large influence on the calculated values of θ. The values of δex presented in Figure 5 are a reasonable isotopic gradient to consider when δe in the F. bidentis plants varied between 19‰ and 25‰ and source water was −5.3 ± 0.3‰. Due to the fact that the majority of CA occurs within the mesophyll cytoplasm in C4 plants it is possible that the CO2-H2O oxygen exchange occurs in a range of δex values. Indeed the average δex values may be closer to the isotopic composition of source water (Fig. 5). Thus in C4 plants water at the site of evaporation may not be the only water that influences Δ18O.
The difference between the measured and predicted values of Δ18O is also due to uncertainty in determining the pCO2 within the mesophyll cytoplasm (pm) that is used to calculate ɛ in Equation 4. Knowing the internal conductance to CO2 (gw) is necessary to accurately calculate values of pm; however, in C4 plants it is difficult to determine gw with the traditional Δ13C measurements because of the low Δ13C values (Evans and von Caemmerer, 1996). We have used Δ18O, as described by Gillon and Yakir (2000a), under low light conditions when θ = 1 would be expected to estimate values of gw in F. bidentis (Table II). However, Figure 5 shows that dramatically varying gw has only a small influence on the calculated θ values and although gw is important for estimating pm, uncertainty in this value would not be sufficient to raise the measured θ values to the values predicted by the in vitro CA assays.
The Influence of Light on CA Activity and Isotopic Equilibrium
The increase in CAleaf activity at low photosynthetic rates is attributed to the increase in substrate availability for CA due to the lack of photosynthetic CO2 drawdown at the low irradiances. The increase in pm causes CAleaf to increase, assuming kCA remains constant with changing light conditions, because CA activity is generally limited by CO2. Additionally, at low light the residence time of CO2 (τ = pm/Fin) is greater as pm increases and the gross flux of CO2 into the leaf (Fin = gt pa) decreases due to the reduction in gt caused by the drop in gs (Table II). Therefore, the number of hydration reactions per CO2 increases at low light as demonstrated by CAleaf/Fin in Equation 9. It is unknown if CA activity in C4 leaves is modulated with changes in irradiances, but potentially the redox statues of mesophyll chloroplast could regulate CA activity (Lee et al., 2004). Under high light conditions, stomatal conductance (gs) in F. bidentis and tobacco is high compared to Z. mays (0.42 ± 0.04, 0.30 ± 0.06, and 0.18 ± 0.01, respectively; see Table II). In Z. mays the low gs causes the Δ18O to be largely influenced by the stomatal fractionation whereas in F. bidentis and tobacco the higher values of gs allow for the greater potential influence of CA on Δ18O (Fig. 4). Therefore, in Z. mays the low gs influences the values of θ more so than in F. bidentis or tobacco.
Using Equation 9, C4 plants with less CA (e.g. grasses) would be predicted to have a much larger shift in θ due to the changing light conditions. This is in contrast to F. bidentis where the high CAleaf activity predicted that θ would be close to 1 under all light conditions (Table II). The variation in θ under different light conditions may have important implications for predicting the influence of plant communities on the δ18O of atmospheric CO2.
CONCLUSION
The amount of CA activity in a leaf plays an important role in determining C18OO discrimination during C4 photosynthesis. We have shown that Δ18O and the extent of CO2-H2O isotopic equilibrium (θ) in F. bidentis leaves, which contain high levels of in vitro CA activity relative to rates of net CO2 assimilation, is sensitive to small changes in CA activity even when rates of net CO2 assimilation are unaffected. We conclude that the cytoplasmic localization of the majority of CA in C4 species has important implications for predicting Δ18O and the estimation of θ under different environmental conditions. It appears that in vitro CA activity on whole leaf extract of F. bidentis may not represent the CA activity associated with the CO2-H2O oxygen exchange and therefore may not be a good predictor of θ. The ability to accurately predict θ in C4 species will be limited by the uncertainties in CA activity involved in the oxygen exchange and knowing the isotopic composition of water at the site of exchange.
MATERIALS AND METHODS
Growth Conditions
Flaveria bidentis plants were previously transformed with antisense RNA constructs targeted to either the nuclear-encoded gene for the SSU (anti-SSU plants) or a putative cytosolic CA (anti-CA plants; Furbank et al., 1996; von Caemmerer et al., 1997b, 2004). The segregating T1 generations of anti-CA primary transformants with photosynthetic rates similar to wild type were grown during the summer months in a glass house under natural light conditions (27°C day and 18°C night temperatures). The wild-type tobacco (Nicotiana tabacum) plants were also grown in a glass house under similar conditions. Anti-CA (segregating T2 generation from primary transformants 15, 12, and 8) and anti-SSU plants (segregating T2 generation from primary transformant 136-13) with low photosynthetic capacities and wild-type plants were grown under 0.96 kPa of CO2 in a controlled environment growth cabinet at an irradiance of 400 μmol quanta m−2 s−1 at plant height and air temperature of 27°C during the day and 18°C at night with a day length of 14 h. Zea mays plants were grown in similar growth cabinet conditions at ambient CO2 concentrations. All plants were grown in 5 L pots in garden mix with 2.4 to 4 g Osmocote/L soil (15/4.8/10.8/1.2 N/P/K/Mg plus trace elements: B, Cu, Fe, Mn, Mo, and Zn; Scotts Australia Pty) and watered daily.
Gas Exchange Measurements
The uppermost fully expanded leaves were placed into the leaf chamber of the LI-6400 (LI-COR) and equilibrated under measurement conditions for a minimum of 1.5 h (Cousins et al., 2006). Air entering the leaf chamber was prepared by using mass flow controllers (MKS Instruments) to obtain a gas mix of 90.5 kPa dry N2 and 4.8 kPa O2 (Cousins et al., 2006). A portion of the nitrogen/oxygen air was used to zero the mass spectrometer to correct for N2O and other contaminates contributing to the 44 and 46 peaks. Pure CO2 (δ18O = 24‰ VSMOW) was added to the remaining air stream to obtain a CO2 partial pressure of approximately 52 Pa. Low oxygen (4.8 kPa) was used to minimize contamination of the 46 (mass-to-charge ratio) signal caused by the interaction of O2 and N2 to produce NO2 with the mass spectrometer source.
The gas mixtures were fed to the inlet of the LI-6400 console and a flow rate of 200 μmol s−1 was maintained over the leaf. The remaining air stream was vented or used to determine the isotopic composition of air entering the leaf chamber (Cousins et al., 2006). The efflux from the leaf chamber was measured by either replacing the match valve line with a line connected directly to the mass spectrometer or by placing a tee in the match valve line allowing flow to both the mass spec and the match valve simultaneously. Gas exchange parameters were determined by the LI-6400 and pCO2 leaving the chamber was subsequently corrected for the dilution of CO2 by water vapor (von Caemmerer and Farquhar, 1981).
Online Isotopic Measurements
The efflux from the leaf chamber and the gas mix supplied to the LI-6400 system was linked to a mass spectrometer through an ethanol/dry ice water trap and a thin, gas permeable silicone membrane that was housed in a temperature-controlled cuvette. The masses (mass-to-charge ratio) 44 and 46 were monitored continuously and the oxygen isotope discrimination during CO2 exchange, Δ18O, was calculated from the ratio of mass 46 to 44 in the reference air, determined before and after each sample measurement, entering the chamber (Re), and the composition of the sample air leaving the leaf chamber (Ro) as (Evans et al., 1986)
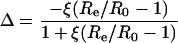 |
(10) |
where ξ = pe/(pe − po), and pe and po are the pCO2 of dry air entering and leaving the leaf chamber, respectively. A summary of the symbols used in the text are listed in Table I. Zero values for the 44 and 46 peaks were determined before and after the sample measurements were subtracted from both the sample and reference measurements prior to determining the mass ratios. The zero values were typically 1% of the 44 and 6% of the 46 peak.
Collection and 18O Isotopic Measurements of Water Vapor
A line connected directly to the exhaust port of the LI-6400 was used to cryogenically trap transpired water in a modified glass collection line submerged in an ethanol/dry ice bath. Water vapor was collected for 45 min (netting 50–100 μL of water depending on transpiration rates) and allowed to warm to room temperature in a sealed collection tube. The tube was subsequently centrifuged and placed into an ice water bath for 30 min. The liquid water was removed from the tube and stored at 4°C in screw-top sample vials (Alltech) until measured. To measure the δ18O of the collected water, 10 mL head space vials (Alltech) with crimped tops containing butyl septa (Alltech) were flushed with 1.9 kPa CO2 (in a N2 background) at 2 L min−1 for 3 min and allowing the pressure inside the vials to remain at atmospheric levels. Water samples (25 μL) were injected through the septa with a gas-tight syringe (SGE) and the CO2-H2O was allowed to equilibrate for 48 h on a shaker at room temperature (Fessenden et al., 2002).
Prior to the isotopic measurements the vials were placed for a minimum of 2.5 h on a temperature block set a 25°C. The CO2 samples were analyzed by injecting 200 μL of the headspace gas into a 500 μL N2 purged gas-tight temperature-controlled cuvette containing a Teflon gas permeable membrane linked to a mass spectrometer (micromass ISOPRIME, Micromass). Masses 44 and 46 were monitored continuously and the zero values determined before and after the sample measurements were subtracted from the values prior to determining the mass ratios. The zero values were typically 3% to 4% of the 44 and 46 peak. Two standard laboratory waters were measured during each measurement to calibrate our measured values against known standards. Our standard waters (S1 = −6.44 and S2 = −22.83) were calibrated by the Stable Isotope Facilities in the Earth Environment Group within the Research School of Earth Sciences at The Australian National University.
The measured δ18O value (δm) was corrected for the contribution of oxygen from the CO2 used for equilibration and normalized against VSMOW as outlined in Scrimgeour (1995). The dilution corrected value was obtained by
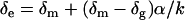 |
(11) |
where δg is the signal of the equilibrating gas, α is the fractionation factor for 18O between water and CO2 (41.1‰ at 25°C), and k is the ratio of oxygen atoms in the water to the oxygen atoms in the CO2 (k = 78). The δc values were then normalized to VSMOW using the two standard waters (−6.44‰ and −22.83‰) and the following equation:
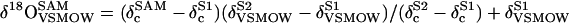 |
(12) |
where the superscripts SAM, S1, and S2 refer to the sample in question and the reference waters 1 and 2, respectively. The subscripts c and VSMOW refer to the dilution corrected values and those calibrate against VSMOW, respectively. The precision of analyses, based on the repeated measurements of gas samples sealed in vials was 0.1‰ (1 sd, n = 8).
CA Activity Measurements
CA activity was measured on leaf extracts using mass spectrometry to measure the rates of 18O2 exchange from labeled 13C18O2 to H216O (Badger and Price, 1989; von Caemmerer et al., 2004; Cousins et al., 2006). Measurements of leaf extracts were made at 25°C with a subsaturating total carbon concentration of 1 mm. The hydration rates were calculated from the enhancement in the rate of 18O loss over the uncatalyzed rate and the nonenzymatic first order rate constant was applied (pH 7.4 appropriate for the mesophyll cytosol). The CA activity was reported as a first order rate constant kCA (mol m−2 s−1 Pa−1) and kCApm gives the in vivo CA activity at that particular cytosolic pCO2. Leaf samples were collected after the gas exchange measurements on the same leaf material and subsequently frozen in liquid nitrogen and stored at −80°C.
Acknowledgments
We thank Chin Wong for helpful advice on collecting transpired water, Hillary Stuart for analyzing the oxygen isotope composition of our CO2 tank, Howard Griffiths, Graham Farquhar, and Matthias Cuntz for their helpful discussions, and Jamaica Ritcher for proofreading portions of this manuscript.
This work was supported by a National Science Foundation international postdoctoral fellowship (to A.B.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Susanne von Caemmerer (susanne.caemmerer@anu.edu.au).
References
- Affek HP, Krisch MJ, Yakir D (2006) Effects of intraleaf variations in carbonic anhydrase activity and gas exchange on leaf (COO)-O-18 isoflux in Zea mays. New Phytol 169: 321–329 [DOI] [PubMed] [Google Scholar]
- Badger M (2003) The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanism. Photosynth Res 77: 83–94 [DOI] [PubMed] [Google Scholar]
- Badger M, Price GD (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45: 369–392 [Google Scholar]
- Badger MR, Price GD (1989) Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol 89: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour MM, Farquhar GD (2004) Do pathways of water movement and leaf anatomical dimensions allow development of gradients in (H2O)-O-18 between veins and the sites of evaporation within leaves? Plant Cell Environ 27: 107–121 [Google Scholar]
- Barbour MM, Roden JS, Farquhar GD, Ehleringer JR (2004) Expressing leaf water and cellulose oxygen isotope ratios as enrichment above source water reveals evidence of a Peclet effect. Oecologia 138: 426–435 [DOI] [PubMed] [Google Scholar]
- Burnell JN, Hatch MD (1988) Low bundle sheath carbonic-anhydrase is apparently essential for effective C-4 pathway operation. Plant Physiol 86: 1252–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Farquhar GD, Wong SC, Stuart-Williams H (2004) Measurement and interpretation of the oxygen isotope composition of carbon dioxide respired by leaves in the dark. Plant Physiol 136: 3350–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitty JA, Furbank RT, Marshall JS, Chen ZH, Taylor WC (1994) Genetic-transformation of the C-4 plant, Flaveria-bidentis. Plant J 6: 949–956 [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S (2006) Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis: insights from antisense RNA in Flaveria bidentis. Plant Physiol 141: 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig H, Gordon LI (1965) Deutrium and oxygen-18 variations in the ocean and the marine atmosphere. In E Tongiorgi, ed, Proceedings of a Conference on Stable Isotopes in Oceanographic Studies and Paleotemperatures. Consiglio Nazionale delle Ricerche, Laboratorie Geologia Nuclear, Pisa, Italy, pp 9–130
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher-plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Evans JR, vonCaemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Farquhar GD, Gan KS (2003) On the progressive enrichment of the oxygen isotopic composition of water along a leaf. Plant Cell Environ 26: 801–819 [PubMed] [Google Scholar]
- Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In JR Ehleringer, AE Hall, GD Farquhar, eds, Stable Isotopes and Plant Carbon-Water Relations. Academic Press, New York, pp 47–70
- Farquhar GD, Lloyd J, Taylor JA, Flanagan LB, Syvertsen JP, Hubick KT, Wong SC, Ehleringer JR (1993) Vegetation effects on the isotope composition of oxygen in the atmospheric CO2. Nature 363: 439–443 [Google Scholar]
- Fessenden J, Cook C, Lott M, Ehleringer JR (2002) Rapid 18O analysis of small water and CO2 samples using a continuous-flow isotope ratio mass spectrometer. Rapid Commun Mass Spectrom 16: 1257–1260 [DOI] [PubMed] [Google Scholar]
- Flanagan LB, Ehleringer JR (1998) Ecosystem-atmosphere CO2 exchange: interpreting signals of change using stable isotope ratios. Trends Ecol Evol 13: 10–14 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, vonCaemmerer S, Ashton AR (1997) Genetic manipulation of key photosynthetic enzymes in the C-4 plant Flaveria bidentis. Aust J Plant Physiol 24: 477–485 [Google Scholar]
- Furbank RT, Chitty JA, von Caemmerer S, Jenkins CLD (1996) Antisense RNA inhibition of RbcS gene expression reduces rubisco level and photosynthesis in the C-4 plant Flaveria bidentis. Plant Physiol 111: 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon J, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the O-18 content of atmospheric CO2. Science 291: 2584–2587 [DOI] [PubMed] [Google Scholar]
- Gillon JS, Yakir D (2000. a) Internal conductance to CO2 diffusion and (COO)-O-18 discrimination in C-3 leaves. Plant Physiol 123: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon JS, Yakir D (2000. b) Naturally low carbonic anhydrase activity in C-4 and C-3 plants limits discrimination against (COO)-O-18 during photosynthesis. Plant Cell Environ 23: 903–915 [Google Scholar]
- Harwood KG, Gillon JS, Griffiths H, Broadmeadow MSJ (1998) Diurnal variation of Delta(CO2)-C-13, Delta(COO)-O-18-O-16 and evaporative site enrichment of delta(H2O)-O-18 in Piper aduncum under field conditions in Trinidad. Plant Cell Environ 21: 269–283 [Google Scholar]
- Hatch MD (1987) C-4 photosynthesis—a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106 [Google Scholar]
- Hatch MD, Burnell JN (1990) Carbonic-anhydrase activity in leaves and its role in the first step of C-4 photosynthesis. Plant Physiol 93: 825–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Edwards GE (1999) The biochemistry of C4 photosynthesis. In R Sage, R Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 49–87
- Ku MSB, Edwards GE (1975) Photosynthesis in mesophyll protoplast and bundle sheath cells of various type of C4 plants. V. Enzymes of respiratory metabolism and energy utilizing enzymes of photosynthetic pathways. Z Pflanzenphysiol 77: 16–32 [Google Scholar]
- Lee K, Lee J, Kim Y, Bae D, Kang KY, Yoon SC, Lim D (2004) Defining the plant disulfide proteome. Electrophoresis 25: 532–541 [DOI] [PubMed] [Google Scholar]
- Ludwig M, von Caemmerer S, Price GD, Badger MR, Furbank RT (1998) Expression of tobacco carbonic anhydrase in the C4 dicot Flaveria bidentis leads to increased leakiness of the bundle sheath and a defective CO2-concentrating mechanism. Plant Physiol 117: 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G, Urey H (1940) The kinetics of isotopic exchange between carbon dioxide, bicarbonate ion, carbonate ion and water. J Am Chem Soc 62: 1019–1026 [Google Scholar]
- Scrimgeour CM (1995) Measurement of plant and soil water isotope composition by direct equilibration methods. J Hydrol 172: 261–274 [Google Scholar]
- von Caemmerer S, Evans JR (1991) Determination of the average partial pressure of CO2 in chloroplast from leaves of several C3 plants. Aust J Plant Physiol 18: 287–305 [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price D, Badger M, Furbank RT (1997. a) Carbon isotope discrimination during C-4 photosynthesis: insights from transgenic plants. Aust J Plant Physiol 24: 487–494 [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT (1997. b) Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isopote discrimination. Plant Physiol 113: 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Quinn V, Hancock NC, Price GD, Furbank RT, Ludwig M (2004) Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant Cell Environ 27: 697–703 [Google Scholar]
- Williams TG, Flanagan LB, Coleman JR (1996) Photosynthetic gas exchange and discrimination against 13CO2 and C18O16O in tobacco plants modified by an antisense construct to have low chloroplastic carbonic anhydrase. Plant Physiol 112: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir D, Sternberg LdL (2000) The use of stable isotopes to study ecosystem gas exchange. Oecologia 123: 297–311 [DOI] [PubMed] [Google Scholar]
- Yakir D, Wang X-F (1996) Fluxes of CO2 and water between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 380: 515–517 [Google Scholar]