Abstract
Many human and mouse tumor antigens are normal, nonmutated tissue differentiation antigens. Consequently, immunization with these “self” antigens could induce autoimmunity. When we tried to induce immune responses to five mouse melanocyte differentiation antigens, gp100, MART-1, tyrosinase, and tyrosinase-related proteins (TRP) 1 and TRP-2, we observed striking depigmentation and melanocyte destruction only in the skin of mice inoculated with a vaccinia virus encoding mouse TRP-1. These mice rejected a lethal challenge of B16 melanoma, indicating the immune response against TRP-1 could destroy both normal and malignant melanocytes. Cytotoxic T lymphocytes specific for TRP-1 could not be detected in depigmented mice, but high titers of IgG anti-TRP-1 antibodies were present. Experiments with knockout mice revealed an absolute dependence on major histocompatibility complex class II, but not major histocompatibility complex class I, for the induction of both vitiligo and tumor protection. Together, these results suggest that the deliberate induction of self-reactivity using a recombinant viral vector can lead to tumor destruction, and that in this model, CD4+ T lymphocytes are an integral part of this process. Vaccine strategies targeting tissue differentiation antigens may be valuable in cancers arising from nonessential cells and organs such as melanocytes, prostate, testis, breast, and ovary.
It has long been known that elements of the cellular immune response, and CD8+ T lymphocytes in particular, are capable of specifically recognizing and destroying tumor cells. Classical studies showed that mice immunized with irradiated methylcholanthrene-induced sarcoma cells were fully protected against a subsequent challenge with that same tumor, but not with others (1). This protection depended on CD8+ T lymphocytes, whereas CD4+ T lymphocytes often played little, if any, role. Furthermore, adoptive transfer of pure populations of CD8+ T lymphocytes can mediate tumor regression in mice (2–4). Thus, CD8+ T lymphocytes have been the focus of recent efforts toward development of therapeutic anticancer vaccines (2, 5–7).
Recently, molecular targets of tumor-specific CD8+ T lymphocytes have been identified in human and mouse systems (4, 5, 8, 9). One group of antigens consists of nonmutated “self” melanocyte differentiation antigens such as gp100/pmel-17, MART-1/Melan-A, tyrosinase, and tyrosinase-related proteins (TRP) 1 and TRP-2. These antigens are expressed by both normal and malignant melanocytes (8). A pitfall in attempts to target these antigens with cancer vaccines may be central and peripheral tolerance to self-antigens, which may not be adequately modeled in many mouse tumor models using “foreign” antigens such as ovalbumin, viral proteins, or xenogeneic forms of target antigens. Self-reactive T lymphocytes can be physically or functionally deleted in the thymus or in the periphery, leaving behind only limited numbers of functionally impaired T lymphocytes (10, 11).
It is nevertheless possible to mount a potent immune response to self-antigens as evidenced in patients afflicted with autoimmune disease, as well as in animal models, where autoantigens can be targeted by immune responses powerful enough to destroy thyroid cells, pancreatic beta cells, or myelin sheaths. In patients afflicted with autoimmune vitiligo, affected skin areas are progressively depleted of melanocytes, leading to a total loss of pigment. Upon treatment of melanoma patients with interleukin (IL) 2, approximately 20% of responding melanoma patients, but none of responding renal cancer patients, developed vitiligo (12). The relationship, if any, between vitiligo and tumor regression has not been elucidated, but it is interesting that IL-2 is one of the prime cytokines secreted by CD4+ T lymphocytes. We hypothesized that deliberate induction of self-reactivity might result in antitumor effects. To test this hypothesis, we attempted to induce autoimmune vitiligo by using murine homologues of five known human melanocyte differentiation antigens and to determine the role of CD8+ and CD4+ T lymphocytes in autoimmune disease and antitumor effects.
MATERIALS AND METHODS
Animals and Cell Lines.
Six- to 10-week-old female C57BL/6n (H-2b) mice were obtained from Frederick Cancer Research Center (Frederick, MD). β2-microglobulin (β2m) knockout mice, major histocompatibility complex (MHC) class II knockout mice, and perforin knockout mice, severely deficient in CD8+ T lymphocytes, CD4+ lymphocytes, and both CD8+ T lymphocytes and Natural Killer function, respectively, were obtained from Taconic Farms. All mice were maintained in a barrier facility. The spontaneous murine melanoma B16 expresses gp100, MART-1, tyrosinase, TRP-1, and TRP-2 as shown by fluorescence-activated cell sorting and Western blotting (data not shown). All tumor lines were maintained in CM [RPMI medium 1640 with 10% heat-inactivated fetal bovine serum (Biofluids, Rockville, MD), 0.03% l-glutamine, 100 μg/ml of streptomycin, 100 μg/ml of penicillin and 50 μg/ml of gentamicin sulfate (National Institutes of Health Media Center)].
Recombinant Viruses and Plasmid DNA.
All recombinant vaccinia viruses (rVVs) used in this study were generated and purified as described (4, 13). rVVmgp100, rVVmMART-1, rVVmTyr, rVVmTRP-1, and rVVmTRP-2 were based on the plasmid pSC65 in which the strong synthetic early/late promoter pSE/L drives expression of the antigen and the weaker early/late p7.5E/L drives expression of the Escherichia coli LacZ gene (14, 20). The baculovirus encoding mTRP-1 was a kind gift of Alan N. Houghton, Memorial Sloan Kettering Center, New York (15). Cloning of the genes for mgp100 and mMART-1/Melan-A and TRP-2 has been described (16, 17). Expression of plasmid DNA and rVV was confirmed by immunostaining and Western blotting analysis of transfected and infected cells by using antisera kindly provided by V. Hearing, National Cancer Institute, Bethesda, MD (18). Interestingly, monkey kidney cells infected with rVV encoding mTyr, the rate-limiting enzyme in the synthesis of eumelanin, turned intensely black within 48 hr. Recombinant plasmid DNA delivery by gene gun was described (19).
Tumor Protection Assay.
Mice were vaccinated i.v. with 1–2 × 107 plaque-forming units of rVVmTRP-1, or control virus rVVLacZ, and boosted 3 weeks later. Three to 5 weeks after the last vaccination, mice were injected s.c. on the ventral side with 1 × 105 B16 tumor cells in 100 μl of PBS. Starting on day 7, the product of perpendicular tumor diameters was determined every other day until tumors became larger than 1 cm2. All experiments were performed at least twice in a blinded, randomized fashion.
Western Blotting, Dot Blotting, and Histological Analysis.
For Western blotting, cell lysates were prepared by lysing indicated cell lines in PBS with 0.02% Triton X-100 and 1% aprotinin. Sample and SDS-containing loading buffer (1:1) were mixed, boiled for 2 min, and applied on 4–20% Tris-glycine SDS/PAGE gel (NOVEX, San Diego, CA). Samples were run and blotted onto poly(vinylidene difluoride) membrane (NOVEX) and blocked in 5% powdered fat-free milk in PBS. Washes between primary antibody and horseradish peroxidase-labeled secondary antibody (Amersham Pharmacia) were done with 1% BSA in PBS before staining with diaminobenzidine substrate (Sigma), or incubation with chemiluminescence reagent (Western blotting Chemiluminescence Reagent Plus, NEN). For dot blotting, indicated 25-mer peptides were solubilized in 10% dimethyl sulfoxide in PBS, and 10 μg of peptide was applied directly on a nitrocellulose membrane and dried 10 min. For histology, tissues were fixed in 10% buffered formaldehyde and embedded in methacrylate, and 4-μm sections were stained with hematoxylin and eosin.
ELISA Testing of Mouse Sera.
B16 melanoma cells were washed and resuspended at 2 × 105 cells/ml in PBS (Biofluids) and then lysed by three cycles of freeze and thaw. B16 lysate at 1 × 104 cell equivalents (50 μl) was plated into wells of poly(vinyl chloride) microtiter plates (Dynex Technologies, Chantilly, VA) and dried overnight at 37°C. Wells were blocked with 100 μl of 5% BSA (GIBCO/BRL) in PBS (Biofluids) for 1 hr at 37°C. Fifty microliters of 1:2,500 experimental mouse sera in PBS with 1% BSA was added to each well and incubated for 2 hr at 37°C. Goat anti-mouse IgG2b horseradish peroxidase conjugate was used at a dilution of 1:4,000 (Boehringer Mannheim). Five milligrams of OPD (o-phenylenediamine dihydrochloride, Sigma) and 50 μl of 3% hydrogen peroxide (Sigma) were added to 10 ml of the substrate buffer [5.1 g of citric acid (Sigma)] with 13.8 g of sodium dibasic heptahydrate (Mallinckrodt) in 1,000 ml of dH2O). Per well, 50 μl of this substrate was added, and the colorimetric reaction was stopped with 50 μl of 4 M H2SO4 after 10 min. The wells were read at 492 nm in a Titertek Multiscan MCC/340 plate scanner.
RESULTS
Immunization with rVVmTRP-1 Induces Vitiligo.
To determine the ability of melanocyte differentiation antigens to induce a self-specific immune response, we constructed rVV encoding each of five murine homologues of known human antigens recognized by T lymphocytes: gp100/pmel17, MART-1/Melan-A, tyrosinase, TRP-1/gp75, and TRP-2. Because these antigens all are expressed by normal melanocytes, we monitored vaccinated animals for autoimmune symptoms, particularly pigmentation abnormalities. Normal C57BL/6 mice immunized with rVV encoding mgp100, mMART-1, mTyr, or mTRP-2 did not show any evidence of depigmentation (0/15 mice in each case). Mice vaccinated with rVV encoding mTRP-1 developed profound skin depigmentation within 3 weeks after their second immunization. (168/206 mice). There was no vitiligo, however, in mice vaccinated with plasmid DNA encoding mTRP-1 (0/15 mice), or any of the other four melanocyte differentiation antigens (0/10 mice in each case), even after repeated immunizations.
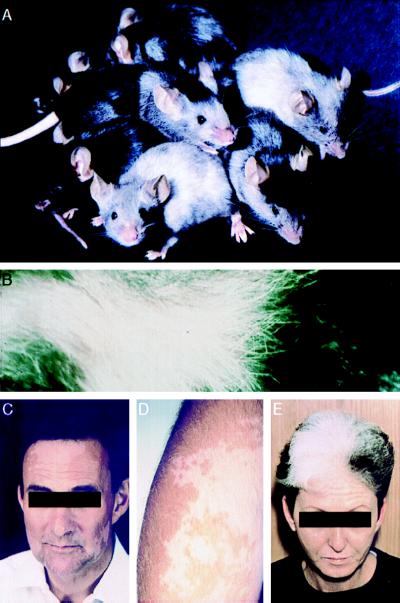
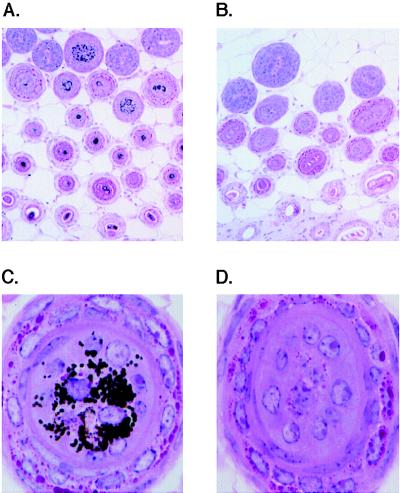
Mice vaccinated with rVVmTRP-1 typically exhibited a bilateral loss of pigment, the site and pattern of which differed from mouse to mouse (Fig. 1). This pattern resembled vitiligo that occurred in some melanoma patients that responded to IL-2 treatment (12) (Fig. 1). Histopathological examination of depigmented skin of mice with vaccination-induced coat color changes revealed complete absence of pigment in hair follicles (Fig. 2 B and D) as compared with skin from mice vaccinated with control rVVLacZ (Fig. 2 A and C). In mice with early signs of vitiligo, depigmented skin areas bordered areas with no apparent signs of pigment loss. Interestingly, mice with pronounced skin depigmentation revealed no inflammation or loss of pigment in melanocyte-containing tissues of the eye, inner ear, and substantia nigra in the brain. This result parallels observations in vitiligo patients, where no histopathological or functional changes were observed in these tissues that require pigmented cells for full function (ref. 12, and data not shown.)
Figure 1.
Vitiligo-like depigmentation in mice vaccinated twice with rVVmTRP-1. Coat color changes vary in degree, site, and pattern (A). A close-up of depigmented mouse fur is shown (B). These patterns resemble those seen in patients with metastatic malignant melanoma that have been treated with IL-2C–E.
Figure 2.
Immunization with rVVmTRP-1 induces destruction of cutaneous melanocytes. Mice were vaccinated twice with rVVmTRP-1 (B and D) and developed a patchy and permanent loss of coat color pigmentation. Histologic comparison is with mice receiving control virus rVVLacZ twice (A and C). [Magnifications: ×10 (A and B) and ×100 (C and D).]
Depigmentation was permanent (>17 months) and generally progressed slowly after the initial rapid patch-wise depigmentation, resulting in coats varying from black with sharply defined white areas to virtually completely white (Fig. 1A). Additionally, priming of the mice was long-lived, because boosting 11 months after the first immunization resulted in coat color changes within 2–3 weeks, identical to mice receiving the boost just 3 weeks after the initial vaccination (data not shown).
Deliberate Induction of Autoreactivity to mTRP-1 Results in Tumor Destruction.
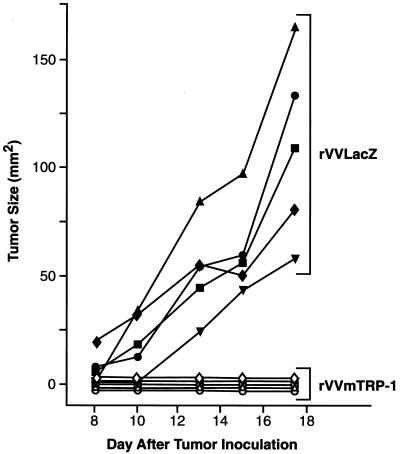
We hypothesized that the deliberate induction of self-reactivity could be useful for treatment of malignancies. To test this hypothesis, mice with vaccine-induced depigmentation were challenged s.c. with B16 melanoma. Mice vaccinated with rVVmTRP-1 were fully protected against tumor challenge, whereas mice vaccinated with control rVVLacZ were not (Fig. 3). In five independent experiments, between 60% and 100% of rVVmTRP-1-immunized mice were completely protected against B16 tumor challenge, with the remainder of mice displaying markedly suppressed tumor growth. In addition, mice were protected against i.v. B16 challenge, although this protection was partial (data not shown). Protection from tumor challenge and vitiligo both depended on a prime-boost regimen: mice vaccinated only once with rVVmTRP-1 never developed depigmentation (>17 months) and were not protected against B16 challenge (data not shown). Treatment of established s.c. tumors was unsuccessful, because tumors grew to sizes that warranted animal euthanasia before a second rVVmTRP-1 immunization could be given. Taken together, these results indicated that deliberate induction of immunity to a self-antigen such as TRP-1 can result in antitumor immunity.
Figure 3.
Immune responses to the normal melanocyte antigen mTRP-1 can prevent melanoma growth. Mice vaccinated with 2 × 107 plaque-forming units of either rVVLacZ or rVVmTRP-1 and boosted 3 weeks later were challenged with 1 × 105 B16 s.c. after an additional 3 weeks. Mice receiving rVVmTRP-1 developed vitiligo and completely rejected tumor challenge. The experiment was repeated three times with similar results.
Immunization with rVVmTRP-1 Induces Class-Switched, mTRP-1-Specific Antibodies.
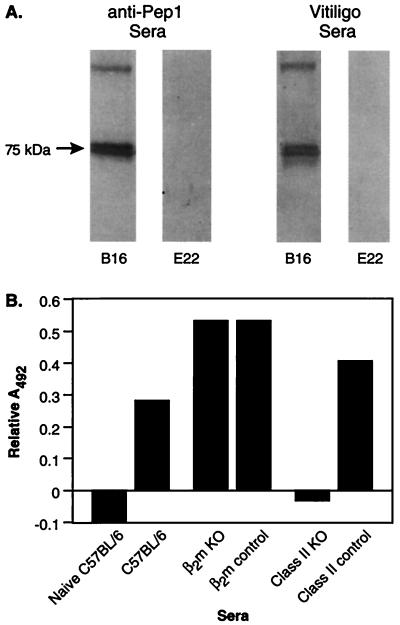
To study the mechanism of TRP-1-specific autoimmunity, sera from mice with depigmentation after immunization with rVVmTRP-1 was assayed for the presence of TRP-1-specific antibodies by Western blotting analysis. Sera from mice immunized with rVVmTRP-1 recognized a single 75-kDa protein present in B16 melanoma lysate, but not in E22 control tumor lysate (Fig. 4A). This reactivity was identical to the reactivity of TA99, a mAb specific for mouse and human TRP-1 (ref. 21, data not shown) as well as the polyclonal rabbit sera αPEP1, raised against a synthetic TRP-1 derived peptide (18) (Fig. 4A). Sera from control-vaccinated mice did not recognize any protein in the lysates (data not shown). To identify the precise mTRP-1 peptide epitope(s) recognized by antibodies in depigmented mice, a library of 25 amino acid peptides overlapping by 10 residues was tested for recognition by sera from depigmented mice in a dot-blotting assay. Two overlapping peptides, peptide 5 (mTRP-161–85, GRGRCVAVIADSRPHSRHYPHDGKD) and peptide 6 (mTRP-176–101, SRHYPHDGKDDREAWPLRFFNRTCQ) were specifically recognized, indicating there was recognition of a linear peptide epitope contained in the shared region of peptides 5 and 6 (data not shown). However, these peptides failed to adsorb out all of the mTRP-1 reactivity from sera of depigmented mice. Moreover, mice immunized with the 15-mer consensus epitope SRHYPHDGKDDREAW covalently linked to keyhole limpet hemocyanin developed high-titered antibodies that were specifically reactive with the full-length mTRP-1 in lysates from B16 melanoma, but these mice never developed vitiligo and were not protected against B16 tumor challenge (data not shown), suggesting the involvement of additional antibody epitopes or immune effector mechanisms, such as T lymphocytes.
Figure 4.
Sera from mice immunized with rVVmTRP-1 recognizes mTRP-1 in melanoma cells. (A) Lysates from B16 melanoma (lanes 1 and 3) and E22 thymoma cells (lanes 2 and 4) were subjected to SDS/PAGE, and stained with the mTRP-1 peptide-specific rabbit sera, αPEP1 (lanes 1 and 2), or sera from depigmented mice vaccinated twice with rVVmTRP-1 (lanes 3 and 4). Both sera specifically detected a 75-kDa TRP-1 band in lysate from B16 tumor, but not in lysate from TRP-1 negative E22 control tumor. (B) Induction of mTRP-1-specific antibodies depends on CD4+ T lymphocytes. MHC class II knockout mice, β2m knockout mice, and nontransgenic litter mates were immunized with 2 × 107 plaque-forming units of either rVVLacZ or rVVmTRP-1 and boosted 3 weeks later. Sera from each group of mice were tested by ELISA. Nonspecific absorption of sera from mice immunized twice with rVVLacZ was subtracted. The experiment was repeated twice with similar results.
To identify whether CD4+ T lymphocytes played any role in the immune response to rVVmTRP-1, ELISA was performed on sera from immunized mice. Normal mice developed high-titered IgG2b-type antibodies that specifically reacted with mTRP-1 in B16 lysates, but mice deficient in CD4+ T-cells did not develop detectable antibodies (Fig. 4B). Similar results were obtained when sera were assayed for IgG2a-type antibodies (data not shown).
This data suggested that CD4+ T lymphocytes and antibodies may play a role in rVVmTRP-1 induced melanocyte-directed autoimmunity. Attempts to identify mTRP-1-specific cytotoxic T lymphocytes were unsuccessful. Splenocytes from vaccinated and depigmented mice that were stimulated repeatedly with B16 melanoma transfected to express the costimulatory molecule CD80/B7–1, and in the presence of recombinant IL-2, failed to display TRP-1-specific reactivity (data not shown). However, it cannot be excluded that mTRP-1-specific CD8+ T cells are present in rVVmTRP-1-vaccinated mice.
Induction of Autoimmunity and Tumor Protection after Vaccination with rVVmTRP-1 Depends on CD4+ T Lymphocytes.
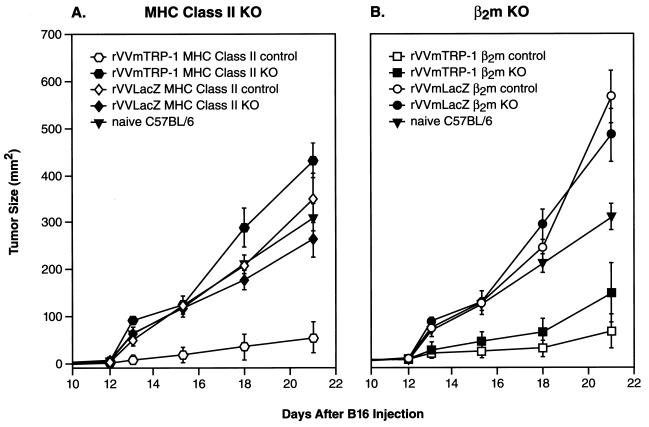
To determine the components of the immune response against mTRP-1, we studied the involvement of T lymphocyte subsets in the development of autoimmune vitiligo and tumor protection after vaccination with rVVmTRP-1. MHC class II knockout mice, severely deficient in CD4+ T lymphocytes (22), as well as normal litter mates were vaccinated with rVVmTRP-1. Two vaccinations with rVVmTRP-1 induced vitiligo in 55% of normal litter mates (11/20 mice). In contrast, MHC class II knockout mice did not develop any sign of vitiligo (0/20 mice) and were not protected from B16 tumor challenge (Fig. 5A). Similarly, mice depleted of CD4+ T lymphocytes by injection of anti-CD4 mAb GK1.5 and vaccinated with rVVmTRP-1 did not develop vitiligo (0/26 mice) and were not protected from tumor challenge, whereas 60% (6/10 mice) of control mice demonstrated depigmentation and rejected B16 challenge.
Figure 5.
Autoimmune and antitumor effects of immunization with rVVmTRP-1 are mediated by CD4+ T lymphocytes. β2m knockout mice, MHC class II knockout mice, or respective nontransgenic litter mates were immunized with 2 × 107 plaque-forming units of either rVVLacZ or rVVmTRP-1, boosted 3 weeks later and challenged with 1 × 105 B16 s.c. after an additional 3 weeks. Both β2m knockout mice and nontransgenic litter mates receiving rVVmTRP-1 developed vitiligo and were protected against B16 tumor challenge (B). In the same experiment, MHC class II knockout mice never developed vitiligo after immunization with rVVmTRP-1, nor were they protected against B16 tumor challenge (A). Nontransgenic litter mates receiving rVVmTRP-1 developed vitiligo and were protected against B16 tumor challenge, indicating a critical role for CD4+ T lymphocytes in the induction of vitiligo and tumor protection. The experiment was repeated twice with similar results.
In contrast, β2m knockout mice, severely deficient in CD8+ T lymphocytes (23), that were vaccinated with rVVmTRP-1 still developed vitiligo (5/20), although this rate was significantly lower than observed in nontransgenic litter mates (17/20). Challenge of vaccinated β2m knockout mice with B16 melanoma revealed robust protection against melanoma growth, indicating a surprisingly minor role, if any, for CD8+ T lymphocytes in the induction of antitumor immune reactivity after vaccination with rVVmTRP-1 (Fig. 5B). The lower rate of vitiligo may be caused by a well-characterized deficiency of β2m knockout mice in the FcRn-receptor, of which β2m forms an integral part (24, 25). This receptor protects IgG-type antibodies from clearance from the body, and its absence in β2m knockout mice could explain the reduced effects of vaccination with rVVmTRP-1. To further clarify the role of CD8+ T lymphocytes, perforin knockout mice, deficient in CD8+ T lymphocyte and NK cell lytic function, were similarly vaccinated with rVVmTRP-1 and followed for depigmentation. In two independent experiments, 6/7 and 6/8 perforin knockout mice developed vitiligo, a rate comparable to nontransgenic litter mates and normal C57BL/6 mice, suggesting that perforin-dependent killing by CD8+ T-cells and Natural Killer cells was not essential for the induction of autoimmunity to mTRP-1. Together, these results strongly pointed to a pivotal role for CD4+ T lymphocytes in the induction of both the autoimmune and antitumor response against the self-antigen mTRP-1.
DISCUSSION
Our results indicate that the deliberate induction of autoimmune reactivities to a nonmutated tissue differentiation antigen can lead to tumor destruction. Vaccination with rVVmTRP-1 induced both autoimmunity and tumor protection in a manner that depended on both antibody and CD4+ T lymphocytes. In humans afflicted with autoimmune vitiligo, tolerance to melanocyte antigens apparently has been broken. One of a number of putative target antigens in this disease is tyrosinase, and elevated levels of class-switched, IgG-type melanoma-specific antibodies have been detected in sera from vitiligo patients (26). Several groups have postulated a role for antibodies in the pathogenesis of human vitiligo (26, 27).
Houghton and colleagues (21) observed that systemic administration of the TRP-1-specific mAb TA99 to mice resulted in antitumor effects as well as depigmentation (21). Furthermore, immunization with lysates of baculovirus-infected insect cells expressing mTRP-1, or plasmid DNA xeno-immunization with human TRP-1, resulted in the induction of antibody responses in vivo, suggesting a requirement for altered forms of the protein, because purified mTRP-1 protein did not elicit antibodies (15, 28).
Four lines of evidence pointed to the involvement of CD4+ T lymphocytes in the breaking of tolerance to mTRP-1 in our studies: (i) ELISA results revealed the presence of mTRP-1-specific antibodies of the IgG2b isotype in sera of rVVmTRP-1 immunized mice with vitiligo, suggesting antibody isotype switching. (ii) The inability to isolate TRP-1-specific CD8+ T lymphocytes by using methods that can generate CD8+ T lymphocytes to other melanocyte antigens (4, 9). (iii) Absence of vitiligo and protection against tumor challenge in MHC class II knockout mice upon vaccination with rVVmTRP-1. (iv) Absence of vitiligo and protection against tumor challenge in mice that were antibody-depleted of CD4+ T lymphocytes upon vaccination with rVVmTRP-1. Apparently, tolerance to mTRP-1 is not absolute, but can be broken in a process that requires the activity of CD4+ T lymphocytes.
CD4+ T lymphocytes can drastically influence the initiation, maintenance, and termination of immune responses through secretion of cytokines and expression of surface molecules such as CD40 ligand. Collectively, these signals can increase antigen-presenting cell proliferation, chemotaxis, and antigen processing, as well as local MHC class I and II expression, expression of costimulatory molecules such as CD80/B7–1, and secretion of IL-12 (29, 30). In addition, CD4+ T lymphocytes can secrete specific patterns of cytokines that “steer” the immune response to a predominantly cellular, type-1 response (including interferon γ) or mainly humoral, type-2 response (including IL-4, IL-5, and perhaps IL-10) (31, 32). Once the immune response subsides, CD4+ T lymphocytes can play a determining role in the induction and maintenance of immunological memory (33–35).
The ability of CD4+ T lymphocytes to steer and amplify immune responses may explain their requirement in immunity against the self tumor antigen mTRP-1, to which some degree of immunological tolerance is likely to exist. Different groups recently have shown that immune responses to less immunogenic proteins tend to display a more pronounced dependency on “help” from CD4+ T lymphocytes (11, 36–39). In addition, classic mouse models of autoimmunity, such as experimental allergic encephalitis and systemic lupus erythematosus, typically show a strong dependence on CD4+ T lymphocytes, which can be transferred to induce disease in healthy animals (40–42). CD4+ T cells also can play a regulatory role by suppressing disease in afflicted recipients (42–45). Specific tolerization of autoreactive CD4+ T lymphocytes can completely abrogate autoimmune disease (10, 46, 47), further highlighting the prominent role for CD4+ T lymphocytes in the immunity to self-antigens.
With the cloning of a host of tumor antigens that are self, the role of CD4+ T lymphocytes in the antitumor immune response induced by antigen-specific cancer vaccines needs to be more clearly defined. Mice vaccinated with the self-antigen mTRP-1 develop autoimmune vitiligo and reject B16 melanoma expressing mTRP-1 with both phenomena revealing an absolute dependency on CD4+ T lymphocytes. However, the specificity of these CD4+ T lymphocytes remains unclear. We have been unsuccessful in isolating mTRP-1-specific CD4+ T lymphocytes by using cytokine-release and proliferation assays. Furthermore, plasmid DNA encoding mTRP-1 was not successful at inducing vitiligo. It therefore is possible that the CD4+ T lymphocytes are specific for vaccinia virus, especially if there is some physical association of TRP-1 with the vaccinia-derived helper epitope. Alternatively, the help could come from vaccinia-specific bystander CD4+ T cells that provide help locally. In either case, the helper functions could cause B lymphocytes to produce high-titered, class-switched mTRP-1-specific antibodies that are then solely responsible for tumor protection. Alternatively, mTRP-1-specific CD4+ T lymphocytes may recognize mTRP-1 released by the tumor and processed and presented by resident antigen-presenting cells.
When developing cancer vaccines using self-antigens, autoimmune destruction of normal cells expressing these antigens could be useful for cancers arising from nonessential organs, such as prostate, breast, thyroid, testis, or ovary. The autoimmune consequences of treatment targeting mTRP-1 caused vitiligo, although the mice had a normal life span. The first cohort of mice with vitiligo have been observed for 17 months, and they appear healthy, with normal size and weight. Histologically, there was no destruction of pigmented cells in the eye or ear. Likewise, melanoma patients who developed vitiligo did not experience ocular or aural changes (12, 48). Thus, the autoimmune consequences of melanoma-specific immune responses that cross-react with normal melanocytes may be acceptable when observed in conjunction with tumor regression.
Acknowledgments
We thank Dr. A. N. Houghton and Dr. V. Hearing for generous supply of recombinant baculovirus encoding mTRP-1 and mTRP-1-specific antisera, respectively, Dr. J. W. Yewdell for reading the manuscript, Mr. P. J. Spiess for expert technical assistance, and Ms. M. Blalock for expert assistance with graphics.
ABBREVIATIONS
- β2m
β2 microglobulin
- IL
interleukin
- MHC
major histocompatibility complex
- TRP
tyrosinase-related protein
- rVV
recombinant vaccinia virus
References
- 1.Prehn R T, Main J M. J Natl Cancer Inst. 1957;18:769–774. [PubMed] [Google Scholar]
- 2.Barth R J J, Bock S N, Mule J J, Rosenberg S A. J Immunol. 1990;144:1531–1537. [PubMed] [Google Scholar]
- 3.Melief C J, Kast W M. Immunol Rev. 1995;145:167–177. doi: 10.1111/j.1600-065x.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 4.Overwijk W W, Tsung A, Irvine K R, Parkhurst M R, Goletz T J, Tsung K, Carroll M W, Liu C, Moss B, Rosenberg S A, Restifo N P. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon T, Coulie P G, van den Eynde B. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 6.Pan Z K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F, Topalian S L, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg S A. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 9.Bloom M B, Perry-Lalley D, Robbins P F, Li Y, el-Gamil M, Rosenberg S A, Yang J C. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akkaraju S, Ho W Y, Leong D, Canaan K, Davis M M, Goodnow C C. Immunity. 1997;7:255–271. doi: 10.1016/s1074-7613(00)80528-2. [DOI] [PubMed] [Google Scholar]
- 11.Guerder S, Matzinger P. J Exp Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg S A, White D E. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 13.Earl P L, Cooper N, Moss B. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith L A, editors. New York: Wiley; 1991. pp. 16.16.1–16.16.17. [Google Scholar]
- 14.Chakrabarti S, Sisler J R, Moss B. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 15.Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S, Houghton A N. Proc Natl Acad Sci USA. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai Y, Yang J C, Spiess P, Nishimura M I, Overwijk W W, Roberts B, Restifo N P, Rosenberg S A. J Immunother. 1997;20:15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson I J, Chambers D M, Tsukamoto K, Copeland N G, Gilbert D J, Jenkins N A, Hearing V. EMBO J. 1992;11:527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winder A, Kobayashi T, Tsukamoto K, Urabe K, Aroca P, Kameyama K, Hearing V J. Cell Mol Biol Res. 1994;40:613–626. [PubMed] [Google Scholar]
- 19.Irvine K R, Rao J B, Rosenberg S A, Restifo N P. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 20.Bronte V, Carroll M W, Goletz T J, Wang M, Overwijk W W, Marincola F, Rosenberg S A, Moss B, Restifo N P. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara I, Takechi Y, Houghton A N. J Exp Med. 1995;182:1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. Nature (London) 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 24.Christianson G J, Brooks W, Vekasi S, Manolfi E A, Niles J, Roopenian S L, Roths J B, Rothlein R, Roopenian D C. J Immunol. 1997;159:4781–4792. [PubMed] [Google Scholar]
- 25.Junghans R P, Anderson C L. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Y H, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N. Lancet. 1994;344:1049–1052. doi: 10.1016/s0140-6736(94)91709-4. [DOI] [PubMed] [Google Scholar]
- 27.Naughton G K, Eisinger M, Bystryn J C. J Exp Med. 1983;158:246–251. doi: 10.1084/jem.158.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber L W, Bowne W B, Wolchok J D, Srinivasan R, Qin J, Moroi Y, Clynes R, Song P, Lewis J J, Houghton A N. J Clin Invest. 1998;102:1258–1264. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 30.Ridge J P, Di Rosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 31.Romagnani S. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 33.Di R F, Matzinger P. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 35.von Herrath M G, Yokoyama M, Dockter J, Oldstone M B, Whitton J L. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuji K, Obata Y, Takahashi T, Arata J, Nakayama E. J Immunol. 1997;159:159–166. [PubMed] [Google Scholar]
- 37.Wada H, Ono T, Uenaka A, Monden M, Nakayama E. Immunology. 1995;84:633–637. [PMC free article] [PubMed] [Google Scholar]
- 38.Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 39.Ossendorp F, Mengede E, Camps M, Filius R, Melief C J. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar V, Stellrecht K, Sercarz E. J Exp Med. 1996;184:1609–1617. doi: 10.1084/jem.184.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Silva H D, Van Driel I R, La Gruta N, Toh B H, Gleeson P A. Immunology. 1998;93:405–408. doi: 10.1046/j.1365-2567.1998.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitham R H, Bourdette D N, Hashim G A, Herndon R M, Ilg R C, Vandenbark A A, Offner H. J Immunol. 1991;146:101–107. [PubMed] [Google Scholar]
- 43.Olivares-Villagomez D, Wang Y, Lafaille J J. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van de Keere F, Tonegawa S. J Exp Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornton A M, Shevach E M. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohashi P S. Curr Opin Immunol. 1996;8:808–814. doi: 10.1016/s0952-7915(96)80009-4. [DOI] [PubMed] [Google Scholar]
- 47.Nicolle M W, Nag B, Sharma S D, Willcox N, Vincent A, Ferguson D J, Newsom-Davis J. J Clin Invest. 1994;93:1361–1369. doi: 10.1172/JCI117112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim, C. J., Chan, C. C. & Rosenberg, S. A. (1999) J. Immunother. Emphasis Tumor Immunol., in press.