Abstract
Mouse cyclophilin C-associated protein (CyCAP) is a member of the scavenger-receptor cysteine-rich domain superfamily and is 69% identical to the human Mac-2 binding protein. Here, we show that CyCAP is a widely expressed secreted glycoprotein that modulates the host response to endotoxin. Gene-targeted CyCAP-deficient mice are more sensitive to the lethal effects of endotoxin. In response to endotoxin, CyCAP-deficient mice overproduced interleukin 12 and interferon-γ systemically and tumor necrosis factor α locally; these are proinflammatory molecules that also promote T helper 1 responses. Furthermore, macrophages stimulated in vitro with endotoxin in serum deficient in CyCAP secreted more tumor necrosis factor α, supporting the proposal that CyCAP specifically down-modulates endotoxin signaling.
Murine cyclophilin C-associated protein (CyCAP) was identified on the basis of its ability to bind the peptidylprolyl isomerase cyclophilin C (cypC). This interaction was shown in cellular extracts and was inhibited by the immunosuppressive drug cyclosporin A (CsA; ref. 1). CyCAP was identified independently as a cell-surface-associated antigen on mouse macrophages and was named MAMA (2).
CyCAP is a member of the scavenger-receptor cysteine-rich (SRCR) domain superfamily (2, 3), a family defined by a cysteine-rich domain first identified in the class A type I scavenger receptor (SR-AI; ref. 4). This evolutionarily conserved domain is found in diverse transmembrane and secreted glycoproteins, including CD5, CD6, M130, complement factor 1, WC1 antigen, and the speract receptor (5). Many of these proteins are expressed by immune cells (e.g., B cells, T cells, and macrophages) that are implicated in host defense and immune regulation. It is likely that SRCR domains are involved in mediating specific protein–protein interactions (5), although this has been established clearly for only one family member, CD6. The SRCR domain of CD6 mediates binding to the activated leukocyte cell adhesion molecule (6).
Among SRCR domain family members, CyCAP is most closely related to the human Mac-2 binding protein (hMac-2-BP; also known as L3 antigen; ref. 7), the melanoma-associated antigen (7), and the 90K tumor-associated antigen (8). Based on sequence similarity, it has been proposed that CyCAP is the mouse homologue of hMac-2-BP (2, 5). Although the sequence similarity is striking (69% identity at the protein level), it was not known whether these two proteins are functionally homologous or whether more closely related proteins exist. Indeed, CyCAP was identified first in cell extracts (1), and MAMA was proposed to be a membrane protein (2), whereas hMac-2-BP is a secreted glycoprotein (7–11).
hMac-2-BP is expressed in many tissues, is found in serum, breast milk, saliva, and urine (8, 12), and may function as a homo-multimer of several million daltons (12). Levels of hMac-2-BP are elevated in the serum of some patients with breast and ovarian cancer (13) and in patients infected with HIV as they progress to AIDS (14), suggesting that hMac-2-BP may be involved in the host response to tumors and infections (8). Ectopic expression of hMac-2-BP in two tumor cell lines suppresses their growth in nude mice (15). In vitro, purified hMac-2-BP stimulates natural-killer and lymphokine-activated-killer activity of human peripheral blood lymphocytes (8) and induces production of interleukin (IL)-1 and IL-6 by human blood monocytes (16). CyCAP expression in macrophages can be up-regulated by adherence and by the inflammatory cytokines tumor necrosis factor α (TNF-α) and interferon-γ (IFN-γ; ref. 2). These studies are consistent with a role for CyCAP/hMac-2-BP in immune regulation and inflammation. Additionally, hMac-2-BP binds to immobilized endotoxin receptor CD14 in a serum-dependent and endotoxin/lipopolysaccharide (LPS)-dependent fashion (17), and the hMac-2-BP ligand, Mac-2/galectin-3, can bind LPS in vitro (18). These data are consistent with a role for CyCAP/hMac-2-BP in the inflammatory response, likely through interactions with CD14 and LPS. To investigate this possibility, we have characterized the biochemical nature and expression of CyCAP and investigated the role of CyCAP in the host response to endotoxin by using CyCAP −/− mice.
MATERIALS AND METHODS
Mice.
Mice were maintained in the animal facility of the Stanford Medical School Veterinary Service Center. (C57BL × CBA)F1 mice were obtained from The Jackson Laboratory.
Cell Culture.
MM55 mouse kidney cells (ATCC CRL 6436) and the HT-29 human colon carcinoma cell line (ATCC HTB 38) were grown in DMEM with 10% fetal calf serum, 2 mM glutamine, and penicillin/streptomycin. Peritoneal exudate cells (PECs) were collected from peritoneal lavages of unstimulated mice or mice 3 days after i.p. injection with 4% thioglycolate broth. Bone-marrow-derived macrophages were obtained by differentiation of nonadherent bone-marrow cells in DMEM with 10% L cell conditioned medium (as a source of macrophage colony-stimulating factor).
Metabolic Labeling and Immunoprecipitation.
For pulse–chase labeling, MM55 cells were starved for cysteine and methionine for 20 min, pulsed for 10 min with 0.5 mCi of l-[35S]methionine and l-[35S]cysteine (1,000–1,300 Ci/mmol; Amersham Pharmacia) in cysteine- and methionine-free DMEM with 5% dialyzed fetal calf serum, and chased in medium with a 5-fold excess of cold methionine and cysteine. For steady-state labeling, cells were incubated with 200–300 μCi/ml l-[35S]methionine and l-[35S]cysteine for 7–16 h. Supernatants were collected, and cells were lysed with 50 mM Tris, pH 7.5/150 mM NaCl/0.5% Triton X-100/1 mM phenylmethylsulfonyl fluoride. Cell lysates and media were precleared with anti-Rat IgG protein A Sepharose (Sigma). CyCAP was immunoprecipitated with a rat polyclonal serum against CyCAP purified from MM55 mouse kidney cell supernatants. Macrophage antigens Mac-1 and Mac-2 were immunoprecipitated with monoclonal antibodies M1/70 (19) and M3/38 (20), respectively. Immune complexes were collected on anti-Rat IgG protein A Sepharose.
cypC-GST-Glutathione Agarose Precipitation of CyCAP.
CyCAP was affinity-isolated with cypC-glutathione S-transferase (GST) fusion protein and glutathione agarose as previously described (1). CyCAP can bind to cypC only in the absence of CsA (1).
Northern and Southern Hybridization.
Hybridizations were performed as described (21). Total RNA from mouse kidney and liver was prepared by the LiCl/urea method (22) and resolved on 1% formaldehyde/agarose gels and transferred to Genescreen (NEN). Filters were hybridized with [α-32P]dCTP-labeled CyCAP and cypA cDNA probes prepared by random priming (Ready To Go, Amersham Pharmacia). For low-stringency Southern hybridization, filters were hybridized with 32P-labeled probes in 5× standard saline citrate (0.15 M sodium chloride/0.015 M sodium citrate, pH 7), 50 mM sodium phosphate (pH 6.8), 5× Denhardt’s solution, 2.5% dextran sulfate, 100 μg/ml denatured salmon sperm DNA, and 25% formamide at 42°C and washed with 0.2× standard saline citrate and 0.1% SDS at 52°C.
Generating CyCAP −/− Mice.
The CyCAP genomic locus was isolated from a 129SV mouse library in the λ FIX II vector (Stratagene) by using a 32P-labeled CyCAP cDNA probe as described in the supplier’s manual. Introns were localized by PCR by using primers designed to span the coding sequence, and the junctions were verified by sequencing (23). A 7.4-kb HpaI fragment of the CyCAP genomic clone was replaced with the neomycin-resistance gene under the control of the phosphoglycerate kinase promoter (PGKneo bpA, a gift from P. Soriano, Fred Hutchinson Cancer Research Center, Seattle), leaving CyCAP exon 1, exon 2, and 30 nt of exon 3, which code for the first 27 amino acids of CyCAP. This fragment was cloned into the NotI site of pPGK-thymidine kinase (a gift from P. Soriano), creating the final targeting vector.
Embryonic stem cells (E14.1a cells, a gift from Richard Murray, DNAX) were cultured, electroporated with the targeting vector, and selected in G418 (GIBCO/BRL) and ganciclovir (Syntex, Palo Alto, CA) as described (24). Genomic DNA from doubly resistant clones was digested with BamHI and screened by Southern analysis (21) by using a 5′ flanking probe for the presence of a 15-kb hybridizing band, indicative of a correctly targeted allele (data not shown).
Targeted embryonic stem cell clones, E14-159 and E14-274, were injected into C57BL/Ka blastocysts and implanted into pseudopregnant (C57BL × CBA)F1 females. Chimeric male offspring were bred to C57BL/Ka females, and agouti offspring were analyzed for the presence of the targeted allele by Southern blot analysis. Heterozygote offspring were mated to obtain homozygote CyCAP −/− and +/+ mice, which were interbred to obtain the mice used in these studies.
LPS Survival Curves.
Mice (8-week-old females) were injected i.p. with 7.5 μg per gram of body weight (gbw) or 10 μg/gbw E. coli 0111:B4 LPS (Difco) in pyrogen-free saline. Mice were monitored for 7 days. P values were calculated with the Mantel–Cox log rank test.
Measuring Cytokine Levels After LPS Treatment.
Wild-type and CyCAP-deficient mice (E14-159 line; 8- to 9-week-old females) were injected i.p. with 10 μg/gbw LPS. Concentrations of IL-12 p70 and IFN-γ in serum and concentrations of TNF-α in peritoneal lavages were determined by ELISA (Genzyme). Statistical analyses on cytokine release was done by using a two-tailed t test.
TNF-α Release by PECs in Vitro.
PECs were obtained from 8- to 12-week-old unstimulated CyCAP −/− mice, pooled, and cultured in Iscove’s serum-free medium containing LPS and 5% serum from CyCAP −/− or +/+ mice for 6 h. TNF-α levels in the media were determined by ELISA. The mouse sera used for culturing were age- and sex-matched and negative for TNF-α, as determined by ELISA. In an independent experiment, peritoneal lavages of individual mice were kept separate and treated as above, except that the cell concentration varied with each animal from 1 × 106 cells to 5 × 106 cells per ml and that one dose of 0.1 μg/ml LPS was used for stimulation. Statistical analysis on TNF-α release from individual mice was performed by using a paired t test.
Statistical Analyses.
P values were calculated with the statview (Abacus Concepts, Berkeley, CA) and excel (Microsoft) programs.
RESULTS
CyCAP Is the Mouse Homologue of hMac-2-BP.
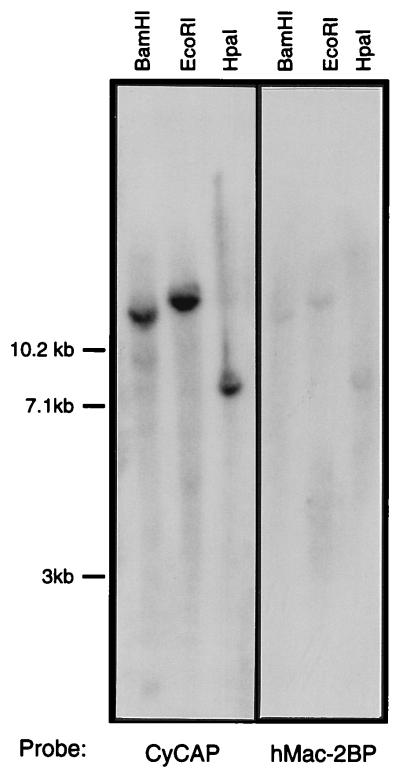
The issue of whether CyCAP is the mouse homologue of hMac-2-BP was addressed by Southern blot analysis of mouse genomic DNA with CyCAP and hMac-2-BP probes at low stringency. As shown in Fig. 1, the two probes hybridize to the same bands, and no new bands are detected, indicating that CyCAP is the most similar sequence to hMac-2-BP in the mouse genome.
Figure 1.
Low-stringency Southern blot analyses. Southern blots of mouse genomic DNA digested with the indicated enzymes were hybridized with 32P-labeled CyCAP and hMac-2-BP cDNA probes under low stringency as described in Materials and Methods.
Biosynthesis, Secretion, and Tissue Distribution of CyCAP.
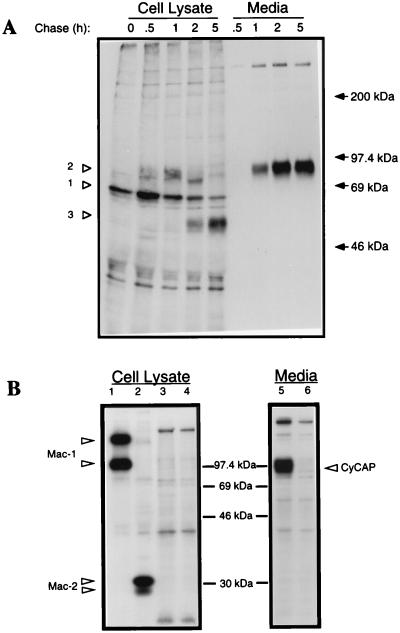
Earlier work suggested that CyCAP was a transmembrane protein, because it was isolated originally from cellular extracts (1) and independently identified as a macrophage cell-surface molecule named MAMA (2). However, we were unable to surface-label CyCAP by biotinylation or iodination or to detect it by flow cytometry with polyclonal rat serum to CyCAP (data not shown). To clarify the cellular localization of CyCAP, MM55 kidney cells were pulse-labeled with [35S]methionine and [35S]cysteine, and the biosynthesis of CyCAP was monitored. This pulse–chase analysis showed that CyCAP is a secreted protein appearing in the medium after 1 h (Fig. 2A). The intracellular maturation of CyCAP proceeds from an initial 64-kDa polypeptide predicted from the amino acid sequence (Fig. 2A, open arrowhead 1) to a posttranslationally modified form of 94 kDa which comigrates with the secreted form (Fig. 2A, open arrowhead 2). A cellular form of 50-kDa, which accumulated late in the chase, may be a degradation product (Fig. 2A, open arrowhead 3). Secreted CyCAP digested with N-glycanase resulted in a 64-kDa protein (data not shown), which most likely represents the completely deglycosylated form.
Figure 2.
Biosynthesis and secretion of CyCAP. (A) Pulse–chase analysis of CyCAP in MM55 kidney cells. Cells were pulsed for 10 min with [35S]cysteine and [35S]methionine and chased for the indicated times. CyCAP was immunoprecipitated from cell lysates and media with polyclonal rat serum against CyCAP. Control precipitations with normal rat serum were negative (data not shown). Proteins were resolved by using SDS/7.5% PAGE. Closed arrows represent positions of molecular-mass standards (Rainbow, Amersham Pharmacia). (B) CyCAP secretion from thioglycolate-elicited peritoneal macrophages. PECs were metabolically labeled overnight, and cypC-GST-glutathione agarose was used as an affinity reagent for CyCAP in the absence of CsA (lanes 3 and 5) or in the presence of CsA (lanes 4 and 6). Antibodies to macrophage markers Mac-1 and Mac-2 were used as positive controls (lanes 1 and 2).
Metabolically labeled thioglycolate-elicited peritoneal macrophages produced and secreted CyCAP efficiently (Fig. 2B, lane 5). Indeed, CyCAP was found to be secreted from diverse cell types including a bone-marrow stromal line (AC.6.2.1), a cytotoxic T cell line (AR-1), B cell hybridomas (FOX-NY and NS-1), and IL-2-activated killer cells (data not shown). As determined by Northern blot analysis, CyCAP mRNA was expressed in all tissues examined (brain, kidney, liver, spleen, testis, and thymus), but CyCAP was most abundant in the liver and highly expressed in kidney and spleen (data not shown). CyCAP was detected also in both mouse serum and urine by using an ELISA in which CyCAP was captured with cypC-GST fusion protein (1) and detected with a rat anti-CyCAP polyclonal serum (data not shown). Thus, we found that CyCAP is a widely expressed secreted protein rich in N-linked carbohydrates.
Production of CyCAP-Deficient Mice.
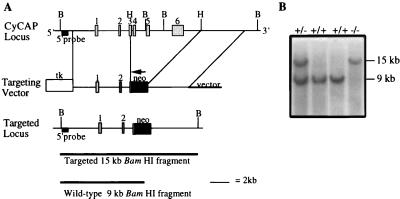
To probe the physiological function of CyCAP, we developed a CyCAP-deficient mouse through homologous recombination in embryonic stem cells. The CyCAP gene was isolated from a 129SV mouse λ library, and the exon organization was determined by PCR and DNA sequencing. CyCAP cDNA is contained in six exons spanning approximately 9.5 kb (Fig. 3A). Exons 3 (191 nt) and 4 (131 nt) comprise the SRCR domain; this split-exon organization is also found in the SRCR domain of SR-AI (25).
Figure 3.
Generation of CyCAP −/− mice. (A) The CyCAP genomic organization (Top), targeting vector (Middle), and targeted locus (Bottom). Exons are numbered 1–6, and restriction-enzyme sites are indicated. B, BamHI; H, HpaI. The expected size fragments for BamHI-digested DNA hybridized with the indicated 5′ probe for wild-type and targeted alleles are shown. (B) Southern blot analysis of representative tail DNA. Genomic tail DNA was digested with BamHI and hybridized with the 5′ probe indicated in A.
Embryonic stem cells were electroporated with the targeting vector (depicted in Fig. 3), and G418- and ganciclovir-resistant clones were screened by Southern blot analysis of BamHI-digested DNA with a 5′ flanking probe (data not shown). Correctly targeted clones were used to generate chimeras that were bred to C57BL/Ka mice. The offspring were bred to obtain homozygous CyCAP −/− and +/+ mice. Southern blot analysis of mouse tail DNAs yielded the expected 15-kb fragment for the targeted allele and a 9-kb fragment for the wild-type allele (Fig. 3B).
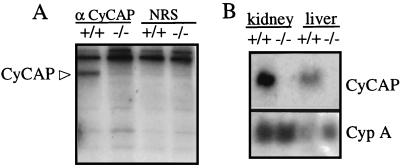
The disruption of the CyCAP gene resulted in the expected loss of CyCAP protein, as shown by indirect immunofluorescence of frozen sections of kidney and small intestine (data not shown) and by immunoprecipitation of CyCAP from metabolically labeled bone-marrow-derived macrophage cultures (Fig. 4A). Similarly, CyCAP mRNA was not detected by Northern analysis (Fig. 4B).
Figure 4.
CyCAP −/− mice do not express CyCAP protein or RNA. (A) CyCAP is secreted from macrophages from wild-type (+/+) but not CyCAP-deficient (−/−) mice. Medium from metabolically labeled bone-marrow-derived macrophages was incubated with rat polyclonal serum to CyCAP (α-CyCAP) or normal rat serum (NRS). (B) CyCAP RNA is expressed in CyCAP +/+ but not CyCAP −/− mice. Northern blot analysis of total RNA from kidney and liver of wild-type and CyCAP-deficient mice probed sequentially with full-length 32P-labeled CyCAP and cypA cDNA probes.
CyCAP −/− mice were fertile and apparently healthy. Complete blood counts with differentials performed on CyCAP −/− mice at 4 weeks and 12 weeks of age were in the normal range (data not shown).
CyCAP −/− Mice Show Increased Susceptibility to the Toxic Shock Effects of LPS.
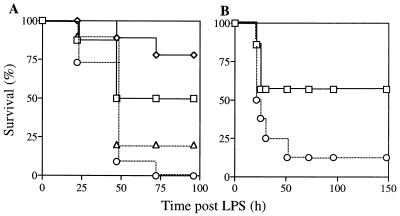
To determine whether CyCAP plays a role in the host response to endotoxin, CyCAP-deficient mice were challenged with E. coli LPS, and morbidity and mortality were monitored for 7 days. In six separate experiments, CyCAP −/− mice showed increased susceptibility to the lethal effects of endotoxin, including two experiments with mice backcrossed onto a 129SV background. Fig. 5 shows two representative experiments. In the first experiment, no CyCAP −/− mice survived the challenge of 10 μg/gbw LPS, whereas 50% of wild-type animals survived (Fig. 5A). Only 20% of CyCAP −/− mice survived a challenge of 7.5 μg/gbw, compared with 78% of wild-type mice. For the second experiment, CyCAP −/− mice derived from an independent embryonic stem cell clone were challenged, and only 12.5% of these mice survived, compared with 57% of wild-type mice (Fig. 5B). These endotoxin-challenge experiments establish that CyCAP-deficient mice are hypersensitive to the lethal effects of endotoxin.
Figure 5.
CyCAP-deficient mice are more sensitive to LPS. (A) CyCAP +/+ and CyCAP −/− (E14-159 line) mice were injected i.p. with LPS. (Squares) CyCAP +/+ mice (n = 8) injected with 10 μg/gbw LPS. (Diamonds) CyCAP +/+ mice (n = 9) injected with 7.5 μg/gbw LPS. (Circles) CyCAP −/− mice (n = 11) injected with 10 μg/gbw LPS. (Triangles) CyCAP −/− mice (n = 10) injected with 7.5 μg/gbw LPS. Survival was compared by using the Mantel–Cox log rank test. For the 10-μg/gbw dose, P = 0.0209; for the 7.5-μg/gbw dose, P = 0.0093. (B) LPS challenge of an independently derived CyCAP-deficient line (E14-274) with 10 μg/gbw LPS. (Squares) CyCAP +/+ mice (n = 7). (Circles) CyCAP −/− mice (n = 8).
CyCAP −/− Mice Produce Increased Levels of Proinflammatory Cytokines in Response to LPS.
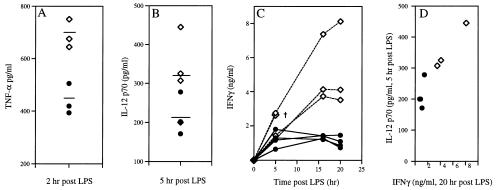
The exposure of mice to LPS triggers the release of proinflammatory cytokines, including TNF-α, TNF-β, IL-1α/β, IL-6, IFN-γ, and IL-12 (26, 27). Specific blockade of TNF-α, IL-1, IFN-γ, and IL-12 through antibodies or antagonists can diminish LPS-induced lethality (27–29). To test the possibility that the increased mortality of CyCAP-deficient mice after endotoxin challenge was caused by overproduction of one or more of these cytokines, we examined the levels of TNF-α, IL-12, and IFN-γ produced after i.p. LPS challenge. Serum TNF-α in normal and mutant mice reached similar peak levels by 2 h and returned to baseline levels by 6 h (data not shown). However, peritoneal lavages of CyCAP −/− mice showed clear and significant increases in TNF-α production in all three independent experiments. The differences in the peak levels of TNF-α in peritoneal lavages in one representative experiment are shown in Fig. 6A.
Figure 6.
In vivo cytokine levels in CyCAP −/− mice in response to LPS. Mice were challenged with 10 μg/gbw LPS, and cytokine levels were determined by ELISA. (A) Peak TNF-α levels in peritoneal lavages; three mice per group; P = 0.0056. (B) Peak IL-12 p70 serum levels 5 h after challenge. (C) Time course of IFN-γ production in serum. The dagger (†) indicates that the mouse died before the next time point. (D) Correlation between IL-12 p70 levels at 5 h and IFN-γ levels at 20 h; Y correlation coefficient, r = 0.942. (Diamonds) CyCAP-deficient mice. (Circles) Wild-type mice.
IL-12 has been shown to act early in the inflammatory response, in part through the induction of IFN-γ (30, 31). Both IL-12 p70 heterodimer and IFN-γ peak levels were elevated in serum from CyCAP-deficient mice in response to LPS (Fig. 6 B and C). IFN-γ levels were 5- to 10-fold higher in CyCAP-deficient mice in two independent challenges, and the IL-12 p70 level at 5 h correlated with the IFN-γ peak at 20 h (Fig. 6 C and D and data not shown). Because IL-12 p40 homodimer has been shown to act as an antagonist of the p70 heterodimer (29), we examined whether the elevated IL-12 p70 in CyCAP-deficient mice was caused by decreased levels of p40. We found similar levels of IL-12 p40 in CyCAP-deficient and wild-type mice (data not shown). These data show that the increased mortality of CyCAP-deficient mice in response to endotoxin challenge is associated with increased production of TNF-α, IL-12 p70, and IFN-γ.
PECs Secrete More TNF-α in Response to LPS when Cultured in Serum Without CyCAP.
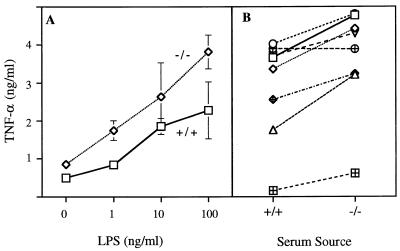
TNF-α in the peritoneal cavities of the CyCAP −/− mice can be produced by either mast cells or macrophages, both of which are resident in the peritoneum and both of which are known to secrete TNF-α (32–34). To test whether CyCAP plays a role in the cellular response to LPS, PECs were isolated from CyCAP −/− mice and stimulated with LPS in medium with 5% serum from either wild-type or CyCAP-deficient mice. As shown in Fig. 7A, consistently more TNF-α was released when LPS stimulation took place in CyCAP −/− serum, i.e., in the absence of CyCAP. When PECs from eight CyCAP −/− mice were tested individually, we observed an increase in TNF-α secretion in 7 of 8 mice in the presence of CyCAP−/− serum (Fig. 7B). Thus, in the absence of CyCAP, PECs overresponded to the LPS stimulus.
Figure 7.
TNF-α release from PECs stimulated with LPS in the presence of serum from wild-type or CyCAP-deficient mice. (A) PECs from CyCAP-deficient mice were pooled and stimulated in vitro for 6 h with indicated concentrations of LPS in the presence of serum from CyCAP −/− (diamonds) or wild-type (squares) mice. TNF-α values represent means of duplicate samples ± SD. (B) PECs from eight CyCAP-deficient mice (cell numbers from 1 × 106 to 5 × 106 cells per ml) were stimulated with LPS (0.1 μg/ml) in serum from CyCAP +/+ or −/− mice, and TNF-α levels were measured. Data were analyzed by using a paired t test; P = 0.002.
DISCUSSION
CyCAP was discovered as a cellular protein that can associate with cypC in a manner that is inhibited by the immunosuppressant drug CsA (1). The cyclophilin:CsA complex mediates immunosuppression by binding and inhibiting the phosphatase activity of calcineurin (1, 35–37). Here, we undertook studies to probe the natural function of CyCAP.
Our studies to define the function of CyCAP derive in part from the reported similarity between CyCAP and hMac-2-BP. Initially, we examined the degree of sequence relatedness between CyCAP and hMac-2-BP by low-stringency Southern blot analysis. We found that CyCAP and hMac-2-BP cDNA probes recognize the same bands in Southern blots of mouse genomic DNA, and no new bands were detected. Additionally, the mouse CyCAP gene reported here has an intron–exon structure and promoter sequence identical to the mouse 90K gene, which was isolated by virtue of hybridization to hMac-2-BP cDNA (ref. 38 and data not shown). These data establish CyCAP as the sequence most similar to hMac-2-BP in the mouse genome.
Although these proteins share sequence similarity, the different cellular localizations reported in the literature suggested different cellular functions. hMac-2-BP has been well characterized by many groups as a secreted glycoprotein (7–11), whereas MAMA (CyCAP) was characterized as a macrophage membrane protein (2). In this report, we show conclusively that CyCAP, like hMac-2-BP, is a secreted protein. However, it is possible that, under certain conditions, secreted CyCAP may become associated with membrane proteins.
Additional observations extend the sequence similarity of CyCAP and hMac-2-BP to suggest true homology. We found that CyCAP, like Mac-2-BP, is a highly glycosylated secreted protein expressed by diverse cell types and tissues and found in urine and serum (7–12). In addition, human Mac-2/galectin-3 bound a protein of the same apparent molecular mass as CyCAP when incubated with proteins secreted from mouse kidney cells, suggesting that this protein–protein interaction may be conserved across species (data not shown). It seems likely that these two proteins will prove to be true functional homologues.
Several groups have proposed that hMac-2-BP may be involved in host defense to pathogens (8, 12, 16, 38). Biochemical studies showing an interaction between hMac-2-BP and LPS and its receptor CD14 (17) suggested that the role of hMac-2-BP in defense may be through the host response to endotoxin. The central role of CD14 in endotoxic shock is illustrated by CD14 transgenic mice that are hypersensitive to endotoxin (39) and by CD14-deficient mice that are resistant to endotoxin (40). Given these data and the similarity between CyCAP and hMac-2-BP, we tested the hypothesis that CyCAP is involved in the host response to bacterial endotoxin. We disrupted the gene encoding CyCAP in embryonic stem cells and created CyCAP-deficient mice. We found that a CyCAP deficiency increased the relative risk of death from endotoxin 3.6-fold (7.5 μg/gbw LPS challenge), suggesting that CyCAP plays a protective role in the response to endotoxin.
Endotoxic shock is mediated in part through proinflammatory cytokines such as TNF-α, IL-12, and IFN-γ. In response to LPS, CyCAP-deficient mice produced more TNF-α locally and more IL-12 p70 and IFN-γ systemically than wild-type controls. The modest increase in the levels of TNF-α in peritoneal lavages was followed by a dramatic increase in serum IFN-γ, suggesting an amplification of the initial inflammatory signal. TNF-α may be acting locally to prime subsequent increases in IFN-γ. Consistent with this idea is the fact that the LPS-inducible expression of IFN-γ is abrogated in mice deficient in lymphotoxin α/TNF-α, indicating that signaling through the TNF receptor is important for subsequent expression of IFN-γ in vivo (41). We propose that the elevated cytokine levels in CyCAP-deficient mice are directly mediating the observed increase in mortality in response to LPS.
Interestingly, another SRCR domain superfamily member, SR-A, has been shown to play a role in the clearance of endotoxin in vivo, predicting a protective role for this protein in endotoxin-mediated shock (42). Indeed, bacillus-primed mice lacking both SR-AI and SR-AII are hypersensitive to LPS and produce elevated levels of TNF-α and IL-6 (43). Because the targeted disruption of the SR-A gene results in a failure to produce both isoforms of this receptor (including an SRCR-minus form), it is not yet known whether this function requires the SRCR domain. Given the similar endotoxin-sensitive phenotype of SR-A and CyCAP-deficient mice, it is tempting to speculate that the SRCR domains may be important for this function.
IL-12 p70 produced by macrophages is a known inducer of IFN-γ by T cells and natural-killer cells (30, 31), and anti-IL-12 antibodies can decrease IFN-γ levels in vivo during endotoxemia (26), suggesting that IL-12 is the proximal inducer of IFN-γ production. Indeed, we found that IL-12 p70 levels correlated with subsequent peak levels of IFN-γ.
IL-12 p70 is a key mediator in the generation of T helper 1 (Th1) cells (30, 31). The observation that loss of CyCAP increases IL-12 p70 production in vivo raises the possibility that CyCAP may play a role in promoting Th2 and/or inhibiting Th1 responses. Several studies of people infected with HIV-1 have suggested that a Th2 cytokine response correlates with more rapid progression of the disease (44), and, provocatively, serum levels of hMac-2-BP are increased during the progression to AIDS (14), suggesting that overproduction of CyCAP/hMac-2-BP may down-modulate Th1 immunity. This possibility is in apparent contrast to reports that hMac-2-BP has tumor suppressive (15) and natural killer-inducing properties (16). CyCAP-deficient mice allow direct testing of whether CyCAP is involved in regulating the nature of the Th response to different pathogenic agents.
To dissect the mechanism of CyCAP action, we tested whether cultured PECs could recapitulate the enhanced responsiveness of CyCAP-deficient mice to LPS seen in vivo. Indeed, PECs produced more TNF-α in the absence of CyCAP. This increased production occurred whether the PECs were derived from CyCAP +/+ or −/− mice, indicating that the defect in CyCAP −/− mice is not intrinsic to the macrophage (Fig. 7 and data not shown). These data support the proposal that serum CyCAP acts at the level of the macrophage to down-modulate LPS signaling. However, we cannot exclude the possibility that CyCAP deficiency acts indirectly through other serum components that may be involved in the LPS response.
CyCAP may down-modulate LPS signaling by altering the association of CD14 with its signaling partner, which may be the Toll-like receptor-2 (45). Consistent with this possibility is the finding that LPS signaling through the Toll-like receptor-2 is enhanced by CD14 about 1.5-fold (45), which is the level of down-modulation we found in LPS signaling in vitro in the presence of CyCAP.
Alternatively, CyCAP may alter LPS signaling by interacting with Mac-2/galectin-3. Mac-2 secretion from macrophages is stimulated by LPS (46) and Mac-2 interacts directly with LPS in vitro (18). Perhaps secreted Mac-2 and CyCAP are associated in vivo, and perhaps they, as a complex, bind LPS and decrease its potency. Consistent with this suggestion are the facts that hMac-2-BP is found as a high-molecular-mass complex (12) and that Mac-2 can form dimers (47).
We have shown that failure to express CyCAP results in enhanced inflammatory cytokine production, and others have shown that CyCAP expression is induced by the proinflammatory cytokines IFN-γ and TNF-α (2, 38). These results are consistent with a model in which CyCAP is expressed in response to inflammation as part of a negative-feedback loop to limit the inflammatory response. This regulation distinguishes CyCAP from the secretory leukocyte protease inhibitor, which is also a macrophage product antagonistic to LPS, whose expression, however, is regulated negatively by IFN-γ (48). It remains to be determined whether the down-modulatory effect of CyCAP is specific for endotoxin or whether the effect extends to other bacterial constituents that signal through CD14 (49).
If CyCAP limits the host response to LPS, then perhaps the overexpression of CyCAP would increase survival after endotoxin challenge. Recent clinical trials in humans have examined several therapeutic strategies that directly inhibit the inflammatory cascade to ameliorate sepsis. Unfortunately, results have been disappointing, prompting a rethinking of approaches to the treatment of sepsis (50). Proteins such as CyCAP that down-modulate but do not abrogate the host response to endotoxin may be worth pursuing for the treatment of Gram-negative sepsis.
Acknowledgments
We thank Leo Aguila, Jos Domen, Reina Mebius, Melinda Fagan, and Troy Randall for helpful discussions; Kirston Koths, Irina Conboy, and Patricia Jones for communicating unpublished work, providing reagents, and helpful discussions; Julie Christensen and Andreea Salinas for their tenacity in generating the chimeric mice; Libuse Jerabek for her advice and technical assistance; David Mack and Richard Murray for the cells from the E14 lines and for advice on their growth; and Jack Nunberg for helpful comments on the manuscript. This work was supported by a grant from Systemix/Sandoz Pharmaceutical (to M.T. and I.L.W.) and Public Health Service National Research Service Award 5T32 CA09302 (to M.T.).
ABBREVIATIONS
- CsA
cyclosporin A
- CyCAP
cyclophilin C-associated protein
- cypC
cyclophilin C
- gbw
gram body weight
- GST
glutathione S-transferase
- hMac-2-BP
human Mac-2 binding protein
- IFN-γ
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharide
- PEC
peritoneal exudate cell
- SR-AI
scavenger receptor class A type I
- SRCR
scavenger-receptor cysteine-rich
- TNF
tumor necrosis factor
- Th
T helper
References
- 1.Friedman J, Weissman I. Cell. 1991;66:799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- 2.Chicheportiche Y, Vassalli P. J Biol Chem. 1994;269:5512–5517. [PubMed] [Google Scholar]
- 3.Friedman J, Trahey M, Weissman I. Proc Natl Acad Sci USA. 1993;90:6815–6819. doi: 10.1073/pnas.90.14.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Nature (London) 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 5.Resnick D, Pearson A, Krieger M. Trends Biochem Sci. 1994;19:5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 6.Bowen M A, Bajorath J, Siadak A W, Modrell B, Malacko A R, Marquardt H, Nadler S G, Aruffo A. J Biol Chem. 1996;271:17390–17396. doi: 10.1074/jbc.271.29.17390. [DOI] [PubMed] [Google Scholar]
- 7.Linsley P S, Horn D, Marquardt H, Brown J P, Hellstrom I, Hellstrom K-E, Ochs V, Tolentino E. Biochemistry. 1986;25:2978–2986. doi: 10.1021/bi00358a037. [DOI] [PubMed] [Google Scholar]
- 8.Ullrich A, Sures I, D’Egidio M, Jallal B, Powell T J, Herbst R, Dreps A, Azam M, Rubinstein M, Natoli C, et al. J Biol Chem. 1994;269:18401–18407. [PubMed] [Google Scholar]
- 9.Iacobelli S, Arno E, D’Orazio A, Coletti G. Cancer Res. 1986;46:3005–3010. [PubMed] [Google Scholar]
- 10.Iacobelli S, Bucci I, D’Egidio M, Giuliani C, Natoli C, Tinari N, Rubinstein M, Schlessinger J. FEBS Lett. 1993;319:59–65. doi: 10.1016/0014-5793(93)80037-u. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg I, Cherayil B J, Isselbacher K J, Pillai S. J Biol Chem. 1991;266:18731–18736. [PubMed] [Google Scholar]
- 12.Koths K, Taylor E, Halenbeck R, Casipit C, Wang A. J Biol Chem. 1993;268:14245–14249. [PubMed] [Google Scholar]
- 13.Iacobelli S, Arno E, Sismondi P, Natoli C, Gentiloni N, Scambia G, Giai M, Cortese P, Benedetti Pancini P, Mancuso S. Breast Cancer Res Treat. 1988;11:19–30. doi: 10.1007/BF01807554. [DOI] [PubMed] [Google Scholar]
- 14.Natoli C, Dianzani F, Mazzotta F, Balocchini E, Pierotti P, Antonelli G, Iacobelli S. J Acquired Immune Defic Syndr. 1993;6:370–375. [PubMed] [Google Scholar]
- 15.Jallal B, Powell J, Zachwieja J, Brakebusch C, Germain L, Jacobs J, Iacobelli S, Ullrich A. Cancer Res. 1995;55:3223–3227. [PubMed] [Google Scholar]
- 16.Powell T J, Schreck R, McCall M, Hui T, Rice A, App H, Azam M, Ullrich A, Shawver L. J Immunother. 1995;14:209–221. doi: 10.1097/00002371-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Wright S D. J Inflamm. 1995;45:115–125. [PubMed] [Google Scholar]
- 18.Mey A, Leffler H, Hmama Z, Normier G, Revillard J-P. J Immunol. 1996;156:1572–1577. [PubMed] [Google Scholar]
- 19.Springer T G, Galfre G, Secher D S, Milstein C. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 20.Ho M-K, Springer T A. J Immunol. 1982;128:1221–1228. [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 22.Auffray C, Rougeon F. Eur J Biochem. 1980;107:303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- 23.Mount S M. Nucleic Acids Res. 1982;10:459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Freeden-Jeffrey U, Vieira P, Lucian L A, McNei T, Burdach S E G, Murray R. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emi M, Asaoka H, Matsumoto A, Itakura H, Kurihara Y, Wada Y, Kanamori H, Yazaki Y, Takahashi E, Lepert M, et al. J Biol Chem. 1993;268:2120–2125. [PubMed] [Google Scholar]
- 26.Heinzel F P, Rerko R M, Ling P, Hakimi J, Schoenhaut D S. Infect Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beutler B. In: Tumor Necrosis Factor and other Cytokines in Septic Syndrome. Reinhart K, Eyrich K, Sprung C, editors. Berlin: Springer; 1994. pp. 107–121. [Google Scholar]
- 28.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 29.Mattner F, Ozmen L, Podlaski F J, Wilkinson V L, Presky D H, Gately M K, Alber G. Infect Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 31.Trinchieri G, Gerosa F. J Leukocyte Biol. 1996;59:505–510. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 32.Gifford G E, Flick D A. Ciba Found Symp. 1987;131:3–20. doi: 10.1002/9780470513521.ch2. [DOI] [PubMed] [Google Scholar]
- 33.Echtenacher B, Männel D N, Hültner L. Nature (London) 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 34.Malaviya R, Ikeda T, Ross E, Abraham S M. Nature (London) 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Farmer J D, Jr, Lane W S, Friedman J, Weissman I, Schreiber S L. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 36.Bierer B E. Proc Assoc Am Physicians. 1995;107:28–40. [PubMed] [Google Scholar]
- 37.Ho S, Clipstone N, Timmerman L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree G R. Clin Immunol Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 38.Brakebusch C, Jallal B, Fusco O, Iacobelli S, Ullrich A. J Biol Chem. 1997;272:3674–3682. doi: 10.1074/jbc.272.6.3674. [DOI] [PubMed] [Google Scholar]
- 39.Ferrero E, Jiao D, Tsuberi B Z, Tesio L, Rong G W, Haziot A, Goyert S M. Proc Natl Acad Sci USA. 1993;90:2380–2384. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 41.Amiot F, Fitting C, Tracey K J, Cavaillon J M, Dautry F. Mol Med. 1997;3:864–875. [PMC free article] [PubMed] [Google Scholar]
- 42.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R. Nature (London) 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 43.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clerici M, Shearer G M. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 45.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Nature (London) 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 46.Sato S, Hughes R C. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- 47.Woo H-J, Lotz M M, Jung J U, Mercurio A M. J Biol Chem. 1991;266:18419–18422. [PubMed] [Google Scholar]
- 48.Jin F-Y, Nathan C, Radzioch D, Ding A. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 49.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 50.Bone R C. J Am Med Assoc. 1996;276:565–566. [Google Scholar]