Abstract
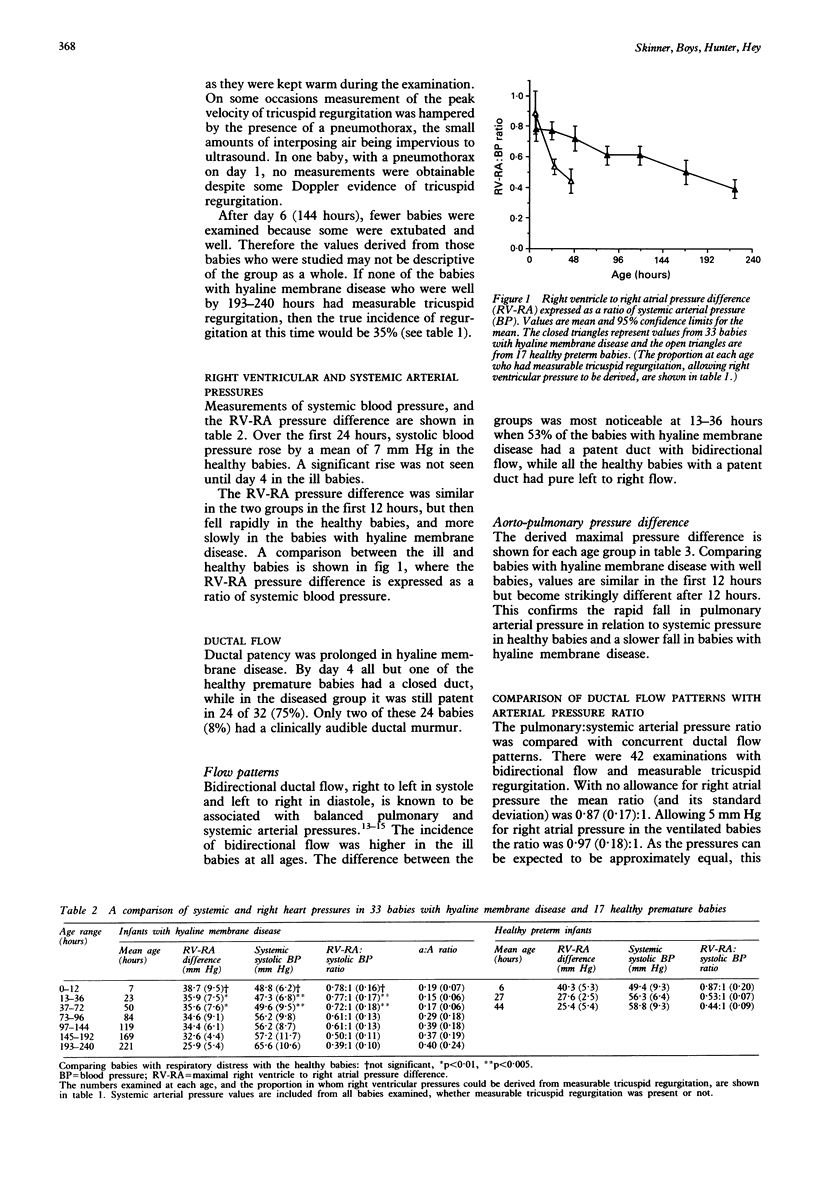
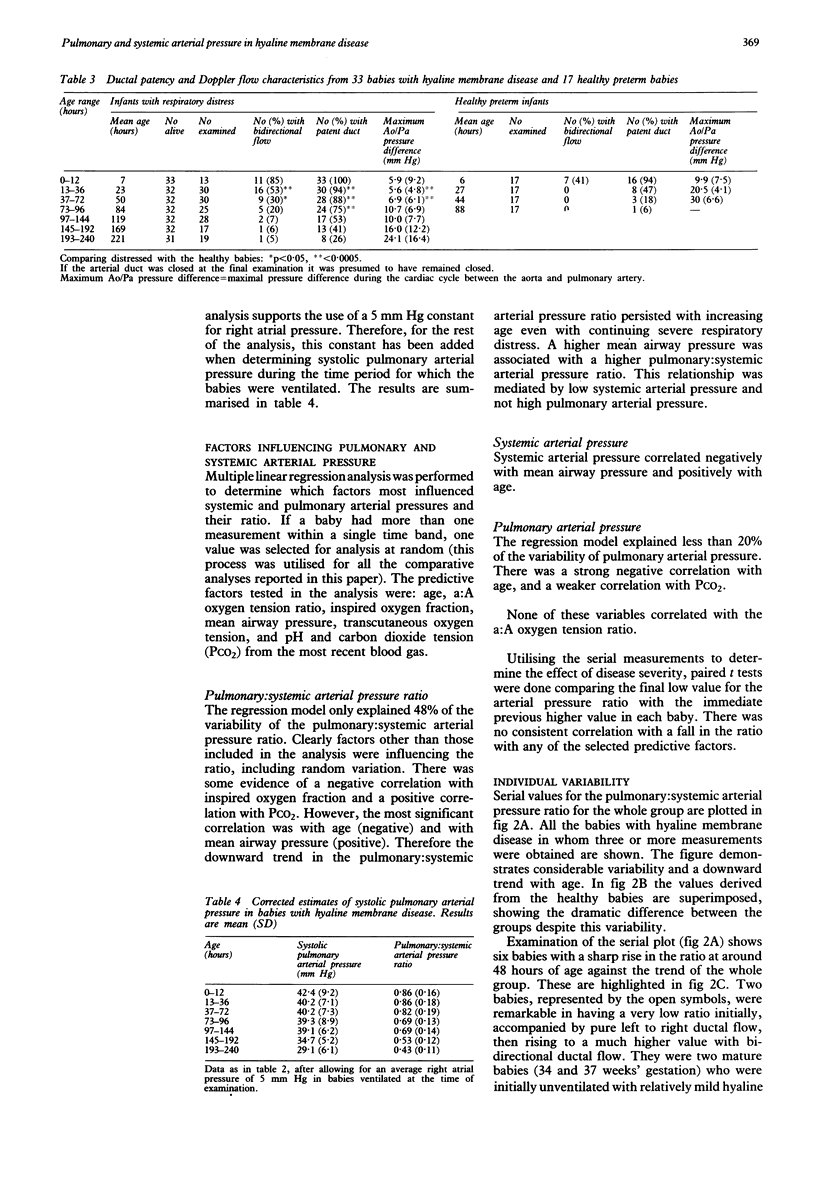
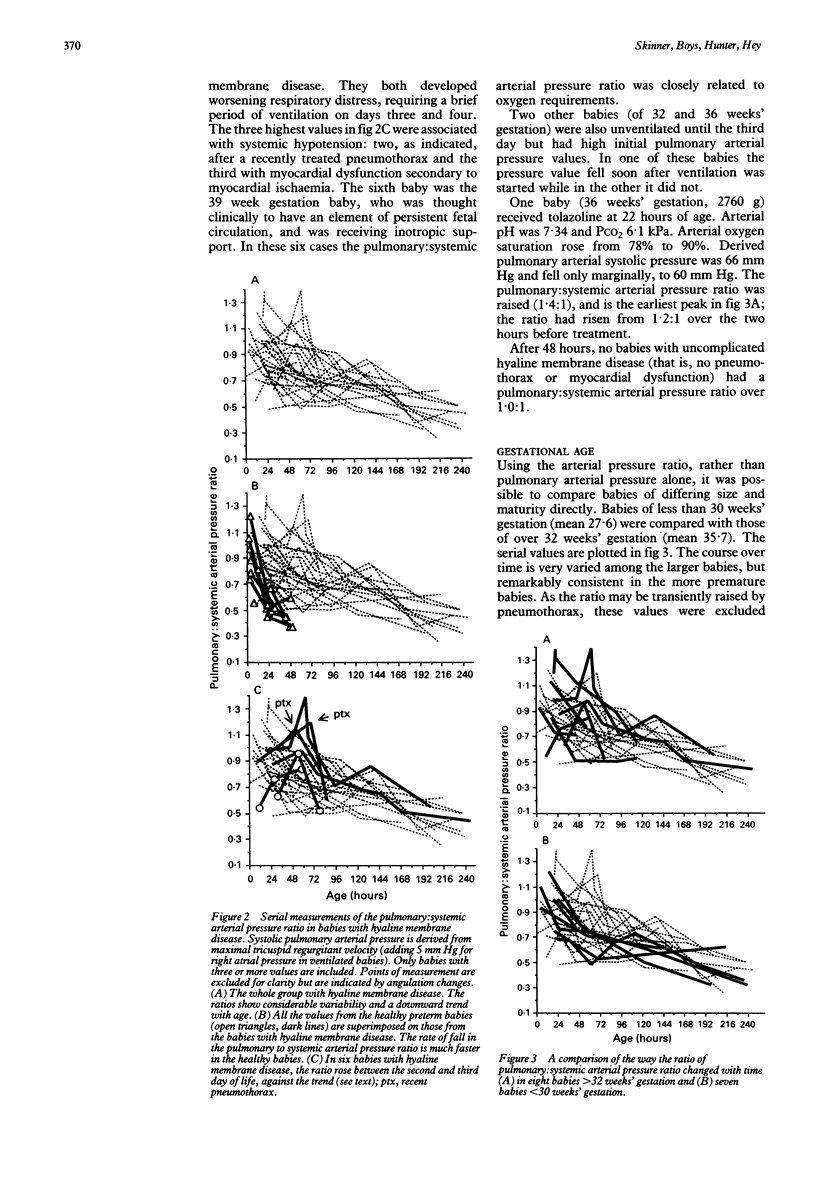
Systolic pulmonary arterial pressure was determined serially over the first 10 days of life in 33 babies with hyaline membrane disease by measuring the peak velocity of pansystolic tricuspid valve regurgitation, using Doppler ultrasound, and applying the Bernoulli equation. Results are presented in age groups 0-12, 13-36, 37-72, and 73-96 hours respectively. The incidence of tricuspid valve regurgitation was 92, 97, 80, and 64% (falling to 35% by day 10) compared with 53, 50, 31, and 0% in 17 healthy premature infants. In comparing healthy babies with those with hyaline membrane disease, no allowance was made for right atrial pressure. The derived 'right ventricle to right atrial (RV-RA) pressure difference', was expressed as a ratio of systemic arterial (systolic) pressure. Over the first three days, this ratio fell much faster in the healthy babies. Values were 0.78:1, 0.77:1, and 0.72:1 in babies with hyaline membrane disease and 0.87:1, 0.53:1, and 0.44:1 in healthy babies. Ductal patency was prolonged in babies with hyaline membrane disease (75% on day 4 compared with 6% in healthy babies). The incidence of bidirectional ductal flow, indicating balanced pulmonary and systemic arterial pressures, was 79, 53, 30, and 20%, and in healthy babies was 41% at 0-12 hours and zero thereafter. Pulmonary arterial pressure was then calculated by adding a right atrial pressure estimate of 5 mm Hg to the RV-RA difference when the babies were ventilated. Babies of lower gestation had lower values. The pulmonary: systemic arterial pressure ratio showed considerable temporal variability, but fell with age and was raised by high mean airway pressure and pneumothorax (through a reduction in systemic pressure), and less noticeably by carbon dioxide tension. It did not correlate significantly with other indices of disease severity. Hyaline membrane disease is associated with delayed postnatal circulatory adaptation characterized by pulmonary hypertension, systemic hypotension, and prolonged ductal patency.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Berger M., Haimowitz A., Van Tosh A., Berdoff R. L., Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985 Aug;6(2):359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- Chu J., Clements J. A., Cotton E. K., Klaus M. H., Sweet A. Y., Tooley W. H., Bradley B. L., Brandorff L. C. Neonatal pulmonary ischemia. I. Clinical and physiological studies. Pediatrics. 1967 Oct;40(4 Suppl):709–782. [PubMed] [Google Scholar]
- Currie P. J., Seward J. B., Chan K. L., Fyfe D. A., Hagler D. J., Mair D. D., Reeder G. S., Nishimura R. A., Tajik A. J. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985 Oct;6(4):750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- Evans N. J., Archer L. N. Doppler assessment of pulmonary artery pressure and extrapulmonary shunting in the acute phase of hyaline membrane disease. Arch Dis Child. 1991 Jan;66(1 Spec No):6–11. doi: 10.1136/adc.66.1_spec_no.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furzan J. A., Gabriele G., Wheeler J. M., Fixler D. E., Rosenfeld C. R. Regional blood flows in newborn lambs during endotracheal continuous airway pressure and continuous negative pressure breathing. Pediatr Res. 1981 May;15(5):874–878. doi: 10.1203/00006450-198105000-00010. [DOI] [PubMed] [Google Scholar]
- Gersony W. M. Neonatal pulmonary hypertension: pathophysiology, classification, and etiology. Clin Perinatol. 1984 Oct;11(3):517–524. [PubMed] [Google Scholar]
- Halliday H. L., Dumpit F. M., Brady J. P. Effects of inspired oxygen on echocardiographic assessment of pulmonary vascular resistance and myocardial contractility in bronchopulmonary dysplasia. Pediatrics. 1980 Mar;65(3):536–540. [PubMed] [Google Scholar]
- Halliday H., Hirschfeld S., Riggs T., Liebman J., Fanaroff A., Bormuth C. Respiratory distress syndrome: echocardiographic assessment of cardiovascular function and pulmonary vascular resistance. Pediatrics. 1977 Oct;60(4):444–449. [PubMed] [Google Scholar]
- Houston A. B., Lim M. K., Doig W. B., Gnanapragasam J., Coleman E. N., Jamieson M. P., Pollock J. C. Doppler flow characteristics in the assessment of pulmonary artery pressure in ductus arteriosus. Br Heart J. 1989 Oct;62(4):284–290. doi: 10.1136/hrt.62.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan C., Eyal F., Mandelberg A., Sapoznikov D., Lewis B. S. Effect of mechanical ventilation and volume loading on left ventricular performance in premature infants with respiratory distress syndrome. Crit Care Med. 1986 Oct;14(10):858–860. doi: 10.1097/00003246-198610000-00004. [DOI] [PubMed] [Google Scholar]
- McIntosh N., Walters R. O. Effect of tolazoline in severe hyaline membrane disease. Arch Dis Child. 1979 Feb;54(2):105–110. doi: 10.1136/adc.54.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musewe N. N., Poppe D., Smallhorn J. F., Hellman J., Whyte H., Smith B., Freedom R. M. Doppler echocardiographic measurement of pulmonary artery pressure from ductal Doppler velocities in the newborn. J Am Coll Cardiol. 1990 Feb;15(2):446–456. doi: 10.1016/s0735-1097(10)80076-2. [DOI] [PubMed] [Google Scholar]
- Musewe N. N., Smallhorn J. F., Benson L. N., Burrows P. E., Freedom R. M. Validation of Doppler-derived pulmonary arterial pressure in patients with ductus arteriosus under different hemodynamic states. Circulation. 1987 Nov;76(5):1081–1091. doi: 10.1161/01.cir.76.5.1081. [DOI] [PubMed] [Google Scholar]
- Peckham G. J., Fox W. W. Physiologic factors affecting pulmonary artery pressure in infants with persistent pulmonary hypertension. J Pediatr. 1978 Dec;93(6):1005–1010. doi: 10.1016/s0022-3476(78)81239-6. [DOI] [PubMed] [Google Scholar]
- Poets C. F., Stebbens V. A., Alexander J. R., Arrowsmith W. A., Salfield S. A., Southall D. P. Oxygen saturation and breathing patterns in infancy. 2: Preterm infants at discharge from special care. Arch Dis Child. 1991 May;66(5):574–578. doi: 10.1136/adc.66.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenscneider T. A., Nielsen H. C., Ruttenberg H. D., Jaffe R. B. Disturbances of the transitional circulation: spectrum of pulmonary hypertension and myocardial dysfunction. J Pediatr. 1976 Oct;89(4):622–625. doi: 10.1016/s0022-3476(76)80404-0. [DOI] [PubMed] [Google Scholar]
- Skinner J. R., Boys R. J., Hunter S., Hey E. N. Non-invasive assessment of pulmonary arterial pressure in healthy neonates. Arch Dis Child. 1991 Apr;66(4 Spec No):386–390. doi: 10.1136/adc.66.4_spec_no.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J. R., Milligan D. W., Hunter S., Hey E. N. Central venous pressure in the ventilated neonate. Arch Dis Child. 1992 Apr;67(4 Spec No):374–377. doi: 10.1136/adc.67.4_spec_no.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjaerpe T., Hatle L. Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur Heart J. 1986 Aug;7(8):704–710. doi: 10.1093/oxfordjournals.eurheartj.a062126. [DOI] [PubMed] [Google Scholar]
- Stahlman M., Blankenship W. J., Shepard F. M., Gray J., Young W. C., Malan A. F. Circulatory studies in clinical hyaline membrane disease. Biol Neonate. 1972;20(3):300–320. doi: 10.1159/000240473. [DOI] [PubMed] [Google Scholar]
- Tarnow-Mordi W., Ogston S., Wilkinson A. R., Reid E., Gregory J., Saeed M., Wilkie R. Predicting death from initial disease severity in very low birthweight infants: a method for comparing the performance of neonatal units. BMJ. 1990 Jun 23;300(6740):1611–1614. doi: 10.1136/bmj.300.6740.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez de Prada J. A., Ruano J., Martin-Duran R., Larman M., Zueco J., Ortiz de Murua J. A., Torres A., Figueroa A. Noninvasive determination of pulmonary arterial systolic pressure by continuous wave Doppler. Int J Cardiol. 1987 Aug;16(2):177–184. doi: 10.1016/0167-5273(87)90249-x. [DOI] [PubMed] [Google Scholar]
- Yock P. G., Popp R. L. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984 Oct;70(4):657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]


