Abstract
There is a great need to develop better mechanism-based therapies for prostate cancer. In this investigation, we studied four human prostate cancer cell lines, LNCaP, DU145, LAPC4, and PC3, which differ in response to the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (vorinostat), a new anticancer drug. Examining the role of intrinsic mitochondrial caspase-dependent apoptosis and caspase-independent, reactive oxygen species (ROS) facilitated cell death, has provided an understanding of mechanisms that may determine the varied response to the histone deacetylase inhibitor. We found striking differences among these cancer cells in constitutive expression and response to suberoylanilide hydroxamic acid in levels of antiapoptotic and proapoptotic proteins, mitochondria membrane integrity, activation of caspases, ROS accumulation, and expression of thioredoxin, the major scavenger of ROS. Identifying these differences can have predictive value in assessing therapeutic response and identifying targets to enhance therapeutic efficacy.
Keywords: mitochondria membrane, reactive oxygen species, suberoylanilide hydroxamic acid, thioredoxin-binding protein, caspase
Prostate cancer is the most common cancer and the second most common cause of death in men in North America and Western Europe (1). Current therapy for metastatic prostate cancer is unsatisfactory (2). Suberoylanilide hydroxamic acid (SAHA; vorinostat) is an orally administered histone deacetylase inhibitor (HDACi) that in phase I/IIa and IIb clinical trials as monotherapy has shown significant antitumor activity in hematological and solid tumors at doses well tolerated by patients (3, 4). The first in vivo evidence of the anticancer activity of SAHA was demonstrated in nude mice bearing human prostate cancer transplants (5). Normal cells are relatively resistant to SAHA (5, 6). In preclinical studies, SAHA has been found to induce caspase-dependent and caspase-independent cell death in different transformed cells (6–9). Why different cells respond in different ways is not well understood. An objective of the present study is to identify molecular targets mediating the anticancer activity of HDACi to increase our understanding of the mechanisms involved in response of prostate cancer cells to this agent.
The intrinsic mitochondria-mediated apoptosis pathway is the best understood induced-death pathway (10–12). Another, albeit less well studied, cellular system that plays an important role in response to stress stimuli that cause cell death is the thioredoxin (Trx)–thioredoxin-binding protein (TBP-2) redox system (13–15). Trx functions as a hydrogen donor for many protein targets, and it is the major scavenger of reactive oxygen species (ROS). TBP-2 binds specifically to Trx and blocks its reducing activity (16).
This study analyzes the intrinsic mitochondrial caspase-mediated apoptotic pathway and the Trx–TBP-2 pathway that modulates ROS accumulation and caspase-independent cell death in response to SAHA (17).
Aberrant expressions of proteins of the apoptotic pathway are associated with resistance to antitumor therapy (8, 18–20). For example, a high level of Bcl-2 in PC3 prostate cancer cells is associated with resistance to therapy (21). Increased expression of XIAP and survivin was found in a cisplatin-resistant LNCaP prostate cancer subline (22). It has been shown that HDACi can increase expression of proapoptotic Bcl-2 family proteins as well as decrease expression of antiapoptotic proteins in various transformed cells (7, 8, 18, 22), which, in part can account for the anticancer activity of these agents.
High levels of Trx are associated with cancers resistant to therapy (14). Further, TBP-2, which blocks Trx activity, is expressed at low levels in many human cancers (6, 23). SAHA, as well as other HDACi, radiotherapy, and chemotherapy cause the production of ROS, which can facilitate cancer cell death (6, 7, 19, 24, 25). SAHA induces TBP-2 in transformed cells, associated with a decrease in Trx activity, which can facilitate ROS-induced cell death (6).
This work used four human prostate cancer cell lines, LNCaP, DU145, LAPC4, and PC3, which differ in sensitivity to HDACi-induced cell death from very sensitive (DU145) to resistant (PC3) (26–31). We found striking differences among these prostate cancer cells in the expression of proteins regulating cell death pathways, which provide a better understanding of the molecular basis for differences in response to the HDACi among these transformed cells. This work shows that the four human prostate cancer cells have substantial differences in expression of antiapoptotic and proapoptotic proteins, mitochondrial transmembrane potential (MTP), mitochondrial protein activation, ROS accumulation, and levels of Trx and TBP-2 in culture without and with SAHA. Both caspase-dependent and caspase-independent HDACi-induced cell death occurred in these prostate cancer cells. This work indicates that SAHA-induced cell death involves different targets in the different cells, and it identifies protein markers that may be predictive of response of prostate cancer to therapy and targets for enhancing the efficacy of SAHA and other drugs in treating prostate cancers.
Results
Effect of SAHA on Growth and Death of Human Prostate Cells.
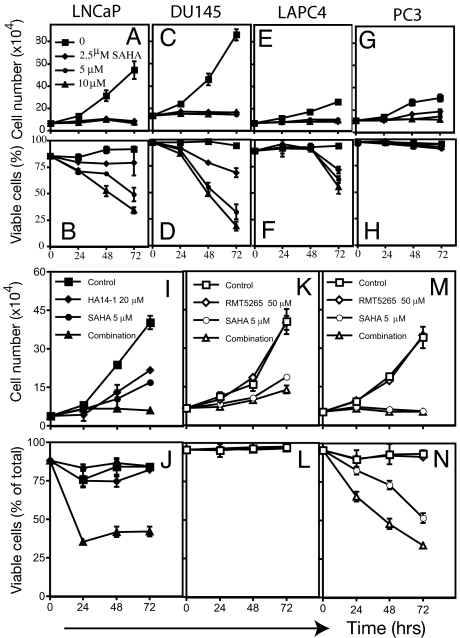
SAHA (2.5 μM) completely inhibited cell growth of LNCaP, DU145, and LAPC4 after 72 h in culture (Fig. 1 A, C, and E). PC3 cell growth was inhibited at 5 μM and completely inhibited at 10 μM (Fig. 1G). LNCaP, DU145, LAPC4, and PC3 had different sensitivity to SAHA-induced cell death: LNCaP, 50% ± 3%; DU145, 77% ± 4%; LAPC4, 40% ± 6%; and PC3, <5% cell death in culture with 5 μM SAHA for 72 h. LNCaP, 25% ± 5%; DU145, 30% ± 2%; and LAPC4, 28% ± 2% in culture with 2.5 μM SAHA for 72 h (Fig. 1 B, D, and F) SAHA caused an accumulation of acetylated histone H3 and H4 in each of the four human prostate cell lines (data not shown) (17).
Fig. 1.
Effect of SAHA on cell growth and viability on human prostate cancer cell lines. (A) Cells were cultured with SAHA at the indicated concentrations for 72 h. Cell number and viability were determined as described in Materials and Methods. (A, C, E, and G) Cell growth of LNCaP (A), DU145 (C), LAPC4 (E), and PC3 (G). (B, D, F, and H) Cell viability of LNCaP (B), DU145 (D), LAPC4 (F), and PC3 (H). (I) Cell growth of PC3 cells cultured with HA14-1 (Bcl-2 inhibitor) or SAHA alone or in combination (HA14-1 plus SAHA). (J) Cell viability of PC3 cells in culture with HA14-1 or SAHA alone or in combination. (K) Cell growth of PC3 cells in culture with RMT5265 inhibitor of XIAP or SAHA alone or in combination. (L) PC3 cell viability cultured with RMT5265 or SAHA alone or in combination. (M) Cell growth of DU145 cells cultured with SAHA or RMT 5265 alone or in combination. (N) Cell viability of DU145 cells cultured with SAHA or RMT5265 alone or in combination.
LAPC4 cells express wild-type androgen receptor (AR), LNCaP express a mutant AR, and both DU145 and PC3 are AR negative (28). SAHA caused a marked decrease in AR expression in LAPC4 and LNCaP cells. Culture of LAPC4 or LNCaP cells with 10 μM hydroxyflutamide, an AR antagonist, did not alter the sensitivity of these cells to SAHA-induced cell death (data not shown).
Consistent with our previous reports (32), SAHA induces p21waf1 protein in LNCaP, DU145, and PC3 cells. p21waf1 is not detectable, and it is not induced by SAHA in LAPC4 (data not shown). SAHA induces G1 arrest in the three cell lines but not in LACP4, consistent with p21waf1 having an important role in SAHA-induced G1 arrest. In LAPC4 cells, SAHA induces a G2 arrest (data not shown). Expression of p21waf1 does not protect these prostate cancer cells from HDACi-mediated cell death because SAHA induced greater cell death of LNCaP and DU145, which express p21waf1, than LAPC4, which does not express p21waf1.
Expression of Antiapoptotic Proteins.
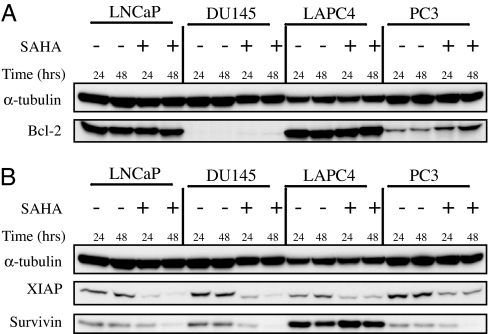
The mitochondrial apoptotic pathway is regulated by antiapoptotic and proapoptotic factors. Mitochondrial membrane integrity depends on the Bcl-2 family of proteins. The expression of the multidomain antiapoptotic protein Bcl-2 was assayed in the four prostate cell lines cultured without and with SAHA. Bcl-2 protein was not constitutively detectable in DU145 cells and not induced by SAHA (Fig. 2A). In PC3 cells, SAHA causes an increased accumulation of Bcl-2. Bcl-2 is expressed in LNCaP and LAPC4, and its expression is not detectably altered by SAHA (Fig. 2A). The lack of Bcl-2 in DU145 is associated with marked sensitivity and the increased levels of Bcl-2 in PC3 with resistance to SAHA-induced cell death. The data shown in Figs. 2 and 3 are studies at 5 μM SAHA. Cells cultured with 2.5 μM SAHA showed similar results with respect to each of the antiapoptotic and proapoptotic proteins (data not shown).
Fig. 2.
Expression of antiapoptotic proteins in LNCaP, DU145, LAPC4, and PC3. Cells were cultured without (−) and with (+) 5 μM SAHA for 24 and 48 h. Western blots of Bcl-2 (A) and XIAP (B) (inhibitor of apoptotic proteins) and survivin are shown; α-tubulin is indicated as the loading control.
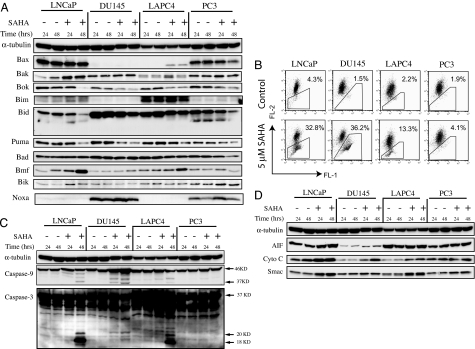
Fig. 3.
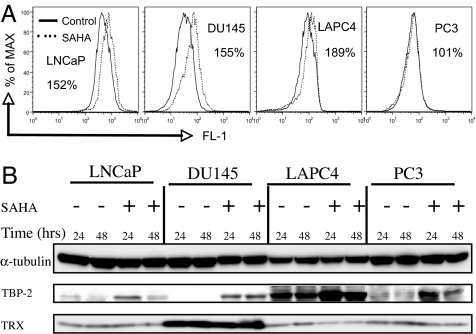
Expression of proapoptotic proteins in LNCaP, DU145, LAPC4, and PC3. (A) Western blot of the indicated Bcl-2 family of proapoptotic proteins in cells cultured without (−) and with (+) 5 μM SAHA for 24 and 48 h; α-tubulin is indicated as the loading control. (B) Mitochondrial transmembrane potential measured as indicated in Materials and Methods. Percentage figures indicate the proportion of cells as a percentage of total with decreased FL-2, an indictor of mitochondrial membrane integrity. (C) Western blots of caspase-9 and caspase-3, with the activation of caspases indicated by the presence of 37-kDa fragment of caspase-9 and of the 20-kDa and 18-kDa fragments of caspase-3. α-Tubulin is indicated as the loading control. (D) Western blots of mitochondrial proapoptotic proteins AIF, cytochrome c, and Smac in cells cultured without (−) and with (+) 5 μM SAHA for 24 and 48 h; α-tubulin is indicated as the loading control.
The antiapoptotic proteins, XIAP (inhibitor of apoptosis) and survivin, are expressed in all four cell lines (Fig. 2B). Constitutive survivin expression is greater in LAPC4 than the other three cell lines. SAHA caused a marked decrease in XIAP levels in all four cell lines and in survivin levels in LNCaP, DU145, and PC3, but not LAPC4. High levels of survivin in LAPC4 are associated with decreased sensitivity to SAHA-induced cell death (Fig. 1F). It has been reported that high levels of survivin are associated with resistance to HDACi-induced cell death of leukemia cell lines (21). A SAHA-induced decrease in the levels of XIAP and survivin does not make PC3 cells (with increased levels of Bcl-2) sensitive to HDACi-induced cell death.
The role of Bcl-2 in the resistance of PC-3 cells to SAHA-induced cell death was evaluated further by using the Bcl-2-selective inhibitor, HA14-1 [ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate] (33). SAHA or HA14-1 added to the cell cultures inhibits cell growth on average 55% and 45%, respectively, compared with >85% when both SAHA and HA14-1 were added to the culture (Fig. 1I). Neither SAHA nor HA14-1 alone induces significant cell death in PC3 cells after 72 h in culture (8% and 11% cell death, respectively), whereas the addition of both HA14-1 and SAHA causes >70% PC-3 cell death within 24 h (Fig. 1J).
XIAP Is Inhibited by Smac.
Inhibition of XIAP with Smac mimic, RMT5265 (34), does not alter PC3 resistance to SAHA-induced cell death (Fig. 1 K and L). The XIAP inhibitor RMT5265 does increase the sensitivity of DU145 to SAHA-induced cell death (Fig. 1N).
Expression of Proapoptotic Protein and Mitochondrial Membrane Permeability.
The multidomain proapoptotic proteins, e.g., Bak and Bax, and the BH3 domain-only proapoptotic proteins, e.g., Bim, Bid, Puma, Bad, Bmf, Bik, and Noxa, inactivate prosurvival proteins, like Bcl-2 (see refs. 6–10, 12, 18, 19, 34, and 35). Stress stimuli including SAHA lead to the BH3 domain-only proteins inactivating Bcl-2 through direct protein–protein interaction, which can, in turn, cause decreased MTP with release of cytochrome c, which induces caspase-9 activation.
There are striking differences among the four prostate cancer cell lines in (i) the expression of proapoptotic proteins and (ii) the effect of SAHA on MTP (Fig. 3). MTP was determined with a fluorescence dye, JC-1, which aggregates in normal mitochondria and emits red fluorescence, but it cannot accumulate in mitochondria losing transmembrane potential, and it emits a diffuse cytoplasmic green signal in dead cells.
In LNCaP cells, the proapoptotic proteins Bax, Bak, Bok, Bim, Bid, Puma, Bad, Bmf, and Bik were expressed. Noxa was not expressed in LNCaP or LAPC4 cells, and it was expressed in DU145 and PC3. In LNCaP cells, SAHA increases the levels of Bak, Bmf, Bim, and Bik (Fig. 3A), it induces loss of MTP 32.8% with SAHA compared with 4.3% in cells cultured without inhibitor (Fig. 3B), and it leads to caspase-9 and caspase-3 activation, as indicated by the presence of 37-kDa caspase-9 fragment and 20-kDa and 18-kDa caspase-3 fragments (Fig. 3C).
In DU145, there was no detectable expression of Bax or Bim, and neither is induced by SAHA (Fig. 3A). The HDACi does up-regulate Bmf but none of the other proapoptotic proteins assayed. In DU145 cells, SAHA causes a marked increase in cells losing MTP [36.2% compared with 1.5% without inhibitor (Fig. 3B)] and activation of caspase-9 and caspase-3 (Fig. 3C).
LAPC4 cells had no detectable constitutive expression of Bax and Noxa. SAHA increases Bax, Bak, Bim, and Bmf expression, moderately induces loss of MTP [13.3% compared with 2.2% without inhibitor (Fig. 3B)], and causes activation of caspase-9 and caspase-3 (Fig. 3C).
PC3 cells, like LNCaP, express all of the proapoptotic proteins assayed. However, unlike LNCaP cells, SAHA does not increase the expression of these proteins in PC3 cells, and it neither significantly increases loss of MTP (4.1% compared with control 1.9%) (Fig. 3B) nor causes caspase-9 or caspase-3 activation (Fig. 3C).
Mitochondrial Protein Expression and Release.
Associated with loss of MTP is disruption of the mitochondrial membrane and release of mitochondrial proteins including cytochrome c, AIF, and Smac. Smac neutralizes the caspase inhibitory activity of XIAP (10). Cytochrome c activates caspase-9 and, downstream, the effector caspases, such as caspase-3, causing cleavage of a number of nuclear and cytoplasmic proteins that lead to the morphological changes characteristic of apoptosis (10). The four human prostate cancer cell lines expressed AIF, cytochrome c, and Smac (Fig. 3D). SAHA increased the levels of each of these proapoptotic proteins in LNCaP, DU145, and, to a lesser extent, LACP4 cells, but not in PC3 cells (Fig. 3D). SAHA caused a release of AIF, cytochrome c, and Smac into the cytoplasm of LNCaP, DU145, and LAPC4, but not PC3 (data not shown). These findings are consistent with greater sensitivity of LNCaP and DU145 than LAPC4 and resistance of PC3 to HDACi-induced cell death.
Effects of Inhibition of Caspase Activity on SAHA-Induced Prostate Cancer Cell Death.
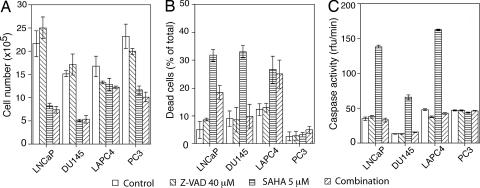
In SAHA-cultured cells, caspase-9 and caspase-3 are activated in LNCaP, DU145, and LAPC4, but not PC3 (Fig. 3C). Addition of N-benzyloxycarbonyl-valyl-alanyl-aspartyl (z-VAD-fmk) to cultures with SAHA inhibited caspase activation in the three prostate cancer cell lines (Fig. 4C). Addition z-VAD to cultures without SAHA did not alter significantly cell growth (Fig. 4A) or cell viability (Fig. 4B), and z-VAD did not prevent SAHA inhibition of cell growth in LNCaP, DU145, LAPC4, or PC3 cells (Fig. 4A). z-VAD did prevent SAHA-induced DU145 cell death but not SAHA-induced cell death of LAPC4 and decreased cell death of LNCaP ≈50%. These results indicate that the HDACi-induced cell death of LAPC4 and, LNCaP, but not DU145, is to a significant extent caspase-independent. These findings further emphasize that human prostate cancer cells differ in pathways regulating cell death, and such differences have important implications for therapeutic strategies for prostate cancer.
Fig. 4.
Effect of z-VAD, the pan-caspase inhibitor, on cell growth (A), cell death (B), and caspase activity (C) in LNCaP, DU145, LAPC4, and PC3. Cells were cultured without (control), with 5 μM SAHA, with 40 μM z-VAD, and with 5 μM SAHA plus 40 μM z-VAD (combination).
Trx–Trx-Binding Protein Pathway.
SAHA, as well as other HDACi and chemotherapy agents, can cause an accumulation of ROS, which can facilitate caspase-independent cell death (6, 19, 24, 25, 35, 36). Trx functions as a hydrogen donor and is a potent scavenger of ROS (13–15, 37). Previous studies showed that SAHA can induce TBP-2 in transformed cells (23). TBP-2 specifically binds to Trx and inactivates its capacity to reduce ROS (15).
SAHA causes an accumulation of ROS in LNCaP, DU145, and LAPC4, but not PC3 cells (Fig. 5A). The HDACi induces TBP-2 protein in all four prostate cancer cells (Fig. 5B). SAHA-induced death of LACP4 and, to a large extent, LNCaP cells, is caspase activation-independent (Fig. 4B). Increased levels of TBP-2 are associated with reduced Trx activity and increased ROS accumulation in LAPC4 and LNCaP cells (Fig. 5 A and B). The increased ROS could facilitate cell death (9). The present data do not establish why SAHA-induced cell death of DU145 cells is caspase-dependent and that of LAPC4 and, to some extent, LNCaP, are not. There are several differences among these cell lines in expression of both proapoptotic and antiapoptotic proteins, but which of these differences account for cell death that is primarily caspase-dependent or caspase-independent is not clear. One can speculate that the lack of Bcl-2 expression in DU145 may be a factor making these cells particularly sensitive to SAHA-induced caspase-dependent death. In LAPC4 cells, the high constitutive levels of Bcl-2 and survivin may be factors contributing to caspase-independent cell death. It is likely that the relative importance of these cell death pathways in the anticancer activity of SAHA is cell context-dependent.
Fig. 5.
Effect of SAHA on accumulation of ROS and expression of thioredoxin (Trx) and Trx-binding protein (TBP-2). (A) Percent figure represents the ratio of ROS in cells cultured with SAHA relative to cells cultured without the HDACi. (B) Western blot analysis of effect of SAHA on expression of TBP-2 and Trx proteins in LNCaP, DU145, LAPC4, and PC3 cells cultured without (−) and with (+) 5 μM SAHA for 24 and 48 h. α-tubulin was indicated as loading control.
Discussion
This study has found that prostate cancer cell lines have striking differences in expression of proteins that modulate mitochondria-mediated apoptosis and ROS-facilitated cell death. These differences determine, in part, the sensitivity of these different prostate cancer cells to the HDACi SAHA-induced cell death, as well as the molecular pathways involved.
Among the four cell lines, LNCaP and DU145 are more sensitive than LAPC4 to SAHA-induced cell death, and PC3 is resistant. Bcl-2 levels play an important role in PC3 resistance because chemically blocking Bcl-2 made these cells sensitive to the HDACi. DU145, the most sensitive to SAHA-induced cell death among the four cell lines, had no detectable Bcl-2. A high level of the antiapoptotic protein, survivin, is present in LAPC4 cells, which are less sensitive than DU145 or LNCaP to the HDACi.
The four cell lines differed in constitutive expression of TBP-2. TBP-2 is not detectable in DU145, and it is relatively well expressed in LAPC4. ROS accumulated in LNCaP, DU145, and LAPC4, but not PC3, cells cultured with SAHA. SAHA-induced TBP-2 expression in all four cell lines and decreased Trx levels most markedly in LACP4 cells. SAHA-induced LAPC4 cell death was not blocked by z-VAD, the pan-caspase inhibitor, suggesting that it was caspase-independent cell death. TBP-2 inactivation of Trx can play a role in caspase-independent cell death. By comparison, in DU145 cells, z-VAD inhibited caspase activation and blocked SAHA-induced cell death, suggesting caspase-dependent cell death.
Further evidence suggesting that the difference in the pathway of SAHA-mediated cell death is related to induced changes in the expression of proapoptotic and antiapoptotic proteins is suggested by the fact that SAHA caused more changes in proapoptotic proteins in sensitive LNCaP and DU145 cells than in the resistant PC3 cells. In LNCaP cells, SAHA increased proapoptotic proteins Bax, Bim, Bmf, Bik, cytochrome c, and Smac, decreased antiapoptotic XIAP and survivin, activated caspase-9 and -3, and increased mitochondrial transmembrane permeability. In PC3-resistant cells, SAHA did not alter MTP, increase cytochrome c or AIF levels, or activate caspase-3 or -9.
In the clinical trials with oral-administered SAHA (3), patients with significant anticancer effects had average maximum concentrations of SAHA of 2.5–3.0 μM with a range of up to 4–5 μM SAHA. It is not possible to relate directly the concentrations of an effective level of HDACi in vitro to that in vivo, but the range of concentrations active in patients with solid tumors is comparable with those used in this work.
Transformed cells in culture can undergo changes in gene and protein expression from the prostate cancers from which they are derived. Accordingly, findings in cancer cells in culture may have little relevance to cancer cells in human primary tumors. In fact, there are few quantitative data to suggest this conclusion (38). Comparing the gene expression profile between tumor prostate cancer tissue (in vivo) with LNCaP cells treated with and without anticancer drugs, 1.4–3.6% of genes were differentially expressed in the two sets of prostate cells. These authors (38) suggest that LNCaP cells should be a good model for many aspects of the study of prostate cancers. Several microarray studies have attempted to determine patterns of gene expression that predict therapeutic response (38–41). In general, this work has identified patterns of differentially expressed genes, but so far it has not been able to establish a set of marker genes that can reliably predict therapeutic response. It is encouraging that gene expression profiling appears to be possible with circulating tumor cells, which clearly are more accessible than pre- and posttherapy prostate biopsy material (40, 41).
The studies reported to date (40, 41) indicate that an assay for specific proteins in circulating tumor cells could be a promising technique and clearly one that requires further development. This work suggests that assays of specific proteins, such as Bcl-2, TBP-2, and cytochrome c, could be useful guides to therapeutic markers and for which immunohistochemical assay techniques are available. Further, the present findings suggest that targeting proteins, such as Bcl-2 down-regulation or TBP-2 up-regulation, could enhance the therapeutic efficacy of SAHA and other anticancer agents in the therapy of prostate cancers.
Materials and Methods
Cell Culture.
Human prostate cancer cell lines LNCaP, DU145, and PC3 were obtained from American Tissue Culture Collection (Manassas, VA) and cultured in RPMI medium 1640, MEM α, and F-12K medium with 10% FBS, respectively. Human prostate cancer cell line LAPC4 was provided by Charles Sawyers (University of California at Los Angeles, CA) and cultured in Iscove's medium with 10% FBS (29).
Cell Growth and Viability.
To begin, 5 × 104 cells per well were seeded in a 24-well plate. After 24 h, SAHA (17) was added to cultures as indicated in the figure legends. On days 1, 2, and 3 of SAHA culture, cells were collected with trypsin digestion (6). Cell number and viability were determined by Guava PCA-96 machine with ViaCount Flex reagent (Guava Technologies, Hayward, CA), according to the manufacturer's protocol, or by trypan blue exclusion as described in ref. 6. At least three independent analyses were performed for all determinations of cell growth and viability. The antitumor effects of combining SAHA with other reagents were examined in the prostate cancer cell lines as follows: 5 × 104 cells per well were seeded in a 24-well plate and cultured overnight. The cultured cells were treated with the concentration of SAHA indicated for each experiment with or without various other reagents, including Bcl-2 antagonist HA14-1 (Raylight, La Jolla, CA), or XIAP inhibitor RMT5256 [a gift from Xiaodong Wang (University of Texas Southwestern Medical Center, Dallas, TX)]. Cell growth and viability on days 1, 2, and 3 of culture were determined as described earlier.
Cell Cycle Analysis.
Cell cycle analysis was performed as described in ref. (6).
Cell Fractionation and Measurement of Caspase-3 Activity.
Cells were cultured with 5 μM SAHA with or without 40 μM pan-caspase inhibitor z-VAD (EMD Biosciences, San Diego, CA). z-VAD was added to cells cultured 1 h before SAHA. Cells were processed, and caspase activity was determined as described in ref. 42. Three independent experiments were performed for each analysis.
Measurement of Mitochondrial Transmembrane Potential.
In a 10-cm-diameter cell culture dish, 106 cells were seeded. After 24 h, the cells were treated with 5 μM SAHA. Cells were collected by trypsin digestion at 48 h, centrifuged at 500 × g for 5 min at 4°C, and washed once with PBS. Cells were incubated with 5 μg/ml JC-1 (Invitrogen, Carlsbad, CA) at 37°C for 15 min. After incubation, the cells were centrifuged at 500 × g for 5 min, resuspended in PBS, and analyzed by using flow cytometry with FL-2 to detect the red fluorescence signal, which is generated from JC-1 concentrated in mitochondria in normal cells and FL-1 to measure green fluorescence, which is emitted by JC-1 diffused in the cytoplasm in cells with loss of transmembrane potential (43).
ROS Measurements.
ROS measurements were performed as described in ref. 6.
Measurement of Trx.
Trx was measured by using the end point insulin assay for biological samples as described in ref. 44.
Western Blotting.
In a 15-cm-diameter cell culture dish, 2–3 × 106 cells were seeded. After 24 h, 5 μM SAHA was added to the culture. Cells were collected by trypsin digestion at 24 and 48 h, washed with PBS, and lysed in RIPA buffer (50 mM Tris·Cl, pH 7.5/250 mM NaCl/5 mM EDTA, pH 8.0/0.5% Nonidet P-40) with proteinase inhibitor tablet (Roche Diagnostics, Indianapolis, IN). Protein concentration was determined by using the Braford method. Forty micrograms of protein was resolved by SDS/PAGE and transferred onto a PVDF membrane. The membrane was blocked with 5% BSA and blotted with the following antibodies and visualized by ECL method. The antibodies against the following proteins used in the study were as follows: Bax, Bim, and Bcl-2 (BD PharMingen, San Diego, CA); Bak, Bok, Bid, Bad, Bmf, Puma, cytochrome c, AIF, Smac, XIAP, and survivin (Cell Signaling, Danvers, MA); Noxa, caspase-3, and caspase-9 (EMB Biosciences, San Diego, CA); androgen receptor (DAKO, Carpinteria, CA); and p21waf1 (Neomarkers, Fremont, CA). The antibodies against TBP-2 and Trx were obtained from MBL (Woburn, MA) and American Diagnostic (Stamford, CT), respectively.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (CA-008748), the Jack and Susan Rudin Foundation, and the David H. Koch Foundation, and by a Prostate Cancer Research Award, the Experimental Therapeutics Center at Memorial Sloan–Kettering Cancer Center, and the DeWitt Wallace Research Fund.
Abbreviations
- AR
androgen receptor
- HDACi
histone deacetylase inhibitor
- MTP
mitochondrial transmembrane potential
- ROS
reactive oxygen species
- SAHA
suberoylanilide hydroxamic acid
- Trx
thioredoxin
- z-VAD
N-benzyloxycarbonyl-valyl-alanyl-aspartyl fluoromethylketone.
Footnotes
Conflict of interest statement: Memorial Sloan-Kettering Cancer Center and Columbia University jointly hold patents on hydroxamic based polar compounds, including SAHA, that were exclusively licensed to Aton Pharma, Inc., a biotechnology company acquired by Merck, Inc. in April, 2004. P.A.M. was a founder of Aton and has a financial interest in Merck's further development of SAHA.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Sawyers CL. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 3.Kelly WK, O'Connor OA, Krug L, Chiao J, Heaney M, Curley T, MacGregor-Cortelli B, Tong W, Secrist JP, Schwartz L, et al. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, Macgregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, et al. J Clin Oncol. 2006;24:166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 5.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 6.Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, Ngo L, Holmgren A, Jiang X, Marks PA. Proc Natl Acad Sci USA. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone RW, Licht JD. Cancer Cell. 2003;4:13–18. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 8.Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, Altucci L, Nervi C, Minucci S, Pelicci PG. Nat Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 9.Dokmanovic M, Marks PA. J Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Wang X. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 11.Marks PA, Jiang X. Cell Cycle. 2005;4:e8–e11. doi: 10.4161/cc.4.4.1564. [DOI] [PubMed] [Google Scholar]
- 12.Willis SN, Adams JM. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arner ES, Holmgren A. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 14.Powis G, Mustacich D, Coon A. Free Radic Biol Med. 2000;29:312–322. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 16.Nishinaka Y, Nishiyama A, Masutani H, Oka S, Ahsan KM, Nakayama Y, Ishii Y, Nakamura H, Maeda M, Yodoi J. Cancer Res. 2004;64:1287–1292. doi: 10.1158/0008-5472.can-03-0908. [DOI] [PubMed] [Google Scholar]
- 17.Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, Marks PA. Proc Natl Acad Sci USA. 1998;95:3003–3007. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peart MJ, Tainton KM, Ruefli AA, Dear AE, Sedelies KA, O'Reilly LA, Waterhouse NJ, Trapani JA, Johnstone RW. Cancer Res. 2003;63:4460–4471. [PubMed] [Google Scholar]
- 19.Hail N., Jr Apoptosis. 2005;10:687–705. doi: 10.1007/s10495-005-0792-8. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Carroll PR, Dahiya RR. J Natl Cancer Inst. 2005;97:103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka K, Rocchi P, Miyake H, Fazli L, Vessella B, Zangemeister-Wittke U, Gleave ME. Mol Cancer Ther. 2005;4:1689–1698. doi: 10.1158/1535-7163.MCT-05-0064. [DOI] [PubMed] [Google Scholar]
- 22.McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Prostate. 2002;51:133–140. doi: 10.1002/pros.10061. [DOI] [PubMed] [Google Scholar]
- 23.Butler LM, Zhou X, Xu W-S, Scher HI, Rifkind RA, Marks PA, Richon VM. Proc Natl Acad Sci USA. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. IUBMB Life. 2001;52:29–33. doi: 10.1080/15216540252774739. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Rahmani M, Dent P, Grant S. Mol Cell Biol. 2005;25:5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bookstein R, Shew JY, Chen PL, Scully P, Lee WH. Science. 1990;247:712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 29.Uzgare AR, Isaacs JT. Cancer Res. 2004;64:6190–6199. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
- 30.Denmeade SR, Sokoll LJ, Dalrymple S, Rosen DM, Gady AM, Bruzek D, Ricklis RM, Isaacs JT. Prostate. 2003;54:249–257. doi: 10.1002/pros.10199. [DOI] [PubMed] [Google Scholar]
- 31.Fronsdal K, Saatcioglu F. Prostate. 2005;62:299–306. doi: 10.1002/pros.20140. [DOI] [PubMed] [Google Scholar]
- 32.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 35.Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. Proc Natl Acad Sci USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosato RR, Almenara JA, Dai Y, Grant S. Mol Cancer Ther. 2003;2:1273–1284. [PubMed] [Google Scholar]
- 37.Holmgren A. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 38.Waghray A, Schober M, Feroze F, Yao F, Virgin J, Chen YQ. Cancer Res. 2001;61:4283–4286. [PubMed] [Google Scholar]
- 39.Sasakawa Y, Naoe Y, Sogo N, Inoue T, Sasakawa T, Matsuo M, Manda T, Mutoh S. Biochem Pharmacol. 2005;69:603–616. doi: 10.1016/j.bcp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, et al. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Wong C, Mysel R, Slobodov G, Metwalli A, Kruska J, Manatt CS, Culkin DJ, Kropp BP, Lin HK. Mol Cancer. 2005;4:30. doi: 10.1186/1476-4598-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao Y, Gao Z, Marks PA, Jiang X. Proc Natl Acad Sci USA. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arner E, Holmgren A. Current Protocols in Toxicology. New York: Wiley; 2000. pp. 7.4.1–7.4.14. [DOI] [PubMed] [Google Scholar]