Abstract
Apolipoprotein E4 (ApoE4) is associated with Alzheimer’s disease by unknown mechanisms. We generated six transgenic mice strains expressing human ApoE4 in combination with mutant amyloid precursor protein (APP) and mutant presenilin-1 (PS1) in single-, double-, or triple-transgenic combinations. Diffuse, but not dense, amyloid plaque-load in subiculum and cortex was increased by neuronal but not glial ApoE4 in old (15 months) double-transgenic mice, whereas both diffuse and dense plaques formed in thalamus in both genotypes. Neuronal and glial ApoE4 promoted cerebral amyloid angiopathy as extensively as mutant PS1 but with pronounced regional differences: cortical angiopathy was induced by neuronal ApoE4 while thalamic angiopathy was again independent of ApoE4 source. Angiopathy correlated more strongly with soluble Aβ40 and Aβ42 levels in cortex than in thalamus throughout the six genotypes. Neither neuronal nor glial ApoE4 affected APP proteolytic processing, as opposed to mutant PS1. Neuronal ApoE4 increased soluble amyloid levels more than glial ApoE4, but the Aβ42/40 ratios were similar, although significantly higher than in single APP transgenic mice. We conclude that although the cellular origin of ApoE4 differentially affects regional amyloid pathology, ApoE4 acts on the disposition of amyloid peptides downstream from their excision from APP but without induction of tauopathy.
Alzheimer’s disease (AD) is characterized by pathological accumulations in the brain of extracellular amyloid plaques and intraneuronal accumulations of protein tau known as neurofibrillary tangles. The amyloid peptides Aβ40 and Aβ42 are the major components of the amyloid deposits in parenchyma and vasculature.1 The amyloid peptides are excised from the integral membrane protein amyloid precursor protein (APP) by sequential endoproteolytic cleavages by β- and γ-secretases.2 The exact causes and consequences, in terms of normal processes and mechanisms that are disturbed by the amyloid peptides and causing neurodegeneration, remain primarily unclear.3
Besides APP and presenilins (PS1, PS2), the apolipoprotein E (ApoE) is genetically linked to AD.4 The ApoE4 lipoprotein or its encoding ε4 allele, are epidemiologically associated with AD and confer an increased risk and earlier age of onset relative to the more common ApoE3 protein or ε3 allele.5–7 ApoE is a 34-kd protein abundantly expressed in liver and brain. In the circulation, ApoE-lipoproteins mediate transport of lipids and cholesterol from and to liver and extrahepatic tissues. In contrast to the peripheral functions of ApoE that are well understood, details of its actions in the central nervous system remain primarily unknown. Actually, the epidemiological association of the ε4-allele to AD provided a strong impetus to research, by pointing out our lack of understanding the physiology of lipid and cholesterol homeostasis and of their transport in brain, in particular the contribution of ApoE and various ApoE receptors.8,9
Similar to the physiology of ApoE, its pathological contributions to amyloid and tau pathology in AD have been studied in different types of transgenic mouse models, either deficient in murine ApoE and/or overexpressing human ApoE isoforms. Combination in double-transgenic models carrying also a mutant human APP has revealed shortcomings and even conflicts into the contributions in the pathogenesis of AD, while many aspects and data, including the cholesterol conundrum, remain intriguing if not controversial.10–12
The deficiency of endogenous murine ApoE in combination with mutant APP in double-transgenic mice, significantly delayed amyloid deposition overall, with less parenchymal and almost no vascular amyloid.13–18 Thereby, ApoE was proposed to cause aggregation of amyloid and/or induce conformational changes in the amyloid peptides to form fibrils. In contrast, deposition of amyloid was also delayed by expression of human ApoE, in an isoform-specific manner, while ApoE even appeared to affect the metabolism of APP before plaques developed.13,16,17 Another study demonstrated, however, that expression of human ApoE4 but not of ApoE3, both under control of the transferrin gene promoter, accelerated deposition of amyloid in APP/Swe transgenic mice.19 In a similar study, overexpression of either ApoE isoform under control of the prion gene promoter neither accelerated nor increased the amyloid burden, nor markedly affected the metabolism before amyloid deposition even in the presence of mutant presenilin-1 in triple-transgenic mice.20
In addition to parenchymal amyloid plaques, the vascular deposits presenting as cerebral congophilic amyloid angiopathy (CAA) are a major pathological sign in AD21 with a prevalence of more than 90% in the oldest patients.22 The severity of CAA was, in contrast to parenchymal amyloidosis, strongly associated with the presence of ApoE4 alleles,23 similar to reduced Aβ42 levels in cerebrospinal fluid.24
Although not consistent throughout all studies, the combined clinical, pathological, and experimental findings suggest that ApoE4 favors vascular over parenchymal deposition of amyloid, although the mechanisms are unclear. The shift in amyloid deposition from the parenchyma to the vasculature by human ApoE4 in brain of double-transgenic APP×ApoE4 knockin mice, corroborates this hypothesis.25
ApoE is synthesized and secreted mainly by nonneuronal glial cells and is taken up by neurons by receptor-mediated endocytosis.8,9,26 Neurons can synthesize ApoE as observed in human and mouse brain.27–30 Interestingly, brain regions that express ApoE in neurons also correlate with more severe amyloid and neurofibrillary tangle pathology in AD. The exact contribution or even the importance of neuronal expression of ApoE to amyloid or tau pathology is unknown. We have previously tested this hypothesis in vivo, by deriving transgenic mice that differentially expressed ApoE4 in neurons or glia.28,29 Neuronal but not glial expression of ApoE4 resulted in hyperphosphorylation of protein tau and caused prominent axonopathy by disruption of axonal transport.28,29 A recent independent study not only confirmed our data, but additionally reported proteolytic cleavage of ApoE4 to be involved.31
We investigated the pathological contributions of different cellular origins of ApoE4 further and report here the in-depth analysis of six transgenic mouse strains expressing human ApoE4 in combination with mutant APP[V717I] and mutant PS1[A246E] in either single-, double-, or triple-transgenic combinations. The aim was to assess the contribution of the differential expression of ApoE4 in either neurons or glia cells on the amyloid pathology in parenchyma and vasculature relative to the parental APP[V717I] transgenic mice.32–34 We explored with progressing age, the differential effects of neuronal versus glial ApoE on amyloid pathology, and analyzed the processing of APP as well as the eventual emergence of tauopathy. The data demonstrate that the cellular origin of ApoE4 differentially modulated the amyloid pathology in brain parenchyma and in the vasculature, without affecting the processing of APP. Moreover, despite hyperphosphorylation of protein tau at different AD-related epitopes, no tauopathy was induced by the neuronal expression of ApoE4, demonstrating that the major, if not only, pathological effect of ApoE4 is to be situated in the cellular or extracellular handling of the amyloid peptides, subsequently to their excision from APP.
Materials and Methods
Transgenic Mice
Transgenic mice with neuronal and glial overexpression of human ApoE4, respectively, driven by the mouse thy1 gene promoter and by the GFAP gene promoter, were generated previously.28,29 Two different strategies were used to generate offspring in a F1 background of FVB/N × C57BL6 and with co-expression of human ApoE4 with APP-V717I and PS1-A246E. Homozygous thy1-ApoE4 mice and hemizygous GFAP-ApoE4 transgenic mice in the C57BL6 genetic background28,29 were crossed with APP[V717I] single-transgenic mice and with APP[V717I] × PS1[A246E] double-transgenic mice both in the FVB/N background.32–34 Genotyping by polymerase chain reaction identified all six possible genotypes, ie, double ApoE4 × APP and triple ApoE4 × APP × PS1 transgenic mice, respectively with ApoE4 driven by either the mouse thy1 gene promoter or by the GFAP gene promoter.
Importantly, all transgenic mice analyzed in this study were all females, all hemizygous for the transgenes they express, and moreover, were all from the F1 generations of different crossings resulting in all having an identical mixed FVB/N × C57BL6 genetic background (Table 1). The time line of analysis was defined at ages 4, 6, 8, and 15 months, based on the characteristics of the four parental mouse strains28,29,32–34 and a total of 112 transgenic mice, ie, three or four transgenic mice per age group per genotype, were analyzed. Genotyping of all transgenic offspring for human APP, human PS1, and human ApoE4 was performed by six independent polymerase chain reaction assays on DNA extracted from tail biopsies as described.28,32–34
Table 1.
The Six Genotypes of Transgenic Mice Analyzed
| Genotype | Expression of human ApoE4 | Notation |
|---|---|---|
| APP[V717I] | APP | |
| APP[V717I] × PS1[A246E] | APP.PS1 | |
| Thy1-ApoE4 × APP[V717I] | Neuron | APP.TE4 |
| GFAP-ApoE4 × APP[V717I] | Astrocyte | APP.GE4 |
| Thy1-ApoE4 × APP[V717I] × PS1[A246E] | Neuron | APP.PS1.TE4 |
| GFAP-ApoE4 × APP[V717I] ×PS1[A246E] | Astrocyte | APP.PS1.GE4 |
Brain Sections and Extracts
Anesthetized mice were perfused transcardiacally with ice-cold saline and their brain was rapidly excised. One hemisphere was immersion-fixed overnight in 10 vol of 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C, rinsed, and stored in PBS containing 0.1% azide at 4°C. Sagittal free-floating vibratome sections (40 μm) were transferred to microtiter plates, and kept in PBS containing 0.1% azide at 4°C.
The other hemisphere was snap-frozen in liquid N2 and used for biochemical analysis by differential extraction. Hemispheres were homogenized with a Potter-type mechanical homogenizer (Zipperer GmbH, Staufen, Germany), in 10 vol of ice-cold Tris-proteinase/phosphatase-inhibitor buffer (TPI-buffer), containing 20 mmol/L Tris-HCl (pH 8.5), 20 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L ortho-phenanthroline, 20 mmol/L NaF, 200 μmol/L Na3VO4, and a cocktail of proteinase inhibitors (Roche Diagnostics GmbH, Germany). A portion equivalent to 2 vol of the total homogenate was stored at −70°C and the remainder was centrifuged (100,000 × g, 4°C, 80 minutes).
The supernatant, designated as soluble fraction was aliquoted and stored at −70°C. The pellet containing the membranes was resuspended in 500 μl of ice-cold TPI-buffer containing 1% Triton X-100 (v/v) and after complete solubilization was centrifuged again (100,000 × g, 4°C, 80 minutes). The supernatant, designated as Triton-soluble membrane fraction (MT) was aliquoted and stored at −70°C. The pellet was solubilized in formic acid (80%) and after centrifugation (20,000 × g, 4°C, 60 minutes) the supernatant was dried under vacuum and the residue solubilized by boiling in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer.
Biochemical Analysis of APP and Its Metabolites
Western blotting was performed to quantify full-length human APP (APPm) and the C-terminal fragments of APP (α- and β-CTF, or C83 and C99) as described.34,35 Briefly, the MT fraction was denatured and reduced by incubation at 95°C for 10 minutes after addition of an equal volume of SDS-PAGE sample buffer [final concentrations of 2% SDS and 2.5% 2-mercaptoethanol (2-ME)]. Equal volumes of all samples were applied on polyacrylamide gels, respectively, APPm and CTF on 8% Tris-glycine gels, and on 4 to 12% Bis-Tris NuPAGE gels (Invitrogen, Germany). After electrotransfer to nitrocellulose filter membranes (Hybond-ECL; Amersham Biosciences, UK), membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (pH 7.6) containing 0.1% Tween-20. APPm and CTF were detected by Western blotting with our polyclonal antibody B10.4 directed against the C-terminal 20 residues of APP.34,35 The β-CTF were detected with monoclonal antibody WO2 (The Genetics Company, Switzerland) directed against human amyloid peptide (sequence 4 to 10) after the nitrocellulose blots were microwaved for 5 minutes in PBS to enhance sensitivity.34–36 After incubation with appropriate secondary antibodies, the Western blots were developed by the ECL detection system (Amersham Biosciences) and images were recorded photographically with different exposure times. All blots were reprobed with an antibody against mouse α-tubulin (Amersham Biosciences) as loading controls.
Sandwich enzyme-linked immunosorbent assay (ELISA) for human soluble Aβ40 and Aβ42 was performed as described.34,35 Briefly, a portion of the soluble fraction (25%) was applied on small reversed phase columns (C18 Sep-Pack cartridges; Waters Corp., Milford, MA) and washed with increasing concentrations of acetonitrile (25% and 80%) containing 0.1% trifluoroacetic acid. The 80% eluted fraction contained all amyloid peptides, was dried under vacuum, and after solubilization used for measurement of the soluble amyloid peptides by specific sandwich ELISA (The Genetics Company). Absorbance at 450 nm was measured in a microtiter plate reader (Victor Wallac;2 Perkin Elmer, UK).
Analysis of Human ApoE
Total homogenate was analyzed for human ApoE4 levels by Western blotting. Briefly, total homogenate was denatured and reduced in SDS-PAGE sample buffer as described above. Equal volumes were resolved by 4 to 12% Bis-Tris NuPAGE in MES running buffer (Invitrogen) and proteins electrotransferred to nitrocellulose filter membranes (Hybond-ECL, Amersham Biosciences). Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (pH 7.6) containing 0.1% Tween-20 and incubated overnight with a rabbit antibody against human ApoE (DAKO, Glostrup, Denmark) followed by goat anti-mouse IgG secondary antibody conjugated to horse-radish peroxidase (Bio-Rad Laboratories, Hercules, CA) and development with ECL (Amersham Biosciences). All blots were reprobed with an antibody against mouse α-tubulin (Amersham Biosciences) as loading controls. On all gels for quantitative analysis, serial dilutions of samples were loaded to define the linear response range of the system. Protein bands corresponding to transgenic human ApoE4 and to endogenous mouse α-tubulin were measured by densitometric scanning and analyzed with dedicated software (ImageMaster 1D, Amersham Bioscience).
Histology and Immunohistochemistry for Parenchymal and Vascular Amyloid Pathology, for Intracellular Amyloid, and for Microhemorrhages
Quantitative analysis of amyloid was performed by staining with thioflavinS, with X34 and by immunostaining with biotin-labeled monoclonal antibody (mAb) 3D6 (Aβ-epitope 1 to 5) (Innogenetics, Ghent, Belgium) on 40-μm sagittal free-floating vibratome sections. For each method and per mouse brain, three well-defined sections located between bregma lateral 1.44 to 2.16 were selected. Dense amyloid plaque-load was quantified by thioflavinS and X34-staining33,37,38 while immunohistochemistry with mAb 3D6 was used to measure total amyloid load, ie, dense and diffuse plaques combined, which is ∼10 times higher than the dense plaque load. Brain sections were rinsed in PBS and quenched for endogenous peroxidase by incubation at room temperature for 15 minutes in 1.5% H2O2 in methanol/water (50%, v/v). After blocking with PBS containing 0.1% Triton X-100 and 10% fetal calf serum, sections were incubated with the primary antibody overnight. After rinsing and incubation with the avidin-biotin-peroxidase complex (Vectastain Elite ABC; Vector Laboratories, Burlingame, CA) the signal was developed with 3,3′ diaminobenzidine. Sections were counterstained with hematoxylin.
Quantification of dense and total plaque load was performed routinely in the subiculum, and in other brain regions as indicated. Microscopic images were recorded and digitalized with a 3 CCD color video camera, and analyzed with dedicated software (Leica QWin Standard V2.8; Leica, Cambridge, UK). The plaque-load in the subiculum of individual mice was expressed as percentage of total surface of brain tissue covered by amyloid plaques. The amyloid load in blood vessels in cortex and thalamus was quantified by counting thioflavinS-positive vessels per section (see also Results section).
Immunohistochemical staining for all cellular amyloid peptides, and for APPm and C99 was performed with mAb 6E10 (Aβ-epitope 1 to 16) (Chemicon, Temecula, CA), whereas for intracellular amyloid peptides we used the polyclonal antibody PanAβ (Aβ-epitope 15 to 30) (Calbiochem, San Diego CA) by standard procedures as described, using diaminobenzidine as chromogen.33 The PanAβ antibody did not react with APP or its metabolites in Western blotting (results not shown).
Cortex and thalamus were analyzed histologically for the presence of microhemorrhages by Prussian Blue or Perls iron-staining as described.17 Briefly, 40-μm vibratome sections were mounted, dried, and incubated for 60 minutes with 23.6 mmol/L potassium ferrocyanide in 0.12 N HCl. After rinsing three times in water, sections were counterstained with nuclear fast red (Vector Laboratories). Additional brain sections were analyzed by incubation with 3,3′ diaminobenzidine subsequent to quenching of endogenous peroxidase. As positive controls we stained sections of brain and spleen of very old APP[V717I] single or APP[V717I]×PS1[A246E] double-transgenic mice (27 to 30 months of age).33,39
Analysis of Tau Phosphorylation
Biochemical analysis of phospho-epitopes on endogenous protein tau was performed by Western blotting as described.28,29,40 Briefly, total homogenate was resolved by SDS-PAGE (8% Tris-glycine, Invitrogen) and after electrotransfer to nitrocellulose membranes (Hybond-ECL, Amersham Biosciences), total protein tau and selected phospho-epitopes were detected, respectively, with monoclonal antibodies Tau5, AT8, and AD2. Antibody AT8 (Innogenetics) recognizes phosphorylated serine-202 and threonine-205 whereas AD2 (kindly provided by A. Delacourte, Lille, France) is specific for phosphorylated serine-396/404. Immunohistochemical analysis with AT8 and AD2 was performed on five sections per mouse brain as described.28,29
Statistical Analysis
Variance analyses of the histochemical and biochemical data were performed by analysis of variance tests (SPSS version 12.0; Tukey HSD) and nonparametric Kruskal-Wallis tests (Mann-Whitney U) at a significance level of P = 0.05. Unless otherwise indicated in the figures, double-transgenic strains were compared to the parental APP[V717I] mice and triple combinations were compared to the APP[V717I] × PS1[A246E] double-transgenic mice. Correlations were analyzed by Spearman’s correlation at a significance level of P = 0.01.
Results
Generation and Major Characteristics of the Six Transgenic Mouse Strains
To analyze the synergistic effects of ApoE4 on amyloid pathology and eventually on tauopathy, we have generated and compared six genotypically different transgenic mouse models with amyloid pathology, ie, the parental single APP[V717I] mice and three double-transgenic and two triple-transgenic strains (Table 1). The aim was to express human ApoE4 either in neurons or in glia in combination with neuronal expression of mutant APP[V717I] alone and in addition with mutant PS1[A246E] (Table 1).
The heterozygous APP[V717I] and APP[V717I]×-PS1[A246E] double-transgenic mice and the homozygous PS1[A246E] transgenic mice were generated before in the FVB/N genetic background with all of the transgenes expressed specifically in neurons by the mouse thy1 gene promoter. These mice have been extensively characterized as robust models for the parenchymal and vascular amyloid pathology in AD and for the contribution of mutant PS1.32–34,37,41,42
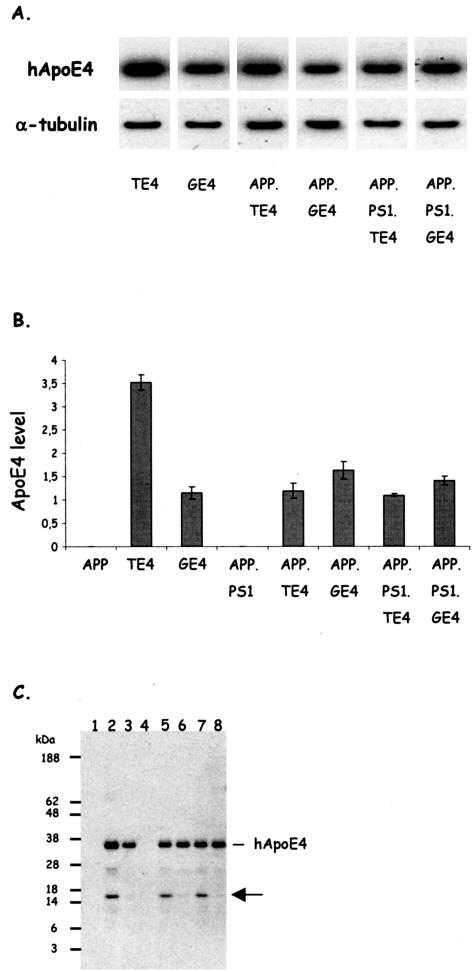
The other parental transgenic mouse strains that overexpress human ApoE4 either in neurons or in glial cells driven, respectively, by the mouse thy1gene promoter and the GFAP gene promoter, were characterized previously28,29 and were back-crossed into the C57BL6 genetic background (n > 11), respectively, to heterozygous and homozygous status at the onset of the current study. The expression of human ApoE4 in neurons of heterozygous offspring of thy1-ApoE4 mice (TE4 mice) or in glia in offspring of GFAP-ApoE4 mice (GE4 mice) resulted in very similar overall levels of ApoE4 in brain (Figure 1). Thereby, the strains are confirmed to differ not quantitatively in transgenic human ApoE4 levels, but only qualitatively in the cell type of expression.28,29
Figure 1.
Expression levels of human ApoE4 and neuron-specific fragmentation. A: Western blotting of total brain extracts of mice with the six genotypes as indicated for human ApoE and for murine α-tubulin, as loading control. B: Concentrations of human ApoE4 in brain of the six strains of transgenic mice (mean ± SEM, n = 4) generated and analyzed in this study and of the two parental strains, ie, thy1-ApoE4 and GFAP-ApoE4.28,29 Human ApoE4 is evidently absent in the parental APP mice and in the APP.PS1 mice. Note that for all of the other genotypes the mice are hemizygous for ApoE4, except for the parental thy1-ApoE4 mice that are homozygous for ApoE4. C: Western blotting showing human ApoE4 and the 16-kd proteolytic fragment (arrow) in the brain of the following transgenic mice: 1, APP; 2, thy1-ApoE4; 3, GFAP-ApoE4; 4, APP.PS1; 5, APP.TE4; 6, APP.GE4; 7, APP.PS1.TE4; 8, APP.PS1.GE4. Note that the 16-kd fragment is only observed in brains of mice that express human ApoE4 in neurons, and that these also appear to contain a fragment of ∼26 kd (see Results for details).31
Importantly, the genetic constellation of the four parental single transgenic mouse strains, ie, thy1-APP, thy1-PS1, thy1-ApoE4, and GFAP-ApoE4, allowed us to generate for each of the six genotypes, the F1 offspring all with an identical mixed genetic background of C57BL6 × FVB/N. Moreover, all of the double- and triple-transgenic mice studied here were heterozygous for all of the human transgenes that they express, similar to previous studies of single- and double-amyloid transgenic mice based on the parental APP[V717I] mice.32–35,37,41,43 The neuronal expression of the human PS1[A246E] transgene is so high that even in hemizygous mice the endogenous PS1 gene product is completely replaced by the mutant human PS1 transgene product due to titering out the needed and limiting co-factors nicastrin, Aph2 and Pen1.34,35,44,45
Based on the characteristics of the parental strains, we analyzed mice from the six genotypes at ages of 4, 6, 8, and 15 months to define the progressive effects of the differential expression of ApoE4 in neurons versus glial cells on the amyloid pathology and eventually on induction of tauopathy. We analyzed biochemically and histochemically a total of 112 transgenic mice, with three to four mice per age point and per genotype, and we restricted the analysis to female mice.
Expression Levels of Human ApoE4 and Neuronal Proteolytic Fragmentation
Because we aimed primarily at defining the consequences of expression of human ApoE4, we first demonstrated that very comparable levels of human ApoE4 were present in total brain extracts in all strains of transgenic mice, independent of the neuronal or glial origin of ApoE4 (Figure 1). The parental thy1-ApoE4 transgenic mice were maintained as a homozygous strain, resulting in human ApoE4 levels that are approximately double those in the other strains, which are all hemizygous for human ApoE4. The parental thy1- and GFAP-ApoE4 mice were used only for generating the F1 multiple transgenic offspring (Table 1) and are included only in the quantitative analysis of ApoE4 for the sake of completeness.
Interestingly, Western blotting with the polyclonal antibody to human ApoE revealed in addition to the mature 34-kd ApoE4 human protein, a 16-kd proteolytic fragment (Figure 1C, arrow). This fragment accumulated in the brain of thy1-ApoE4 transgenic mice and in the double- and triple-transgenic strains derived from it, as opposed to its near complete absence in the brain of GFAP-ApoE4 mice and in their double and triple derivatives (Figure 1C, arrow). Some additional, but less abundant fragments of 22 to 28 kd were also evident in brain extracts of the thy1-ApoE4 mice (results not shown). These data corroborate recent findings on the fragmentation of ApoE in neurons as opposed to glia,31 an aspect that was not further analyzed here.
Neuronal ApoE4 Promotes Diffuse Plaques in the Parenchyma of Subiculum and Cortex
Onset of diffuse amyloid plaque deposition is somewhat variable in individual APP mice, but becomes notable at approximately age 10 to12 months. Diffuse and subsequently dense amyloid plaques develop robustly in all old APP[V717I] transgenic mice that we have analyzed to date, regionally appearing first in subiculum and entorhinal cortex, progressively followed by neocortex and finally thalamus by age 16 to 18 months.32–35,37,41 In APP.PS1 double-transgenic mice, amyloid pathology develops more early (∼6 months) and progresses more rapidly,33,34 like in other comparable double-transgenic mouse models.46,47 Vascular amyloid pathology follows the parenchymal pathology with a delay of some months in both the APP single and APP.PS1 double-transgenic mice.32–35,37,41
Here, we comparatively analyzed the parental APP and APP.PS1 single- and double-transgenic mice to their double- and triple-transgenic combinations containing either thy1-ApoE4 or GFAP-ApoE4 transgenes (Table 1 and 2). The primary aim was to define differential and progressive effects of neuronal and astrocytic ApoE4 on amyloid pathology in aging female mouse brain. The patterns of timing and intensity of amyloid pathology in the cortex are very similar to those in the subiculum and are therefore not presented separately.
Table 2.
Overview of Amyloid Pathology in Transgenic Mice of the Six Genotypes Analyzed in This Study
| Genotype | Plaques subiculum/cortex
|
Plaques thalamus
|
CAA
|
Aβ
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Diffuse | Dense | Diffuse | Dense | Cortex | Thalamus | 40 | 42 | 42/40 ratio | |
| APP.PS1 | ++++ | ++++ | +++ | +++ | ++ | ++ | +++ | +++ | ++ |
| APP.TE4 | ++ | = | ++ | ++ | ++ | ++ | ++ | ++ | + |
| APP.GE4 | = | = | + | + | + | ++ | + | + | + |
| APP.PS1.TE4 | +++ | +++ | +++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ |
| APP.PS1.GE4 | +++ | ++ | +++ | ++++ | ++++ | +++ | ++++ | ++++ | ++ |
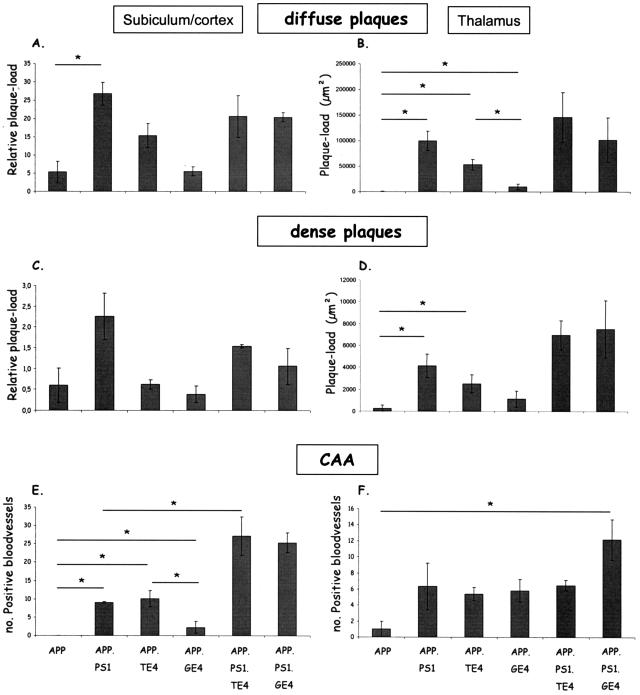
The intensity of each pathological parameter in the indicated brain regions is expressed relative to the pathology in the parental APP[V717I] transgenic model as represented by a proportional number of + signs, with = indicating no difference.
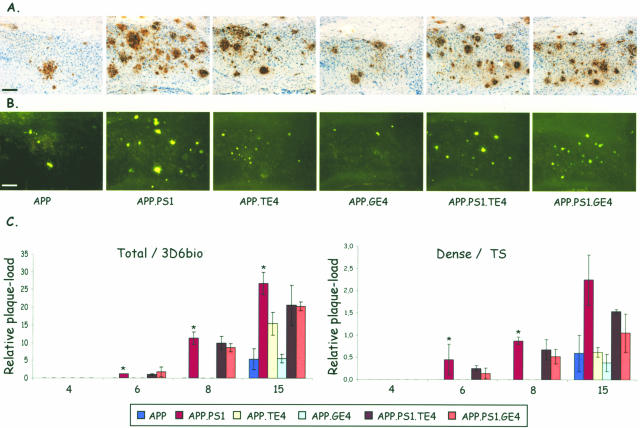
Immunostaining with mAb 3D6 and histological staining with thioflavinS and X34 demonstrated total and dense core amyloid plaques in subiculum (Figure 2, A and B) and cortex of all old APP mice (15 months). In age-matched APP.PS1 double-transgenic mice, the dense and total amyloid plaque load was approximately four times higher (Figure 2) and, as expected, diffuse and dense amyloid deposition was already evident at age 6 months in APP.PS1 double-transgenic mice (Figure 2C). The early pathology is consistent with increased levels of amyloid peptides, combined with a higher Aβ42/40 ratio.33,34 Total plaque-load measured with mAb 3D6 can be equated to diffuse plaque load because it is approximately an order of magnitude higher than dense plaqueload in all genotypes and at all time points in this and previous studies.
Figure 2.
Amyloid plaque-load in subiculum. A and B: Staining with mAb 3D6 (A) and with thioflavinS (B) of the subiculum of the six transgenic mouse strains (age, 15 months). C: Quantification of relative total and dense plaque-load expressed as surface area covered by plaques (percent; mean ± SEM, n = 3 or 4 per genotype and per time point) in the six genotypes at ages 4, 6, 8, and 15 months as indicated on the ordinate. Asterisks denote statistical differences at P < 0.05. Scale bars, 100 μm.
Neuronal co-expression of ApoE4 with mutant APP produced in the oldest APP.TE4 double-transgenic mice (15 months) a trend to increased total plaque-load (P = 0.075), without appreciably affecting the age of onset because no diffuse or dense plaque pathology was observed at age 8 months (Figure 2C). In contrast, the glial ApoE4 expression in APP.GE4 mice did not affect the amyloid pathology, neither in age of onset nor in intensity of diffuse or dense plaques (Figure 2C).
The three genotypes that contained mutant human PS1 (one double- and two triple-transgenic strains) were marked by a similar early onset (at 6 months) of amyloid pathology in the subiculum (Figure 2C) and by a similar intensity in total amyloid plaque-load (Figure 2, A and B; panels 2, 5, and 6), which was evident at all ages. Overall, the neuronal or glial overexpression of human ApoE4 in combination with mutant APP and mutant PS1 neither affected the age of onset nor the dense plaque-load (thioflavinS or X34) (Figure 2C). If anything, human ApoE4, rather independent of its cellular origin, tended to reduce the increase in amyloid pathology provoked by mutant PS1 with a most marked reduction in dense plaques at age 15 months in APP.PS1.GE4 mice (Figure 2C, right).
The data demonstrated not only that mutant PS1 was the much stronger mediator of amyloid pathology than human ApoE4, but moreover pointed to attenuating, differential effects of neuronal versus glial origin of ApoE4. An intracellular interaction of ApoE4 with APP or with Aβ, inside the producing neurons, could alter trafficking and relocation of APP, its C-terminal fragments, and/or intracellular amyloid peptides.48–51 Therefore, we analyzed all of the old transgenic mice (age 15 months) for intra-neuronal accumulations of amyloid by immunohistochemistry with two different antibodies (6E10 and PanAβ). Although both antibodies reacted with neurons in the subiculum, dendate gyrus, and neocortex (Figure 3E), neither antibody revealed major differences among the six genotypes (results not shown) indicating that accumulations of amyloid peptides were not different by intraneuronal ApoE4 expression.
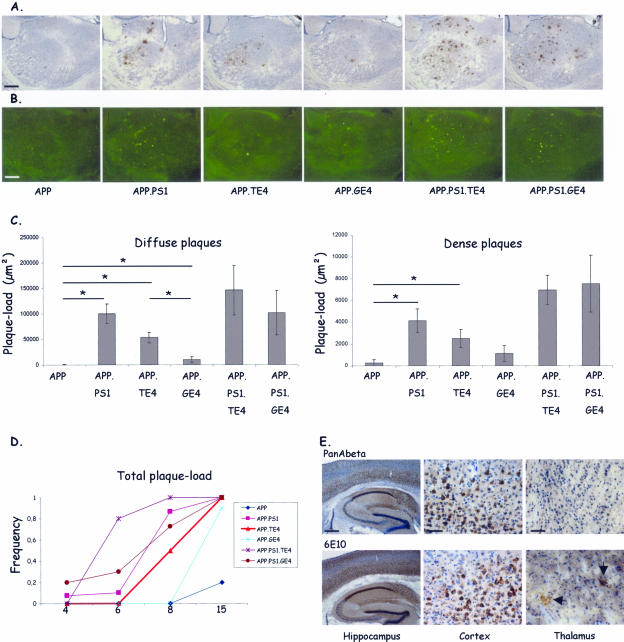
Figure 3.
Amyloid pathology in thalamus. A and B: Staining with mAb 3D6 (A) and with thioflavinS (B) of the subiculum of the six transgenic mouse strains (age, 15 months). C: Quantification of absolute levels (μm2) of plaque-load (mean ± SEM) in 15-month-old mice. *P < 0.05. D: Frequency of presence of diffuse plaques (mAb 3D6) in thalamus: 0 = not present; 1 = present in all three sections analyzed per mouse. E: Immunostaining with polyclonal antibody PanAβ and with mAb 6E10 detects intraneuronal amyloid in hippocampus and cortex but not in thalamus of APP.PS1.TE4 triple-transgenic mouse. Arrows indicate location of amyloid plaques. Scale bars: 200 μm (A, B); 400 μm (E, hippocampus); 50 μm (E, cortex and thalamus).
ApoE4 Accelerates Amyloid Pathology in the Thalamus Similar to Mutant PS1
The very similar patterns of timing and intensity of amyloid pathology in subiculum and cortex contrasted with that in the thalamus. Neither immunohistochemical nor thioflavinS staining revealed any appreciable amyloid pathology in the thalamus of the parental APP[V717I] transgenic mice at age 15 months (Figure 3) as documented before.33 In contrast, in the double-transgenic, and particularly in both types of triple-transgenic mice, an important accumulation of diffuse as well as senile plaques was evident in the thalamus (Figure 3, A and B).
Surprisingly, thalamic deposition of amyloid plaques in contrast to thalamus CAA (detailed in the next section) was promoted more extensively by neuronal than by glial ApoE4 at age 15 months, although not as prominent as by mutant PS1 (Figure 3C) (Table 2). In the thalamus, in contrast to the cortex and subiculum (Figure 2), human ApoE4 independently of its cellular origin, even increased diffuse and dense amyloid plaques synergistically with mutant PS1 in triple-transgenic mice (Figure 3C). Moreover, a marked earlier age of onset was evident in the triple transgenic and also in the APP.TE4 double-transgenic mice. Immunoreactive plaques were already present in the thalamus of APP.TE4 mice at age 8 months, as opposed to the plaque-free thalamus of age-matched parental APP and APP.GE4 mice (Figure 3D). The diffuse nature of these plaques was evidenced by the negative reaction with thioflavinS or X34 in the thalamus before the age of 15 months (results not shown). Again, neuronal ApoE4 was more potent than glia-derived ApoE4 in inducing early diffuse amyloid pathology. Remarkably, this was typical for the thalamus, and not observed in subiculum or cortex, demonstrating important brain-regional differences in ApoE4-mediated amyloid metabolism or disposition.
To delineate in the thalamus, eventual intracellular differences in the contribution to or the origin of the deposited amyloid, we analyzed immunohistochemically with antibodies PanAβ and 6E10 and concentrated on the detection of intraneuronal reaction for Aβ with PanAβ and for APP metabolites that carry the amyloid sequence, ie, mainly APPm, C99, and Aβ. Remarkably, neither antibody revealed important intracellular signals in the thalamus, not even in the APP.PS1.TE4 triple-transgenic mice (Figure 3E). We conclude that local production of Aβ is minimal in the thalamus, and the amyloid depositions there must be primarily due to import from other brain regions, and must be facilitated by ApoE lipoproteins.
CAA in Cortex and Thalamus Is Differentially Affected by Neuronal and Glial ApoE4
Conform our previous studies demonstrating that CAA develops later than parenchymal amyloid deposits, the current analysis by staining with thioflavinS and X34, revealed CAA predominantly in the old mice of all genotypes (Figure 4, A–D). CAA was quantified by counting the number of thioflavinS-positive blood vessels in similarly positioned and equally spaced brain sections (Figure 4E) (see Materials and Methods).33,37 In single APP transgenic mice of 15 months, CAA was evident in the thalamus but not yet in the cortex that is attained only later, ie, at age 16 to 18 months with dramatic increases up to age 24 months.33,37 As expected, mutant PS1 increased both cortical and thalamic CAA in APP.PS1 double-transgenic mice at age 15 months (Figure 4E).33,37
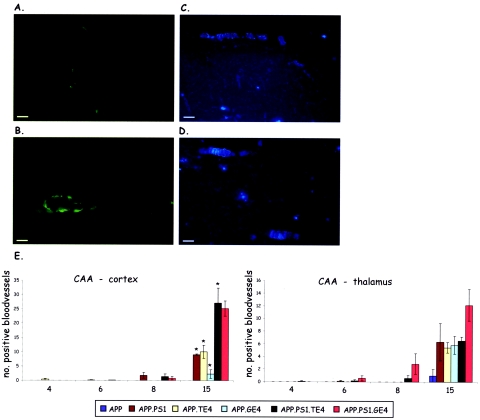
Figure 4.
Amyloid angiopathy (CAA) in cortex and thalamus. A–D: Representative images of vascular amyloid in cortex (A, C) and thalamus (B, D) of an APP.PS1.GE4 triple-transgenic mouse stained with thioflavinS and with X34. E: Quantification of CAA in cortex and thalamus, expressed as number of positive blood vessels per three sections (mean ± SEM) of the six genotypes in function of age (4, 6, 8, and 15 months). Asterisks denote statistical significance at P < 0.05. Scale bars: 50 μm (A, B); 100 μm (C, D).
Surprisingly, neuronal but not glial expression of human ApoE4 significantly increased CAA in cortex of APP.TE4 mice as pronouncedly as mutant PS1 in age-matched APP.PS1 mice (Figure 4E, left; groups of 15 months). In the thalamus, on the other hand, glial ApoE4 promoted thalamic CAA as prominently as neuronal ApoE4 and as mutant PS1 (Figure 4E, right; groups of 15 months). This differential regional effect paralleled closely the total plaque-load in subiculum and cortex, which was also promoted less by glial than by neuronal ApoE4 (compare Figures 2C and 4E, left panels; age group, 15 months). In contrast, in the thalamus the glial ApoE4 provoked very similarly CAA levels as neuronal ApoE4, clearly in excess of its effect on plaque-load (compare Figures 2C and 4E, right panels; age group, 15 months).
For ease of comparison, we have regrouped all of the quantitative pathological data in the oldest mice (age group, 15 months) of all genotypes (Figure 5, Table 2). Thereby, additional important regional differences in the response to neuronal or glial synthesis of ApoE4 were revealed. Remarkably, in conjunction with mutant PS1, both neuronal and glial ApoE4 dramatically increased cortical CAA in both types of triple-transgenic mice (Figure 4E, left, age 15 months, top right bars; Figure 5) while thalamic CAA increased preponderantly due to glial ApoE4 (Figure 4E, right, age group 15 months, top right bar; Figure 5). Since this difference was already evident as a trend at younger age (Figure 4E, right, age groups 6 and 8 months, top right bars), the underlying mechanisms and the pathological repercussions are not anecdotal and concluded to be important.
Figure 5.
Amyloid pathology in transgenic mice of the six genotypes at age 15 months. For clarity and ease of comparison, this figure compiles all data of the amyloid pathology, ie, diffuse and dense plaques and angiopathy as indicated, in the oldest transgenic mice with the six genotypes (age, 15 months). The left panels represent subiculum and cortex, and right panels contain data for the thalamus as indicated. Asterisks denote statistical significance at P < 0.05 between groups connected by the horizontal bars (mean ± SEM).
CAA Is Not Associated with Microhemorrhages
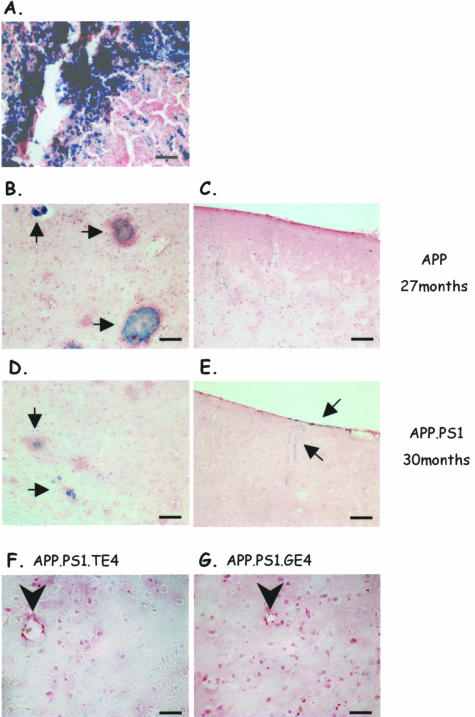
The presence of CAA and its promotion by ApoE might increase the incidence of hemorrhages, and we therefore analyzed all of the transgenic mice of the six genotypes in the oldest age group for the presence of microhemorrhages by Perl’s iron staining.17,25,52–54 As positive control we reanalyzed brain sections of very old APP and APP.PS1 transgenic mice that had been identified by MRI in vivo and were confirmed by postmortem MRI and by histochemistry to show discrete but very limited microhemorrhages in the thalamus (Figure 6, B–E).39 In addition, we stained sections of mouse spleen to validate the histochemical method, which faithfully identified accumulation of iron (Figure 6A).
Figure 6.
Histochemical staining for non-heme iron as index of microhemorrhages. A: Representative image of mouse spleen stained for non-heme iron (blue) as positive control. B and C: Microbleedings (arrows) in thalamus (B) but not in cortex (C) of very old APP transgenic mouse (27 months) included as positive control.39 D and E: Microbleedings in thalamus (D, arrows) and cortical meninges (E, arrows) of a very old APP.PS1 mouse (30 months) included as positive control. F and G: Staining for non-heme iron of thalamus of an APP.PS1.TE4 and APP.PS1.GE4 mouse. Note that no microhemorrhages were observed in thalamus or cortical meninges of any of the old transgenic mice analyzed in the current study (see text for details and discussion). Arrowhead, blood vessel. Scale bars: 50 μm (thalamus); 100 μm (cortex).
In none of the old mice (15 months) of the six transgenic genotypes that were generated and analyzed in the current study, did we detect any convincing signs of the presence of extensive microhemorrhages, either in the cortical meninges or in the thalamus (Figure 6, F and G). Additional brain sections from all transgenic mice were analyzed by enhancing the Perls staining by incubation with diaminobenzidine (see Materials and Methods) only to confirm the negative outcome of the overall analysis (data not shown). We therefore conclude that in all of the six transgenic mouse strains studied here, neither minor nor major hemorrhages were a significant pathological problem, despite the massive occurrence of amyloid angiopathy in thalamus and in other brain regions.
CAA in Cortex but Not in Thalamus Correlates with Soluble Aβ Levels
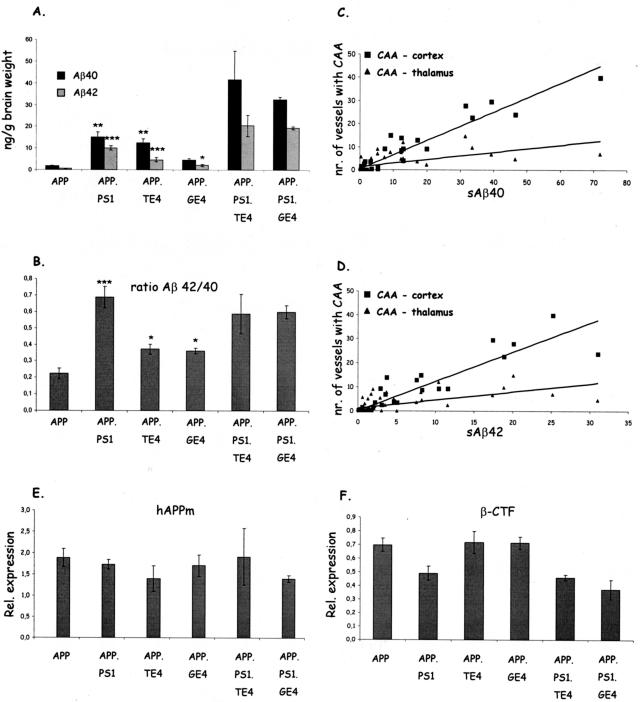
The levels of soluble Aβ peptides, measured by specific sandwich ELISA, were highest in the brain of mice in the old age group, and significantly higher in the complex genotypes relative to the parental APP mice (Figure 7A). In the APP.PS1 mice, the co-expression of mutant PS1 with mutant APP significantly increased both the absolute levels of Aβ40 and Aβ42 (P < 0.01 and P < 0.001, respectively) as well as the Aβ42/40 ratio (P < 0.01) relative to the parental APP mice (Figure 7, A and B). Increased levels were evident earlier in life (results not shown) consistent with the earlier appearance of the amyloid pathology (6 months) in APP.PS1 double-transgenic mice (Figures 2 to 4),33,34,35 and as reported in comparable mouse models.42
Figure 7.
Biochemical analysis of amyloid peptides and processing of APP in brain. A and B: Levels of soluble Aβ40 and Aβ42 (A) and the Aβ42/40 ratio (B), in brain of old transgenic mice (15 months) measured by specific sandwich ELISAs (mean ± SEM, n = 3 or 4). Asterisks denote statistical significance at *P < 0.05, **P < 0.01, ***P < 0.001. C and D: Correlation between Aβ40 (C) and Aβ42 (D) levels with cortical and thalamic CAA over all genotypes analyzed in this study. Spearman rho: (Aβ40 versus CAA-cortex: r = 0.766), (Aβ40 versus CAA-thalamus: r = 0.606), (Aβ42 versus CAA-cortex: r = 0.821), and (Aβ42 versus CAA-thalamus: r = 0.657); all P < 0.01. E and F: Levels of total mature human APP (E) and of β-CTF (C99) (F) in brain of all transgenic mice at age 15 months.
In the APP.TE4 mice, the neuronal expression of human ApoE4 produced a nearly similar rise in soluble Aβ40 as mutant PS1 (P < 0.01), with a less pronounced, significant increase in Aβ42 levels (P < 0.001) (Figure 7A). This contrasted with much lower Aβ40 and Aβ42 levels produced by glial expression of human ApoE4 in the APP.GE4 mice (Figure 7A). Although both neuronal and glial ApoE4 elevated the Aβ42/40 ratio significantly (P < 0.05) relative to the parental APP mice, the increase was less than that inflicted by mutant PS1 (Figure 7B). An important further difference with mutant PS1 was the fact that either type of ApoE4 expression failed to increase Aβ levels at young age (data not shown) nor advanced the onset of amyloid pathology (Figures 2 and 4). Combined, these data indicate that ApoE4 and mutant PS1 operated by different mechanisms on the amyloid metabolism.
Unexpectedly high levels of soluble Aβ40 and Aβ42 were observed in both the triple-transgenic combinations whereby PS1 dominated and obscured any difference between neuronal and glial ApoE4 with respect to absolute levels (Figure 7A) and Aβ42/40 ratio (Figure 7B). This outcome paralleled completely the amyloid pathology in subiculum, cortex, and thalamus described above in the triple-transgenic mice with the notable exception of CAA in the thalamus (Table 2, Figure 5). This was confirmed by analysis of the quantitative data of amyloid plaques and CAA for all of the individual mice over all genotypes for all possible correlations with the levels of soluble Aβ40 and Aβ42 and their ratio. The correlation observed for CAA in the cortex with the concentrations of Aβ40 and Aβ42 was higher (r = 0.766 and 0.821, respectively), than for CAA in the thalamus (r = 0.606 and 0.657, respectively) but both were significant (P < 0.01) (Figure 7, C and D).
Neuronal ApoE4 Did Not Affect Proteolytic Processing of APP or Increase Phosphorylation of Tau
The observation that ApoE4 increased the concentrations of soluble Aβ in brain more strongly when expressed in neurons than in glia in double-transgenic mice (Figure 7A) prompted us to compare the proteolytic processing of APP. We measured the steady-state levels of the major metabolites of APP, ie, membrane-bound APPm, soluble secreted APPs, and the α and β C-terminal fragments (CTF) (C83 and C99, respectively) in brains of all transgenic mice at age 15 months.
The steady-state levels of APPm were similar in all genotypes (Figure 7E), as were the levels of the α-CTF (results not shown). The best argument that neither neuronal nor glial expression of ApoE4 interfered with the proteolytic processing of APP was the similar levels of β-CTF, very similar also to those in the parental APP mice (Figure 7F). Mutant PS1 is known to increase γ-secretase cleavage of β-CTF to produce more Aβ42 peptides (Figure 7A), which was also faithfully reflected in higher Aβ42/40 ratios (Figure 7B) and in lower β-CTF levels in the three genotypes containing mutant PS1 (Figure 7F). The comparable levels of β-CTF in the APP.PS1 mice and in both the triple genotypes further corroborated the conclusion that mutant PS1 increased amyloidogenic processing of APP, whereas ApoE4 did not, not even when co-expressed in the same cells, casu quo neurons in the thy1-ApoE4 combinations.
Finally, we analyzed the phosphorylation of endogenous murine protein tau biochemically and immunohistochemically, with the phosphorylation-specific antibodies AT8 and AD2.40 Both tau epitopes were detected immunohistochemically as a somatodendritic staining of neurons throughout all cortical layers with highest intensity in frontal cortex (results not shown). Occasional neurons stained in CA1 and CA3 and in the molecular layer and hilus of the dentate gyrus, while interestingly also most neurons in the thalamus contained both epitopes. No major reaction was evident with dystrophic neurites in amyloid plaques. Importantly, and confirmed biochemically by quantitative Western blotting, no differential effects were observed between the six genotypes (results not shown). We conclude that moderate neuronal or glial overexpression of human ApoE4 in brain of transgenic mice did not affect the proteolytic processing of APP or induced hyperphosphorylation of endogenous protein tau.
Discussion
The observation that ApoE can be expressed not only in glia but also in neurons of cortex and hippocampus has fuelled the hypothesis that the ε4 allele could increase neuronal synthesis of ApoE4 and thereby promote the earlier development of AD pathology.27,55 In the current study, we addressed experimentally the contribution of ApoE4 to the amyloid and tau pathology in a mouse model for AD. We generated and compared a unique set of six transgenic mouse strains to differentiate the repercussions of neuronal versus glial expression of human ApoE4 on parenchymal and vascular amyloid pathology, and eventually on the emergence of tauopathy.
The latter aspect is most easy to discuss because no pertinent tauopathy became evident in any of the multiple transgenic mouse strains even at age 15 months, which thereby do not differ appreciably from the parental APP[V717I] transgenic mice.33 In a previous study, we demonstrated hyperphosphorylation of tau and progressive axonopathy with Wallerian degeneration, motor defects, and muscle wasting in homozygous thy1-ApoE4 transgenic mice, an outcome recently confirmed independently.28,29,31 The current hemizygous ApoE4 combination models were designed explicitly to 1) avoid the motor defects, 2) to express similar levels of neuronal and glial ApoE4, and 3) to have an identical genetic background.
The ApoE4 strains differ only qualitatively in the cell type of expression and not quantitatively in the level of ApoE4, which is a usual and considerable problem with transgenic mice. Significantly, the evident gene-dosage effect of neuronal ApoE4 demonstrates that expression of human ApoE4 in neurons, even to the same level as in glia cells, affects only the amyloid pathology, but not by interference with processing of APP, and fails to inflict a tauopathy.
The pathological consequences of mutant PS1 in combination with ApoE4 also need not to be discussed in great detail because it is evident that mutant PS1 is a much stronger booster of the amyloidogenic metabolism than ApoE4 in transgenic mice, recapitulating very closely the situation in the corresponding genetic variants of human AD patients. The essential effect imposed by mutant PS1, ie, increased production of amyloid peptides and particularly of Aβ42, was not appreciably modified by ApoE4 even when co-expressed in the same cells, casu quo neurons. Further discussion is restricted here to the most salient aspects revealed by the current analysis, ie, 1) cortical versus thalamic amyloid pathology, 2) parenchymal versus vascular pathology, and 3) potential cellular mechanisms involving ApoE.
Cortical versus Thalamic Amyloid Pathology
In addition to the well-known diffuse and dense plaques in cortex and subiculum in the parental APP[V717I] mice, considerable amyloid pathology was produced in the thalamus of all double- and triple-transgenic mice analyzed here (Figure 3, Table 2). Amyloid plaques have been observed in the thalamus by us and others in APP transgenic mice but this brain region is attained much later than the cortex or the hippocampal formation.33,53,56,57
The mouse thy1 gene promoter construct is devoid of expression in the nuclei of the thalamus28,33,35,58 with the possible exception of a limited population of neurons in the zona incerta.40 Neither APP nor intracellular Aβ was detectable inside neurons in the thalamus, as opposed to neurons in the hippocampal formation and in cortex, ie, the brain regions where amyloid is deposited earlier in life. Because the amyloid peptides appear not to be synthesized locally in the thalamus, they must be produced elsewhere and imported into the thalamus. Thalamic amyloid appears mainly in later stages of the pathology, and interestingly, was considerably augmented and brought forward in time by neuronal ApoE4, eg, from age 15 to 8 months in APP.TE4 double-transgenic mice. In contrast, ApoE4 synthesized by glia was present at the same overall concentrations and affected only marginally the thalamic plaque-load, but was as affective as neuronal ApoE4, and remarkably also as mutant PS1 in promoting thalamic CAA.
The problem of how plaques develop in the thalamus without local cellular synthesis of APP or Aβ, implies that these need to be transported from other regions, ie, likely cortical or hippocampal areas. Moreover, this transport is affected or even mediated by ApoE, possibly by direct binding of Aβ to ApoE-lipoproteins59, facilitating diffusion-mediated drainage, interstitially and/or into the cerebrospinal fluid. Direct Aβ to ApoE lipoprotein interactions occurring during or after co-lateral secretion and reuptake could influence each others fate in terms of diffusion and degradation. Besides possible ApoE isoform effects, not addressed in this study, differences in the composition of neuronal and glial ApoE lipoproteins, secreted or endocytosed, must be considered.
Although the transport mechanisms in brain of amyloid peptides are not understood, the implications of these findings for the pathology in AD are important. Thalamic nuclei are functionally much more than simple relay stations of sensory input to and from the cortex. Direct projections from thalamic nuclei to the hippocampal region and cortex modulate hippocampal neurons.60–62 In addition, projections from the entorhinal cortex to thalamic nuclei confirm a thalamus-limbic-cortex connection that has been implicated in memory and learning.63 Disturbances of thalamic signals could affect processes in the hippocampus and cortex, and vice versa. Obviously, in vivo follow-up studies are required to define the impact of thalamic amyloid pathology on AD-related defects of cognition and neurodegeneration, and on their progression with age.
Parenchymal versus Vascular Amyloid Pathology (CAA)
Amyloid is deposited abluminally in the wall of blood vessels in most if not all AD patients21,22 and while strongly associated with ε4 alleles23 the mechanism remains elusive. CAA is recapitulated, albeit dallied relative to plaques, in our parental APP[V717I] transgenic mice at old age33,37,39 as in other APP transgenic mice.13,17,64 In other models, ApoE deficiency prevented CAA while ApoE overexpression shifted parenchymal to vascular amyloid (see Introduction).17,18,25 Our data demonstrate that also the cellular origin of ApoE is important, and moreover that different brain regions are affected differently. Importantly, the regional differences due to ApoE4 underline that CAA appeared subsequent to the amyloid plaques in all genotypes analyzed, indicating that CAA is secondary to and results from the parenchymal amyloid deposits.
Thalamic CAA was already considerable at 8 months in the APP.PS1.GE4 triple-transgenic combination, which is approximately a year earlier than in the parental APP[V717I] transgenic mice. The anatomical or functional parameters that underlie thalamic CAA are not known and require further analysis. The current observations are, however, reminiscent of our recent study of old APP[V717I] mice by combination of histochemistry and in vivo MRI, allowed us to identify the thalamus as the preferential defective brain region with respect to amyloid-laden blood vessels.39 In this context, a possible association of CAA with microhemorrhages was noted in transgenic mice17,25,53,65 and in AD patients.66,67 Extensive investigation of all of the current old mice of all genotypes did not reveal appreciable microbleedings or major differences due to genotype, demonstrating that amyloid pathology is neither cause nor consequence of cerebrovascular accidents.
Cellular Mechanisms Involved in Neuronal versus Glial ApoE4
Despite a significant increased Aβ42/40 ratio due to ApoE4 in double-transgenic mice relative to the parental APP mice, all parameters analyzed demonstrated that the APP processing was not disturbed. This is direct proof that even neuronal co-expression of ApoE4 with APP did not affect the balance of amyloidogenic to nonamyloidogenic secretase pathways.2,3 Our data contrast with human ApoE knockin mice wherein delayed amyloid plaque deposition was claimed to be due to modulation of APP processing and elevated Aβ40/42 ratio .25 We neither find a delay, but a promotion of amyloid deposition, nor a decrease, but an increase in Aβ42/40 ratio by ApoE4. Our data are in line with a study demonstrating promotion of parenchymal and vascular amyloid by human ApoE4, without affecting the metabolism of APP.19
We conclude that any modulation of the amyloid pathology by ApoE4 must be ascribed to postsynthetic events, taking place after the amyloid peptides are excised from APP as a consequence of intra- or extracellular interaction with ApoE4 lipoproteins that slow down clearance and degradation of Aβ. Moreover, we demonstrate that the cellular origin of ApoE4 plays an important role in determining the fate of the amyloid peptides.
This does not necessarily imply an exclusive intracellular versus extracellular mode of action for neuronal- and glial-produced ApoE4, respectively. Obviously, in both genotypes ApoE4 lipoproteins become localized not only in the pericellular space, but also inside both cell types, as observed by immunohistochemistry (unpublished results). This complex picture is due to superposition of secretion and endocytosis of ApoE lipoproteins via various members of the low-density lipoprotein receptor family.8,26 In addition, intracellular and axonal transport could explain that ApoE4 affects amyloid deposition in the thalamus without being produced there differently in glia and neurons.
A final cellular aspect pertains to the fragmentation of ApoE demonstrated in AD brain, and claimed to be associated with the ε4 allele.68 In vitro as well as in vivo in transgenic mice, accumulation of truncated ApoE correlated with neurodegeneration and tangle-like intracellular inclusions.68,69 Recently, neuronal ApoE fragmentation in transgenic mice was correlated to increased phosphorylation of tau, in contrast to a negative outcome for astrocytic ApoE.31 Our data corroborate the formation of a 16-kd ApoE fragment in a neuron-specific manner, ie, in thy1-ApoE4 mice and not in GFAP-ApoE4 mice. Although this aspect was not further elaborated on in this study, the phenomenon could be informative for the intraneuronal handling of amyloid peptides in relation to the differential induction of amyloid pathology by neuronal or glial ApoE4. On the other hand, despite increased phosphorylation of protein tau on epitopes AT8 and AD2, neither neuronal nor glial expression of ApoE4 triggered any sign of tauopathy in our study (as opposed to Brecht et al31) not even in the triple combination including mutant APP and PS1 indicating that ApoE4-mediated modulation of amyloid pathology is not synergistic with tauopathy.
In conclusion, our study demonstrates a pronounced differential outcome by neuronal or glial overexpression of human ApoE4 in transgenic mouse brain on the amyloid pathology provoked in first instance by mutant APP[V717I]. Most remarkable is the observation that co-expression of human ApoE4 with APP in neurons affected diffuse amyloid plaques and cortical CAA, not only in hippocampus and cortex, but also in the thalamus, where expression of both transgenes in neurons is nearly absent. In contrast to cortex and hippocampus, the thalamic pathology differs in intensity and in age of onset, underlining that ApoE4 exerted an influence over apparently very different mechanisms that provoke plaques and CAA in cortex and thalamus. Our data support the hypothesis that neuronally synthesized ApoE4 and Aβ interact intracellularly, thereby influencing the cellular routing of Aβ, redirecting them to different brain regions. Finally, the evident modulation of pathology in cortex, hippocampus, and thalamus by endogenous genetic factors is likely to contribute to differences in clinical parameters in AD patients with different genetic make-up.
Footnotes
Address reprint requests to Fred Van Leuven, Ph.D., Dr.Sc., Experimental Genetics Group, LEGT_EGG, K.U. Leuven, Campus Gasthuisberg O&N 06, B-3000 Leuven, Belgium. E-mail: fredvl@med.kuleuven.be.
Supported by the Instituut voor Wetenschappelijk en Technologisch onderzoek, the European Commission 6th Framework Program, the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, the Rooms fund, the Katholieke Universiteit Leuven Special Research Fund (BOF), and KU Leuven Research and Development.
References
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Van Leuven F. Secretases as targets for the treatment of Alzheimer’s disease: the prospects. Lancet Neurol. 2002;1:409–416. doi: 10.1016/s1474-4422(02)00188-6. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;2:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;6:363–375. doi: 10.1124/mi.2.6.363. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer’s disease. C R Biol. 2005;328:119–130. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45:403–409. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer’s disease. Neurobiol Aging. 2005;26:355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Holtzman DM. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci. 2004;23:247–254. doi: 10.1385/JMN:23:3:247. [DOI] [PubMed] [Google Scholar]
- DeMattos RB. Apolipoprotein E dose-dependent modulation of beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. J Mol Neurosci. 2004;23:255–262. doi: 10.1385/JMN:23:3:255. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Granholm AC, Kindy MS, Bhat NR, Greig NH, Lahiri DK, Mintzer JE. Cholesterol and Alzheimer’s disease: clinical and experimental models suggest interactions of different genetic, dietary and environmental risk factors. Curr Drug Targets. 2004;5:517–528. doi: 10.2174/1389450043345335. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:15–21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid beta-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol (Berl) 2000;100:451–458. doi: 10.1007/s004010000263. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters Aβ metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Vitek MP, Xu F, Previti ML, Davis J, Van Nostrand WE. Reducing cerebral microvascular amyloid-β protein deposition diminishes regional neuroinflammation in vasculotropic mutant amyloid precursor protein transgenic mice. J Neurosci. 2005;25:6271–6277. doi: 10.1523/JNEUROSCI.1306-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DB, Dunn E, McKinley DD, Stratman NC, Boyle TP, Kuiper SL, Oostveen JA, Weaver RJ, Boller JA, Gurney ME. Human apolipoprotein E4 accelerates beta-amyloid deposition in APPsw transgenic mouse brain. Ann Neurol. 2001;50:468–475. doi: 10.1002/ana.1134. [DOI] [PubMed] [Google Scholar]
- Lesuisse C, Xu G, Anderson J, Wong M, Jankowsky J, Holtz G, Gonzalez V, Wong PCY, Price DL, Tang F, Wagner S, Borchelt DR. Hyper-expression of human apolipoprotein E4 in astroglia and neurons does not enhance amyloid deposition in transgenic mice. Hum Mol Genet. 2001;10:2525–2537. doi: 10.1093/hmg/10.22.2525. [DOI] [PubMed] [Google Scholar]
- Weller RO, Nicoll JA. Cerebral amyloid angiopathy: pathogenesis and effects on the ageing and Alzheimer brain. Neurol Res. 2003;25:611–616. doi: 10.1179/016164103101202057. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. J Neurol Sci. 2005;229–230:37–41. doi: 10.1016/j.jns.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Chalmers K, Wilcock GK, Love S. APOE ε4 influences the pathological phenotype of Alzheimer’s disease by favouring cerebrovascular over parenchymal accumulation of Aβ protein. Neuropathol Appl Neurol. 2003;29:231–238. doi: 10.1046/j.1365-2990.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology. 2004;62:2116–2118. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-β40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer JD, Demattos RB, McCormick LM, O’dell MA, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G, Holtzman DM. The low-density lipoprotein receptor regulates the level of CNS human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem. 2005;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- Xu PT, Gilbert JR, Qiu HL, Ervin J, Rothrock-Christian TR, Hulette C, Schmechel DE. Specific regional transcription of apolipoprotein E in human brain neurons. Am J Pathol. 1999;154:601–611. doi: 10.1016/S0002-9440(10)65305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Van Dorpe J, Spittaels K, Van den Haute C, Moechars D, Van Leuven F. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am J Pathol. 2000;156:951–964. doi: 10.1016/S0002-9440(10)64963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Van Dorpe J, Bruynseels K, Bronfman F, Sciot R, Van Lommel A, Van Leuven F. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am J Pathol. 2000;157:1495–1510. doi: 10.1016/S0002-9440(10)64788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Tesseur I, Brecht WJ, Xu Q, Mullendorff K, Chang S, Wyss-Coray T, Mahley RW, Huang Y. Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Fish JD, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reversé D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Van Den Haute C, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Van Leuven F. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the London mutant of human APP in neurons. Am J Pathol. 2000;157:1283–1298. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I, Van Dorpe J, Smeijers L, Gilis M, Kuiperi C, Laenen I, Caluwaerts N, Moechars D, Checler F, Vanderstichele H, Van Leuven F. Aging increased amyloid peptide and caused amyloid plaques in brain of old APP/V717I transgenic mice by a different mechanism than mutant presenilin1. J Neurosci. 2000;20:6452–6458. doi: 10.1523/JNEUROSCI.20-17-06452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter I, Reversé D, Caluwaerts N, Ris L, Kuipéri C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow PS, Finley D, Varshavsky A. Enhancement of immunoblot sensitivity by heating of hydrated filters. Anal Biochem. 1986;156:147–153. doi: 10.1016/0003-2697(86)90166-1. [DOI] [PubMed] [Google Scholar]
- Willem M, Dewachter I, Smyth N, Van Dooren T, Borghgraef P, Haas C, Van Leuven F. β-Site amyloid precursor protein cleaving enzyme 1 increases amyloid deposition in brain parenchyma but reduces cerebrovascular amyloid angiopathy in aging BACE × APP[V717I] double-transgenic mice. Am J Pathol. 2004;165:1621–1631. doi: 10.1016/s0002-9440(10)63419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styren SD, Hamilton RL, Styren GC, Klunk WE. X34, a fluorescent derivate of Congo Red: a novel histochemical stain for Alzheimer disease pathology. J Histochem Cytochem. 2000;48:1223–1232. doi: 10.1177/002215540004800906. [DOI] [PubMed] [Google Scholar]
- Vanhoutte G, Dewachter I, Borghgreaf P, Van Leuven F, Van der Linden A. Noninvasive in vivo MRI detection of neuritic plaques associated with iron in APP[V717I] transgenic mice, a model for Alzheimer’s disease. Magn Reson Med. 2005;53:607–613. doi: 10.1002/mrm.20385. [DOI] [PubMed] [Google Scholar]
- Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Van Haesendonck C, Borghgraef P, Van Leuven F. Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem. 2005;280:3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dooren T, Dewachter I, Borghgraef P, Van Leuven F. Transgenic mouse models for APP processing and Alzheimer’s disease: early and late defects. Subcell Biochem. 2005;38:45–63. doi: 10.1007/0-387-23226-5_2. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci USA. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Harris CL, Ratovitski T, Davenport F, Slunt HH, Price DL, Borchelt DR, Sisodia SS. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J Biol Chem. 1997;272:28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Kaether C, Steiner H, Haass C. Co-expression of nicastrin and presenilin rescues a loss of function mutant of APH-1. J Biol Chem. 2004;279:37311–37315. doi: 10.1074/jbc.M406228200. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Tseng BP, Kitazawa M, Laferla FM. Amyloid beta-peptide: the inside story. Curr Alzheimer Res. 2004;1:231–239. doi: 10.2174/1567205043332045. [DOI] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla R, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome. Differential effects of ApoE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson FL. Chicago: ASCP Press; Histotechnology, (ed 2) 1996 [Google Scholar]
- Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, Tolnay M, Staufenbiel M, Jucker M. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Roses AD, Gilbert J, Xu PT, Sullivan P, Popko B, Burkhart DS, Christian-Rothrock T, Saunders AM, Maeda N, Schmechel DE. Cis-acting human ApoE tissue expression element is associated with human pattern of intraneuronal ApoE in transgenic mice. Neurobiol Aging. 1998;19:53–58. doi: 10.1016/s0197-4580(98)00030-x. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1-42). J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, Gervais F. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Andra K, Abramowski D, Duke M, Probst A, Wiederhold KH, Bülki K, Goedert M, Sommer B, Staufenbiel M. Expression of APP in transgenic mice: a comparison of neuron-specific promoters. Neurobiol Aging. 1996;17:183–190. doi: 10.1016/0197-4580(95)02066-7. [DOI] [PubMed] [Google Scholar]
- Carter DB. The interaction of amyloid-beta with ApoE. Subcell Biochem. 2005;38:255–272. doi: 10.1007/0-387-23226-5_13. [DOI] [PubMed] [Google Scholar]
- Shibata H. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J Comp Neurol. 1993;337:431–445. doi: 10.1002/cne.903370307. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nuclear reunions thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Kaitz SS. Thalamic connections with limbic cortex. I. Thalamocortical projections. J Comp Neurol. 1981;195:501–525. doi: 10.1002/cne.901950308. [DOI] [PubMed] [Google Scholar]
- Shibata H. Direct projections from the entorhinal area to the anteroventral and laterodorsal thalamic nuclei in the rat. Neurosci Res. 1996;26:83–87. doi: 10.1016/0168-0102(96)01083-8. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci USA. 1999;96:14088–14093. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Stürchler-Pierrat C, Bürki K, Van Duinen SG, Maat-Schieman MLC, Staufenbiel M, Mathews PM, Jucker M. Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nature Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vonsattel JP, Segal AZ, Chiu RI, Clatworthy AE, Liao A, Hyman BT, Rebeck GW. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–965. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- Revesz T, Holton JL, Lashley T, Plant G, Rostagno A, Ghiso J, Fragione B. Sporadic and familial cerebral amyloid angiopathies. Brain Pathol. 2002;12:343–357. doi: 10.1111/j.1750-3639.2002.tb00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, Weisgraber KH, Mucke L, Mahley RW, Huang Y. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioural deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]