Abstract
Helicobacter pylori is a gram-negative bacterium that persistently colonizes more than half of the global human population. In order to successfully colonize the human stomach, H. pylori must initially overcome multiple innate host defenses. Remarkably, H. pylori can persistently colonize the stomach for decades or an entire lifetime despite development of an acquired immune response. This review focuses on the immune response to H. pylori and the mechanisms by which H. pylori resists immune clearance. Three main sections of the review are devoted to (i) analysis of the immune response to H. pylori in humans, (ii) analysis of interactions of H. pylori with host immune defenses in animal models, and (iii) interactions of H. pylori with immune cells in vitro. The topics addressed in this review are important for understanding how H. pylori resists immune clearance and also are relevant for understanding the pathogenesis of diseases caused by H. pylori (peptic ulcer disease, gastric adenocarcinoma, and gastric lymphoma).
INTRODUCTION
The gram-negative bacterium Helicobacter pylori persistently colonizes the human stomach (34, 145, 153, 217). H. pylori colonization of the stomach elicits humoral and cellular immune responses (28, 52, 129, 180), which in most cases do not result in bacterial clearance. In the absence of antibiotic therapy, H. pylori can persist in the human stomach for decades or for an entire lifetime (116). H. pylori is widespread throughout the world and is present in about 50% of the global human population (178, 226). H. pylori-induced gastric inflammation does not cause symptoms in most infected persons (56) but is associated with an increased risk for development of duodenal ulcer disease, gastric ulcer disease, gastric adenocarcinoma, and gastric lymphoma (45, 179, 183, 217, 233). In this review, we examine innate and adaptive immune responses to H. pylori and discuss mechanisms by which H. pylori evades immune clearance.
ANTIBACTERIAL PROPERTIES OF THE HUMAN STOMACH
Humans ingest many microorganisms each day, but most cannot successfully colonize the stomach. One of the most important antibacterial properties of the human stomach is its acidic pH. Under fasting conditions, the human gastric luminal pH is <2, which prevents the proliferation of bacteria within the gastric lumen. Within the gastric mucus layer overlying gastric epithelial cells, a pH gradient exists, ranging from a pH of about 2 at the luminal surface to a pH of between 5 and 6 at the epithelial cell surface (185, 225). After entering the stomach, H. pylori penetrates the gastric mucus layer (203) and thereby encounters a less acidic environment than that which is present within the gastric lumen. H. pylori typically does not traverse the epithelial barrier (97), and it is classified as a noninvasive bacterial organism. Within the gastric mucus layer, most H. pylori organisms are free living, but some organisms attach to the apical surface of gastric epithelial cells and may occasionally be internalized by these cells (10, 97, 119, 173).
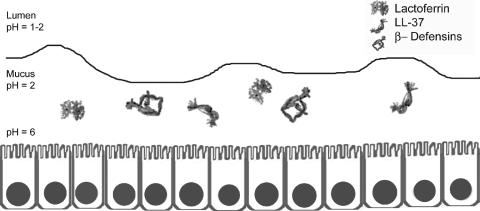
Multiple factors produced by the gastric mucosa limit the proliferation of bacteria (Fig. 1). Antibacterial peptides, including β-defensins 1 and 2 and LL-37, are active against many different species of bacteria (74, 94). Lactoferrin inhibits bacterial growth by restricting the availability of extracellular Fe3+ (133) and can have direct effects on bacterial membrane permeability (13, 175, 253). Lactoferricin, a peptide derived from lactoferrin, also has antimicrobial properties (80). Lysozyme can degrade the peptidoglycan of many bacterial species. Surfactant protein D is capable of aggregating many different types of microorganisms in a calcium-dependent and lectin-specific manner (114, 158, 164). Finally, specific components of human gastric mucin can inhibit bacterial growth; alpha-1,4-GluNAC-capped O-glycans inhibit biosynthesis of cholesteryl-α-d-glucopyranoside, a component of the H. pylori cell wall (112).
FIG. 1.
Antibacterial properties of the stomach. The stomach is intrinsically resistant to bacterial colonization. Factors which contribute to this resistance include gastric acidity, lactoferrin, and antibacterial peptides (LL-37, β-defensin 1, and β-defensin 2). The gastric epithelial layer constitutes a physical barrier that prevents entry of bacteria into the gastric mucosa. Ribbon diagrams of lactoferrin, β-defensins, and LL-37 are derived from published structures (24, 200, 218).
Toll-like receptors (TLRs) are present on the surface of gastric epithelial cells and can recognize pathogen-associated molecular patterns (PAMPs) (21, 201, 216). If bacteria invade and penetrate the gastric epithelial barrier, the alternate pathway of complement is activated, and invading bacteria encounter macrophages and neutrophils. Since most H. pylori organisms localize within the gastric mucus layer and do not invade gastric tissue, contact between H. pylori and phagocytic cells probably occurs infrequently unless there are disruptions in the gastric epithelial barrier.
The antibacterial properties of the human stomach described above prevent most bacterial species from colonizing the stomach. Based on the high prevalence of H. pylori in humans throughout the world, it may be presumed that H. pylori possesses mechanisms to overcome these innate host defenses.
H. PYLORI FACTORS THAT CONTRIBUTE TO GASTRIC COLONIZATION
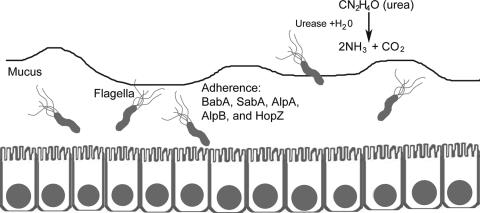
The capacity of H. pylori to colonize the human stomach can be attributed to the production of specific bacterial products (Fig. 2). Numerous H. pylori components have been designated colonization factors based on the demonstration that null mutant strains defective in the production of these factors are impaired in the ability to colonize the stomach in animal models. For example, H. pylori null mutant strains defective in production of urease or flagella are unable to colonize animal models (59, 62). Urease hydrolyzes urea to yield ammonium ions and thereby contributes to the acid resistance of H. pylori (144). Flagella confer the property of motility and enable H. pylori to penetrate the gastric mucus layer. In a recent signature-tagged mutagenesis analysis, 47 H. pylori genes were found to be essential for colonization of the Mongolian gerbil stomach but not essential for growth of H. pylori in vitro (111). Probably many other H. pylori factors are also required for colonization of the stomach.
FIG. 2.
Colonization factors of H. pylori. Multiple bacterial factors contribute to the ability of H. pylori to colonize the stomach. Urease contributes to the acid resistance of H. pylori. Flagella permit bacterial motility, which allows bacterial penetration of the mucus layer. Several outer membrane proteins, including BabA, SabA, AlpA, AlpB, and HopZ, can mediate bacterial adherence to gastric epithelial cells.
Multiple H. pylori outer membrane proteins, including BabA, SabA, AlpA, AlpB, and HopZ, can mediate H. pylori adherence to gastric epithelial cells (Fig. 2). Attachment of H. pylori to gastric epithelial cells results in activation of numerous signaling pathways (87) and permits efficient delivery of toxins or other effector molecules into the cells. Studies in an animal model indicate that attachment of H. pylori to epithelial cells influences the development of gastric mucosal inflammation, production of autoantibodies, and parietal cell loss (90).
H. pylori outer membrane proteins and other surface components are likely targets for recognition by host immune defenses. One mechanism by which H. pylori evades immune recognition may involve a form of antigenic disguise in which the bacteria are coated with host proteins. For example, H. pylori PgbA and PgbB proteins bind plasminogen, and the bacteria can thereby be coated with this host protein (108). Other mechanisms for evading immune recognition may involve phase variation and antigenic variation of surface components. Phase variation has been reported for multiple H. pylori surface components, including outer membrane proteins and lipopolysaccharide (LPS) antigens (14, 198, 210, 241). Genetic rearrangements contribute to antigenic variation in CagY (16), and intragenomic recombination may contribute to antigenic variation in outer membrane proteins (210).
LPS from most bacterial organisms serves as a potent signal for development of an inflammatory response. An important H. pylori adaptation is the synthesis of LPS that is less proinflammatory than LPSs from many other gram-negative species (114, 157, 181). In comparison to LPS from Escherichia coli or Salmonella enterica serovar Typhimurium, H. pylori LPS has approximately 500-fold-lower endotoxic activity (162), and its ability to stimulate macrophage production of proinflammatory cytokines, nitric oxide, and prostaglandins is relatively weak (35, 181). The low biological activity of H. pylori LPS is attributable to modifications of its lipid A component (157, 162). H. pylori strains commonly express LPS O antigens that are structurally related to Lewis blood group antigens found on human cells (19, 154). This similarity in structure between H. pylori LPS and Lewis blood group antigens may represent a form of molecular mimicry or immune tolerance that permits H. pylori LPS antigens to be shielded from immune recognition because of similarity to “self” antigens.
Many H. pylori strains contain a 40-kb region of chromosomal DNA known as the cag pathogenicity island (PAI) (5, 40). Some strains contain an incomplete cag PAI (less than 40 kb in size), and in other strains the cag PAI is completely absent (40, 166). One product of the cag pathogenicity island, CagA, is translocated into gastric epithelial cells and induces numerous alterations in cellular signaling (18, 98, 171, 204, 214). Multiple other products of the cag pathogenicity island have a role in secretion of CagA and in altering gene transcription in gastric epithelial cells (5, 40, 71, 87, 205). In comparison to cag PAI-negative H. pylori strains, cag PAI-positive strains stimulate gastric epithelial cells to produce high levels of proinflammatory cytokines such as interleukin-8 (IL-8) (5, 38, 71, 87, 125, 161, 205). Gastric cancer and peptic ulcer disease occur more commonly in persons infected with cag PAI-positive strains (particularly those strains containing an intact 40-kb cag PAI) than in persons infected with cag PAI-negative strains (33, 70, 166, 236).
Several H. pylori factors are known to interact directly with immune cells and modulate immune responses to H. pylori. These factors include a secreted toxin (VacA) (37, 46, 77, 219), neutrophil-activating protein (HP-NAP or NapA) (68, 197), arginase (83, 256), urease (93, 139, 140), Hsp60 (a GroEL heat shock protein) (81), SabA (235), HcpA (53), CagA (170, 234), and a proinflammatory peptide designated Hp(2-20) (29). Several of these factors act on multiple different types of immune cells. For example, VacA alters the function of T lymphocytes, B cells, macrophages, and mast cells (37, 49, 77, 152, 219, 220, 258), and HP-NAP acts on neutrophils, mast cells, and monocytes (155, 156, 197). The activities of H. pylori factors that interact directly with immune cells will be discussed in greater detail below.
IMMUNE RESPONSE TO H. PYLORI IN HUMANS
Acute Infection
There have been several reports of acute H. pylori infection in humans, and these provide insight into the immune responses to H. pylori that occur within the first few days after infection. Soon after the discovery of H. pylori, two volunteers ingested cultures of the organism (143, 159, 160). Both volunteers developed nausea, vomiting, or fever within 10 days after ingesting H. pylori, and gastric biopsies revealed mucosal inflammation in both volunteers. By 2 weeks postinfection, a sharp rise in the gastric pH to about 7 was detected in one of the volunteers. In 1979, a group of 17 volunteers were probably inadvertently infected with H. pylori (85, 191), and these persons also developed hypochlorhydria in association with gastric mucosal inflammation. Further insight comes from a case in which an endoscopist reported a syndrome of cramping epigastric pain, accompanied by transient fasting achlorhydria and acute neutrophilic gastritis, about 5 days after infection with H. pylori (208). Within 14 days after infection, this individual developed an H. pylori-specific immunoglobulin M (IgM) and IgA immune response.
More recently, 20 human volunteers were experimentally infected with 104 to 1010 CFU of an H. pylori strain (86). Symptoms occurred most frequently during the second week after infection and included dyspepsia (in >50% of subjects), headaches, anorexia, abdominal pain, belching, and halitosis. Gastric biopsies performed 2 weeks after infection showed infiltration of lymphocytes and monocytes, along with significantly increased expression of IL-1β, IL-8, and IL-6 in the gastric antrum (86). Four weeks after infection, the numbers of gastric CD4+ and CD8+ T cells were increased compared to preinfection levels, indicating the development of an early adaptive immune response (168). These cases provide evidence that gastric inflammation develops within a short period of time after H. pylori infection and that the initial colonization of the stomach by H. pylori frequently results in upper gastrointestinal symptoms. Either innate immune responses to H. pylori or early adaptive immune responses could account for the gastric mucosal inflammatory responses and symptoms that accompany acute infection.
Chronic Infection
Gastric mucosal biopsies from humans who are persistently infected with H. pylori reveal an increased concentration of various types of leukocytes compared to biopsies from uninfected humans (45, 55). This inflammatory response to H. pylori has been termed “chronic superficial gastritis” (55, 243). Lymphocytes (both T cells and B cells), macrophages, neutrophils, mast cells, and dendritic cells (DCs) are usually present (27, 55, 222). CD4+ T cells are typically more abundant than CD8+ T cells (22, 132, 146, 184). CD4+/CD25hi regulatory T cells expressing FOXP3 are present in higher numbers in the gastric mucosa of H. pylori-infected persons than in uninfected persons, and these are presumed to play an important role in regulating the inflammatory response (130, 131). Various cell types, including B cells and CD4+ cells, sometimes organize into lymphoid follicles (228). The chronic gastric mucosal inflammatory response to H. pylori probably reflects the combined effects of a cellular immune response and an ongoing stimulation of an innate immune response.
In contrast to the intestine, the stomach does not contain Peyer's patches or M cells (165). Therefore, there is some uncertainty about the location where priming of the immune response to H. pylori occurs. Gastric epithelial cells up-regulate expression of major histocompatibility complex (MHC) class II and costimulatory molecules during H. pylori infection (17, 255), and potentially these cells have a role in antigen presentation. Monocytes, macrophages, and dendritic cells in the lamina propria of the gastric mucosa also may play important roles in antigen presentation (115, 222, 240). Alternatively, priming of the immune response to H. pylori may occur within lymph nodes draining the stomach or may occur at intestinal sites in response to H. pylori antigens or intact organisms that are shed from the stomach.
H. pylori-specific CD4+ T cells are detectable in the gastric mucosae of H. pylori-infected persons but not uninfected persons (52, 54, 132). One study reported that about 15% of CD4+ T-cell clones isolated from the stomachs of H. pylori-infected persons were H. pylori specific, whereas the other T-cell clones did not proliferate in response to antigens in H. pylori lysate (52). Some T-cell clones from H. pylori-infected patients recognize epitopes on parietal cell H+,K+-ATPase (9), and it has been suggested that recognition of H+,K+-ATPase by gastric T cells may contribute to the development of autoimmune gastritis (51).
Levels of numerous cytokines, including gamma interferon (IFN-γ), tumor necrosis factor (TNF), IL-1β, IL-6, IL-7, IL-8, IL-10, and IL-18, are increased in the stomachs of H. pylori-infected humans compared to uninfected humans (47, 126). IL-4 has not been detected in the gastric mucosae of most H. pylori-infected persons (110, 126, 184). The Th1-defining cytokine, IFN-γ, is expressed by a higher proportion of gastric T cells from H. pylori-infected persons than gastric T cells from uninfected persons (22, 91, 110, 126, 211). In one study, 83% of H. pylori-specific gastric T-cell clones produced IFN-γ but not IL-4 upon stimulation with H. pylori antigens, compared to 17% of clones that produced IL-4 (22, 52). Based on the relative abundance of IFN-γ-producing T cells and the relative scarcity of IL-4-producing gastric T cells in the setting of H. pylori infection, it has been concluded that H. pylori infection leads to a Th1-polarized response (22, 211). In the setting of H. pylori infection, multiple cytokines in the gastric mucosa (including TNF, IFN-γ, IL-1β, IL-6, IL-8, and IL-18) are predicted to have proinflammatory effects, whereas IL-10 is an immunoregulatory cytokine that may limit the inflammatory response.
A humoral immune response to H. pylori is elicited in nearly all H. pylori-infected humans (180). In a study of H. pylori-infected human volunteers, H. pylori-specific serum IgM antibodies were present by 4 weeks postinfection (168). Serum IgA and IgG antibodies in persons with chronic H. pylori infection are directed toward many different H. pylori antigens (147, 180). Antibody-secreting cells producing H. pylori-specific IgA or IgM antibodies are detectable in the gastric mucosae of H. pylori-infected persons (147), and secretory IgA antibodies to H. pylori are detectable in gastric juice, which suggests that H. pylori infection elicits a local secretory IgA response in the stomach (96, 147).
Factors Modulating the Immune Response to H. pylori in Humans
The gastric mucosal inflammatory response to H. pylori in humans may be modulated by characteristics of the H. pylori strain. Infection with H. pylori strains containing the cag PAI is typically associated with a more severe inflammatory response than that which accompanies infection with cag PAI-negative strains (33, 230). Similarly, H. pylori strains containing type s1/m1 vacA alleles, containing a gene of unknown function known as jhp0917/0918 (dupA), and expressing certain outer membrane proteins (BabA and HopH [OipA]) are associated with an enhanced inflammatory response (128, 137, 186, 252).
The gastric inflammatory response to H. pylori also may be modulated by characteristics of the human host. H. pylori-associated gastric inflammation in adults is characterized by infiltration of mononuclear cells and neutrophils, whereas in children the inflammatory response often is predominantly lymphocytic with relatively few neutrophils (246). Adults who are persistently colonized with H. pylori for many decades may develop atrophic gastritis (an inflammatory process characterized by loss of glandular structures and parietal cells in the gastric mucosa), which is considered a preneoplastic lesion (45, 117).
No immunodeficiency diseases are known to result in enhanced severity of H. pylori-associated inflammation. For example, gastric inflammation is not more severe in H. pylori-infected humans with IgA deficiency than in immunocompetent hosts (36). However, single-nucleotide polymorphisms in several genes encoding proinflammatory cytokines can influence the clinical outcome of H. pylori infection (Table 1). Polymorphisms that result in elevated levels of IL-1β and TNF-α and reduced levels of IL-10 have been associated with an increased risk of atrophic gastritis and gastric cancer (64, 66, 101, 134, 232, 248). A polymorphism in the promoter region of IL-1 receptor antagonist that leads to reduced expression of IL-1 receptor antagonist has also been associated with an increased incidence of atrophic gastritis and gastric cancer (64, 135, 187). The exact mechanisms by which these polymorphisms affect the risk for gastric cancer are not yet completely understood. Some of the TNF-α and IL-10 polymorphisms associated with increased risk for gastric cancer are considered proinflammatory genotypes (66, 134), and IL-1β is known to be a potent inhibitor of gastric acid secretion (254). These polymorphisms may predispose individuals to develop gastric atrophy and gastric cancer by pathways involving enhancement in the severity of gastric inflammation and a reduction in gastric acid secretion (254).
TABLE 1.
Genetic polymorphisms in cytokine-encoding genes which influence the clinical course of H. pylori infection in humans
| Gene product | Polymorphism(s) | Postulated effect of polymorphism | Reference(s) |
|---|---|---|---|
| IL-1β | IL-1B-31C, IL-1B-511T | Increased expression of IL-1β, which induces expression of proinflammatory cytokines and inhibits acid secretion | 63-66, 134, 135 |
| IL-1RA | IL-1RN*2 | Reduced expression of IL-1 receptor agonist, which increases IL-1β activity | 135 |
| IL-2 | IL-2-330T | Reduced IL-2 expression | 232 |
| IL-10 | IL-10 haplotype ATA | Reduced expression of IL-10, which increases proinflammatory cytokine activity | 66 |
| TNF | TNF-A-308A | Increased TNF expression, which induces expression of proinflammatory cytokines and inhibits acid secretion | 66, 134 |
Although H. pylori is very successful in evading immune clearance, it is possible that the immune response is sometimes successful in clearing H. pylori from the stomach. The frequency with which H. pylori is cleared by the immune response is not known. In regions of the world with a high incidence of H. pylori infection, reinfection occurs commonly following H. pylori eradication (213), which suggests that a protective immune response develops infrequently in H. pylori-infected persons.
INTERACTIONS BETWEEN H. PYLORI AND HOST DEFENSES IN ANIMAL MODELS
H. pylori Infection of Wild-Type Animals
Several animal models of H. pylori infection have been developed, utilizing mice, Mongolian gerbils, guinea pigs, rats, ferrets, beagle dogs, cats, gnotobiotic piglets, or nonhuman primates (reviewed in references 174 and 245). The use of nonhuman primate models is of particular interest because of the close relatedness of these animals to humans. The rhesus monkey can be experimentally infected with H. pylori, and a large proportion of monkeys in certain colonies are naturally infected with H. pylori (57, 58, 148, 209). Histopathological changes that occur in the gastric mucosae of monkeys in response to H. pylori infection are similar to the changes that occur in H. pylori-infected humans (148). However, H. pylori-associated peptic ulceration and gastric adenocarcinoma have not been described in nonhuman primate models (148).
Mongolian gerbils can be experimentally infected with H. pylori and develop gastric inflammation characterized by infiltration of mononuclear cells and neutrophils (149, 172, 247). An attractive feature of the Mongolian gerbil model is that these animals may develop gastric mucosal ulceration or gastric adenocarcinoma in response to H. pylori infection (73, 100, 172, 244), and they thus provide a model for two important H. pylori-associated diseases that occur in humans. Several studies suggest that products of the H. pylori cag pathogenicity island contribute to gastric pathology in the gerbil model (105, 172, 192). Limitations of this model include the relative paucity of gerbil-specific immunologic reagents and the fact that Mongolian gerbils are outbred.
The mouse model is frequently utilized because of low cost, availability of relevant reagents, and the potential for development of knockout mice (142). H. pylori can persistently colonize the stomachs of wild-type mice for periods of at least 15 months. However, wild-type mice do not develop gastric mucosal ulceration or gastric adenocarcinoma in response to H. pylori. One limitation of the mouse model is that only a few human isolates of H. pylori have been successfully adapted to permit efficient colonization of the mouse stomach (20). Infant mice and certain types of knockout mice (e.g., IL-12 knockout mice) seem to be more permissive hosts than are wild-type adult mice and tolerate infection with a broader range of H. pylori strains (89, 99). A different Helicobacter species, H. felis, also can colonize conventional inbred mice and causes more severe gastric inflammation than does H. pylori (196). However, H. felis does not express several important H. pylori virulence factors (249).
The gastric mucosal inflammation that develops in wild-type mice infected with H. pylori consists primarily of lymphocytes and other mononuclear cells. Most of the infiltrating cells are CD4+ T cells, but CD8+ T cells, B cells, dendritic cells, and monocytes are also present (167, 199, 207, 237). The intensity of inflammation that develops in H. pylori-infected mice is relatively mild compared to that which develops in H. pylori-infected humans and is also relatively mild compared to that which develops in H. pylori-infected Mongolian gerbils (121). Neutrophils are typically present in the gastric mucosae of H. pylori-infected humans (194, 246) and gerbils (100) but are less commonly observed in the gastric mucosae of H. pylori-infected mice (138).
C57BL/6 mice have been commonly used for studies of H. pylori. Gastric levels of IFN-γ, IL-12, TNF, and IL-6 are increased in H. pylori-infected C57BL/6 mice compared to uninfected mice, whereas gastric levels of IL-4 are not increased in response to H. pylori infection (150, 212). Upon antigen stimulation ex vivo, splenocytes from H. pylori-infected C57BL/6 mice produce substantially more IFN-γ than IL-4 (107, 127, 207). These patterns of cytokine expression are indicative of a predominantly Th1 response, which is similar to the response which occurs in H. pylori-infected humans.
There is variability among different strains of inbred mice in susceptibility to H. pylori infection (138, 231) and in host responses to H. pylori. Inbred mice are known to have default T-helper responses, and therefore, the genetic backgrounds of inbred mice may influence the T-cell response to H. pylori. C57BL/6 mice have a default Th1 response, whereas BALB/c mice have a default Th2 response (113). This difference may be a factor that helps to explain why BALB/c mice are relatively resistant to H. pylori colonization and why H. pylori-infected BALB/c mice develop relatively mild gastric inflammation compared to H. pylori-infected C57BL/6 mice (109, 196).
H. pylori Infection of Knockout Mice
Mouse knockout models have served as valuable tools for investigating the roles of various components of the immune response to H. pylori. Features of H. pylori infection in selected mouse knockout models are discussed below, and a summary of the data is shown in Table 2. As noted above, inbred mice are known to have default T-helper responses, and consequently, the genetic backgrounds of mouse strains can potentially influence the results of knockout mouse studies. Most studies of H. pylori infection in knockout mice have been performed with C57BL/6 animals, which have a default Th1 response.
TABLE 2.
H. pylori infection of mouse knockout modelsa
| Knockout model (mouse strain[s]) | H. pylori strain | Bacterial density compared to WT | Inflammation compared to control | Reference(s) |
|---|---|---|---|---|
| NOS2−/− (C57BL/6) | SS1 | Similar colonization | More severe gastritis | 32 |
| Gp91phox/NOS2−/− (C57BL/6) | SS1 | Reduced colonization | More severe gastritis | 32 |
| SCID (B- and T-cell deficient) (C57BL/6) | SS1 | Greater colonization | No gastric inflammation | 61 |
| μ-MT (B-cell deficient) (C57BL/6) | SS1 | Reduced colonization | More severe gastritis | 3 |
| MHC class I KO (C57BL/6) | SS1 | Greater colonization | ND | 177 |
| MHC class II KO (C57BL/6) | SS1 | Greater colonization | ND | 177 |
| IFN-γ KO (C57BL/6) | SM326 | ND | No gastric inflammation | 207 |
| IFN-γ KO (C57BL/6) | CPY2052 | Greater colonization | No gastric inflammation | 199,251 |
| IFN-γ KO (C57BL/6 and BALB/c) | SM326 | More frequent recovery of bacteria | ND | 109 |
| IRF-1 KO (C57BL/6) | SS1 | Greater colonization | No gastritis | 212 |
| IL-4 KO (C57BL/6) | SM326 | ND | More severe gastritis | 207 |
| IL-4 KO (C57BL/6 and BALB/c) | SM326 | Similar colonization | ND | 109 |
| IL-4 KO (C57BL/6) | SS1 | Similar colonization | Similar degree of gastritis | 42 |
| IL-4 Tg (IL-4 overexpression) (C3H) | SS1 | Similar colonization | Similar degree of gastritis | 42 |
| IL-10 KO (IL-10+/−, 129 × C57BL/6) | SS1 | Reduced colonization | More severe gastritis | 43 |
| IL-12 KO (C57BL/6) | SS1 | Similar colonization | Similar degree of gastritis | 2 |
| FasL KO (C57BL/6) | SS1 | Similar colonization | Similar degree of gastritis | 107 |
| TNF KO (C57BL/6) | CPY2052 and KP142 | Greater colonization | Similar degree of gastritis | 251 |
| TNF receptor KO (C57BL/6) | Six clinical isolates of H. pylori | ND | Similar degree of gastritis | 229 |
This table summarizes the results of selected studies in which transgenic mouse models were infected with Helicobacter pylori. WT, wild type; ND, not determined; KO, knockout.
SCID (severe combined immunodeficient) mice lack mature T and B lymphocytes due to a defective capacity to express rearranged antigen receptors and are therefore deficient in both humoral and cell-mediated immunity. SCID mice can be successfully colonized by H. pylori, but these mice develop minimal gastric inflammation in response to infection (61). This indicates that an adaptive immune response is required for development of chronic gastric inflammation in response to H. pylori and also indicates that gastric inflammation is not required in order for H. pylori to persistently colonize the stomach. If H. pylori-infected SCID mice receive splenocytes from uninfected C57BL/6 mice through adoptive transfer, the recipient SCID mice develop severe gastric inflammation characterized by a neutrophilic infiltrate (61). The gastritis that develops in H. pylori-infected SCID recipient mice is more severe than that which occurs in H. pylori-infected C57BL/6 mice (61). The transfer of splenocytes to SCID mice potentially induces a severe form of gastritis due to the absence of regulatory cells in the SCID mice (61, 182).
μ-MT (B-cell-deficient) mice infected with H. pylori develop gastritis that is more severe than that which occurs in wild-type mice, and subsequently H. pylori infection is cleared from the stomachs of the B-cell-deficient mice (3). There are several possible reasons why H. pylori-induced gastritis may be more severe in B-cell-deficient mice than in wild-type mice. For example, antibodies produced by wild-type mice may engage the inhibitory IgG receptor (FcγRIIb) on leukocytes and increase expression of anti-inflammatory cytokines such as IL-10 (3).
In comparison to H. pylori-infected wild-type mice, H. pylori-infected mice with defects in IFN-γ expression (IFN-γ−/− mice or interferon response factor 1−/− mice) develop less severe gastric inflammation and have higher bacterial colonization densities (2, 169, 199, 207, 212, 251). This suggests that IFN-γ contributes to increased severity of H. pylori-induced gastric inflammation while also contributing to reducing bacterial colonization. In support of this view, H. pylori-infected SCID mice reconstituted with splenocytes that express IFN-γ developed more severe gastritis than did mice reconstituted with IFN-γ-deficient splenocytes (60). IFN-γ may indirectly modulate the severity of gastritis by activating macrophages to secrete proinflammatory cytokines and also may down-regulate the expression of anti-inflammatory factors such as the anti-inflammatory cytokine transforming growth factor β (215).
In comparison to Helicobacter-infected mice that express IL-10, infected IL-10−/− mice develop more severe gastritis (26, 43). IL-10 is known to be a potent anti-inflammatory and immunoregulatory cytokine, and therefore it seems likely that IL-10 has a role in down-regulating H. pylori-induced inflammation (43). One study reported that H. pylori-infected IL-4−/− mice developed more severe gastritis than did H. pylori-infected wild-type C57BL/6 mice (207). Similarly, H. felis-infected IL-4−/− mice developed significantly more severe gastric inflammation than did H. felis-infected IL-4+/+ mice (151). Although the results of studies analyzing IL-4−/− mice have not been entirely uniform (42, 109), these data suggest that both IL-10 and IL-4 have a role in down-regulating gastric inflammation (26, 72, 151, 207, 257).
A general theme that emerges from studies of H. pylori in mouse models is that there is a reciprocal relationship between the intensity of gastric mucosal inflammation and bacterial load (or colonization density) (32, 43, 61, 188, 199, 251). For example, IFN-γ−/− mice have relatively high bacterial loads and mild gastritis, whereas IL-10−/− mice have relatively low bacterial loads and severe gastritis (43). As will be discussed later in this review, these observations are relevant to understanding the immunologic basis for protective immunity to H. pylori.
Th1 and Th2 Responses in Mice
The data described above, involving experiments with various knockout mice, suggest that expression of IFN-γ (a Th1 cytokine) contributes to enhanced gastric inflammation, whereas expression of certain Th2 cytokines (IL-10 and possibly IL-4) contributes to diminished inflammation. To investigate further the role of Th1 and Th2 responses in modulating gastric inflammation, C57BL/6 mice were initially infected with a nematode that induces a strong Th2 response and then were challenged with H. felis (72). In comparison to mice infected with H. felis alone, the mice coinfected with H. felis and the nematode had reduced gastric expression of Th1 cytokines (IFN-γ, TNF, and IL-1β), increased gastric expression of Th2 cytokines (IL-4, IL-10, and transforming growth factor β), and reduced gastric inflammatory scores (72). These data provide support for the hypothesis that a Th2-polarized response down-regulates the severity of H. pylori-induced gastric inflammation.
Several cytokines affect the expression of gastric hormones that control gastric acid secretion. Expression of gastrin, a hormone that stimulates gastric acid secretion, is stimulated by IFN-γ (257), and expression of somatostatin, a hormone that inhibits gastric acid secretion, is stimulated by IL-4 and inhibited by IFN-γ and TNF (25, 257). Increased expression of IFN-γ (a Th1 response) is expected to result in increased gastrin production, whereas expression of IL-4 (a Th2 response) is expected to result in increased somatostatin production and reduced gastrin secretion. One study used a mouse model of H. felis infection to investigate the effects of IL-4-induced alterations on gastrin and somatostatin expression (257). As expected, administration of IL-4 resulted in increased somatostatin expression and reduced gastrin expression. These changes were accompanied by a reduction in the severity of H. felis-induced gastritis. The modulatory effects of IL-4 on the severity of gastric inflammation were observed in H. felis-infected wild-type mice but not in infected somatostatin knockout mice, which suggested that the IL-4-induced alterations in inflammation were mediated through effects of IL-4 on somatostatin production by D cells (257).
Role of Regulatory T Cells
The gastric mucosal inflammatory response to H. pylori may be regulated in part by regulatory T cells (Tregs) (CD25+ CD45RBlo T cells). CD4+/CD25+ Tregs can suppress cytokine production and proliferation of other T cells (118). One recent study investigated the role of Tregs in a murine model of H. pylori-induced gastritis by reconstituting athymic C57BL/6 nude mice (T-cell-deficient nu/nu mice) with either lymph node cells containing CD25+ cells or lymph node cells depleted of CD25+ cells, 3 weeks prior to H. pylori infection (188). In mice reconstituted with a cell population depleted of Tregs, a relatively severe gastritis occurred by 6 weeks postinfection compared to that which occurred in mice reconstituted with a nonsorted T-cell population (containing both CD25+ and CD25− T cells). The mice reconstituted with a T-cell population lacking CD25+ cells developed a stronger Th1 response, characterized by increased numbers of CD4+ T cells in the mucosa and increased IFN-γ production compared to mice reconstituted with an unsorted T-cell population (188). These data indicate that Tregs have an important role in regulating the gastric mucosal inflammatory response to H. pylori.
Protective Immunity in Animal Models
Protective immunity to H. pylori may be defined as either (i) immunity that protects against H. pylori colonization of the stomach or (ii) an immune response that results in eradication of an established infection. Both prophylactic H. pylori immunization (to prevent future infection) and therapeutic immunization (to eradicate an established infection) have been successfully accomplished in animal models (50, 106, 142, 195).
Several early studies suggested that protection might be mediated by Helicobacter-specific antibodies (30, 48, 69, 122). Subsequently, it was shown that immunization of μ-MT mice (which are unable to produce antibodies) or IgA-deficient mice can result in protective immunity against H. pylori or H. felis infection (3, 31, 67, 76, 84, 221). Therefore, there is now a general consensus that H. pylori-specific antibodies are not required for protective immunity.
Cellular immune responses seem to have an important role in protective immunity against H. pylori. Mice deficient in CD8+ T cells (MHC class I−/− mice) can be successfully immunized and protected against colonization with H. pylori (177), whereas mice deficient in CD4+ T cells (MHC class II−/− mice) were not protected by prophylactic immunization against H. pylori (177). CD4+ T cells from H. felis-immunized mice can mediate protective immunity if adoptively transferred into immunodeficient Rag1−/− mice (84). These data suggest that CD4+ T cells, but not CD8+ cells, are necessary for protection (67, 177).
Several lines of evidence suggest that Th2-type responses might be required for protective immunity against H. pylori. Specifically, persistent H. pylori infections in humans and mice typically result in Th1-polarized responses, whereas successful Helicobacter immunization of animals typically results in Th2-polarized responses (1, 50). In addition, adoptive transfer of Th2 cells from H. felis-infected C57BL/6 mice into infected C57BL/6 mice significantly reduced the bacterial load compared to when Th1 cells were adoptively transferred (151). Conversely, there is evidence that a Th2 response may not be required for protection. Specifically, IL-4 and IL-5 knockout C57BL/6 mice were successfully protected from H. pylori infection following immunization (76). In addition, studies with IL-4 receptor α-chain-deficient BALB/c mice (which lack both IL-4 and IL-13 signaling) suggested that IL-4 and IL-13 are not required for a protective immune response (129). Whether IFN-γ-producing Th1 cells are required for protective immunity is not yet completely clear (75, 199). However, immunization studies using IL-12 and IL-18 knockout mice indicate that these two Th1 cytokines are required for effective protection against H. pylori and suggest that the establishment of an active Th1-type response is required for protection (2, 4, 75). In summary, the role of Th1-type versus Th2-type immune responses in protective immunity to H. pylori infection remains incompletely understood. Differences in the mouse strain backgrounds used in various studies potentially complicate interpretation of the data.
There is evidence that protective immunity against H. pylori in prophylactically immunized mice may require mast cells. In contrast to immunized wild-type mice, immunized mast cell-deficient mice (W/W v mice) were not protected from challenge with H. felis (238). Reconstitution of W/W v mice with bone marrow-derived mast cells restored the ability of W/W v mice to develop a protective immune response following prophylactic vaccination (238). The mechanism by which the mast cells contribute to protective immunity is undefined, but it may be hypothesized that mast cells modulate the activity of T cells or neutrophils through secretion of cytokines or that mast cells have antibacterial activity via the production of nitric oxide or antimicrobial peptides.
Further insight into protective immunity against H. pylori can be gleaned by analyzing levels of H. pylori colonization (bacterial load or bacterial density) in persistently infected knockout mouse models. The levels of H. pylori colonization in SCID mice are significantly higher than those in wild-type mice (61). Conversely, the levels of H. pylori colonization in IL-10 knockout mice are about 100-fold lower than those in wild-type mice (43), and in some cases, H. pylori is completely eradicated from IL-10 knockout mice (104). Control of H. pylori proliferation in IL-10 knockout mice is associated with development of a gastric mucosal inflammatory response that is more severe than that in infected wild-type mice. Therefore, it may be hypothesized that protective immune responses leading to eradication of H. pylori are associated with relatively severe gastric mucosal inflammatory responses.
INTERACTIONS BETWEEN H. PYLORI AND IMMUNE CELLS IN VITRO
As described in the previous sections of this review, H. pylori stimulates a gastric mucosal inflammatory response and resists clearance by host immune defenses. To investigate the molecular mechanisms underlying these phenomena, interactions between H. pylori and various types of immune cells have been analyzed in vitro. Because H. pylori lives in the gastric mucus layer and does not typically breach the gastric epithelial barrier, contact between H. pylori and phagocytic cells may be fairly limited in vivo. Nevertheless, several publications have described ingestion of H. pylori by phagocytic cells in human gastric tissue (10, 97, 119, 173). Interactions between H. pylori and phagocytic cells probably occur when there are disruptions in the gastric epithelial barrier or in the setting of gastric mucosal injury. Interactions between H. pylori and intraepithelial T cells potentially occur commonly even in the absence of gastric epithelial disruptions.
Immune Recognition of H. pylori by Gastric Epithelial Cells
Toll-like receptors recognize conserved microbial components, termed “pathogen-associated molecular patterns,” and play an important role in initiating innate immune responses to bacterial pathogens. At least 13 different TLRs have been described, 10 of which are expressed in humans. Among the TLRs that recognize gram-negative bacteria, some of the most extensively characterized include TLR2 (which recognizes lipoproteins), TLR4 (gram-negative LPS), TLR5 (flagellin), and TLR9 (bacterial CpG DNA motifs) (223). H. pylori adheres to human gastric epithelial cells, and therefore TLRs on gastric epithelial cells would be expected to recognize H. pylori PAMPs in vivo. Gastric epithelial cells in the antrum and the corpus of the human stomach are reported to express TLR4, TLR5, and TLR9 (201). In H. pylori-negative patients, TLR4, TLR5, and TLR9 are expressed at both the apical and basolateral poles of gastric epithelial cells. In contrast, in H. pylori-positive patients, TLR5 and TLR9 are expressed exclusively at the basolateral pole, and TLR4 is expressed at both poles (201). Localization of TLRs to the basolateral poles of epithelial cells would make it unlikely for adherent H. pylori to be recognized by these receptors. Cultured primary human gastric cells express TLR2 and TLR5 but not TLR4 (21).
Several studies have sought to characterize the interactions of H. pylori PAMPs with TLRs in vitro. These studies have used many different cell types, including primary gastric epithelial cells, gastric epithelial cell lines, and cell lines transfected with plasmids that express TLRs and/or TLR accessory proteins. It is possible that some of the gastric epithelial cell lines used in these experiments do not express certain TLR accessory proteins such as CD14 and MD-2, which are required for TLR4 signaling. Different sources of H. pylori PAMPs have been used, including intact bacteria, purified LPS, and flagellin. Because of the many variations in experimental design, the results of these studies have not been uniform. Nevertheless, several general conclusions can be drawn from these studies.
Analyses of the interactions of purified H. pylori LPS with TLRs suggest that, in contrast to LPSs from most other gram-negative bacteria, H. pylori LPS is not well recognized by TLR4 (21, 103, 206). One study provided evidence that H. pylori LPS may act as an antagonist for TLR4 (124). H. pylori LPS induced NF-κB activation in HEK293 cells that expressed TLR2 but not in HEK293 cells that expressed TLR4 (206). These data suggest that H. pylori LPS may be recognized by TLR2 instead of by TLR4. H. pylori Hsp60 also is reported to be recognized by TLR2 (224).
Unlike flagellins from gram-negative organisms such as Salmonella enterica serovar Typhimurium, H. pylori flagellin is not recognized by TLR5 (12, 79, 123). This evasion of TLR5 recognition is attributable to alterations in H. pylori FlaA amino acid sequences in the TLR5 recognition site. If the corresponding amino acids are mutated in FlaA from Salmonella, the resulting Salmonella mutant strain is not recognized by TLR5 (12). Thus, H. pylori expresses at least two PAMPs (LPS and flagellin) that are recognized relatively poorly by TLRs and that may not trigger a strong innate immune response.
Recognition of intact H. pylori organisms by cultured epithelial cells appears to be dependent on TLR2 and TLR5 and to be independent of TLR4 (136, 141, 206). In one study, dominant negative forms of TLR2, TLR4, and TLR5 were expressed in the human gastric cancer cell line MKN45, and the cells then were incubated with H. pylori (206). The expression of chemokines (ΜIP3α, IL-8, and GROα) in these cells in response to H. pylori was dependent on TLR2 and TLR5 signaling but not on TLR4 signaling. These studies suggest that intact H. pylori organisms can be recognized by TLR5, despite poor recognition of H. pylori flagellin by TLR5. Potentially H. pylori components other than flagellin are recognized by TLR5, or perhaps the results are influenced by variations in the methodology used in different studies.
In addition to recognition of H. pylori PAMPs by TLRs, H. pylori peptidoglycan can be recognized by Nod1 (CARD4), an intracellular pathogen recognition molecule (239). There is evidence that the type IV secretion system encoded by the H. pylori cag PAI delivers H. pylori peptidoglycan into epithelial cells. Intracellular recognition of H. pylori peptidoglycan by Nod1 leads to activation of NF-κB and altered gene transcription in host cells (239). Compared to gastric epithelial cells from wild-type mice, gastric epithelial cells from Nod1-deficient mice produced significantly less macrophage inflammatory protein-2 in response to H. pylori (239).
In summary, H. pylori can be recognized in vitro by TLRs as well as the Nod1 receptor, and such recognition probably contributes to initiation of an innate immune response in vivo (Fig. 3). There is no evidence that H. pylori can evade detection by TLRs, but certain H. pylori PAMPs, such as LPS and flagellin, seem to be poorly recognized by TLRs. This may represent a mechanism by which H. pylori down-regulates the intensity of the innate immune response.
FIG. 3.
Innate immune recognition of H. pylori. Innate immune recognition of H. pylori leads to production of proinflammatory cytokines by macrophages (Mφ), DCs, mast cells, and gastric epithelial cells. Innate immune recognition of H. pylori is mediated at least in part through TLRs. In addition, H. pylori peptidoglycan (PG) can be recognized by intracellular Nod receptors (239). Interactions between H. pylori and gastric epithelial cells lead to activation of NF-κB and alteration in gene transcription in the epithelial cells. Production of IL-8 by epithelial cells leads to recruitment of neutrophils (polymorphonuclear leukocytes [PMNs]), which can phagocytose opsonized bacteria and produce reactive oxygen species (ROI) or reactive nitrogen species (RNI). The activation of mast cells results in degranulation and production of proinflammatory cytokines and chemokines.
It should be noted that the interactions of H. pylori with gastric epithelial cells are dependent on characteristics of the H. pylori strain. H. pylori strains possessing certain adhesins bind to gastric epithelial cells more efficiently than do strains that lack these adhesins (102). Strains that possess the cag PAI stimulate epithelial cells to produce relatively high levels of proinflammatory cytokines compared to strains that lack the cag PAI (5, 38, 71, 87, 125, 161, 205, 239). In addition, strains possessing the cag PAI and expressing a functionally active form of VacA can cause structural alterations in gastric epithelial cells (46, 95). Both CagA and VacA have been implicated in increasing the permeability of gastric epithelial monolayers (11, 176). A consequence may be entry of H. pylori antigens into the lamina propria, which would be expected to trigger an inflammatory response.
Interactions of H. pylori with Neutrophils
Neutrophils are recruited when H. pylori initially colonizes the human stomach (85, 191), and the gastric mucosal inflammatory response that occurs in the setting of persistent H. pylori infection is characterized by infiltration of neutrophils (194, 246). Several specific H. pylori factors are known to interact with neutrophils and modulate their function.
H. pylori produces a 150-kDa oligomeric protein known as neutrophil-activating protein (HP-NAP), which is chemotactic for neutrophils and activates neutrophils in vitro (68). HP-NAP stimulates neutrophils to produce reactive oxygen intermediates, and in response to HP-NAP, neutrophils release Ca2+ and phosphorylate cytosolic cellular signaling molecules (197). In addition, HP-NAP induces expression of β2-integrins on the surface of neutrophils (197).
An H. pylori outer membrane protein, SabA, also has an important role in human neutrophil activation (235). Wild-type strains of H. pylori expressing SabA activate neutrophils, whereas mutant and wild-type strains lacking SabA do not (235). There is evidence that binding of H. pylori to neutrophils through SabA-mediated adhesion may stimulate a G-protein-linked signaling pathway and downstream activation of phosphatidylinositol 3-kinase (235).
Whether H. pylori can resist phagocytosis by neutrophils is not yet completely resolved (7, 170), but one study reported that uptake of unopsonized H. pylori by neutrophils was inefficient compared to uptake of latex-coated beads and that H. pylori could inhibit phagocytosis of latex-coated beads or Neisseria gonorrhoeae (189). If nonopsonized H. pylori organisms are phagocytosed by neutrophils, the bacteria are able to resist intracellular killing (7, 227). One mechanism by which nonopsonized H. pylori evades intracellular killing may involve disruption of NADPH oxidase targeting, such that superoxide anions generated in the oxidative burst do not accumulate in the phagosome but instead are released into the extracellular space (7). A catalase-dependent pathway also may have a role in allowing nonopsonized H. pylori to evade intracellular killing (189).
The migration of neutrophils in response to chemokines IL-8 and Groα is mediated through the chemokine receptors CXCR1 and CXCR2 (163). H. pylori can down-regulate the expression of CXCR1 and CXCR2 in human neutrophils in vitro, and this is predicted to have an inhibitory effect on neutrophil migration (202). In summary, multiple H. pylori factors can activate neutrophils, and there is also evidence that H. pylori can interfere with the proper functioning of neutrophils.
Interactions of H. pylori with Mast Cells
In vitro experiments indicate that whole H. pylori bacteria (250) and various H. pylori components can activate mast cells. One H. pylori factor that can activate mast cells is VacA. VacA can induce mast cell chemotaxis and can stimulate mast cell expression of multiple proinflammatory cytokines, including IL-1, TNF, IL-6, IL-13, and IL-10 (49, 220). VacA induces degranulation of the mast cell line RBL-2H3 but does not induce degranulation of murine bone marrow-derived mast cells (49, 220). HP-NAP also can activate mast cells, resulting in β-hexosaminidase release and IL-6 production (156). Activation of mast cells by H. pylori may contribute to the inflammatory response associated with H. pylori infection.
Interactions of H. pylori with Macrophages
Contact between macrophages and intact H. pylori bacteria or H. pylori components results in macrophage activation and the secretion of numerous cytokines and chemokines (81, 93, 140). Macrophages recognize H. pylori LPS via TLR4 (35, 136) and can also be activated by H. pylori proteins, including urease and Hsp60 (81, 93). Macrophage recognition of intact H. pylori can be mediated by TLR2 or TLR4 (141, 216).
Although not all studies have reached identical conclusions (170), at least one study reported that H. pylori is able to inhibit its own uptake by macrophages (190). When nonopsonized H. pylori organisms are internalized by macrophages, they initially localize in phagosomes, which then coalesce into “megasomes” that contain multiple bacteria (8, 193). Ingested H. pylori cells have at least some ability to resist intracellular killing (8). One study reported that phagolysosomal fusion is impaired in H. pylori-infected macrophages through retention of the tryptophan aspartate-containing coat protein on phagosomes, a phenomenon that is expected to result in increased intracellular survival of the bacteria (258).
Phagocytosis of bacteria by macrophages typically results in localization of the microorganisms within phagosomes that contain protein kinase C (PKC) isoform α (39). PKC activation plays a role in the respiratory burst and phagosome-lysosome fusion (120). Upon phagocytosis of nonopsonized H. pylori by macrophages, PKC isoforms ζ and ɛ accumulate on the forming phagosomes, but the conventional PKC isoform α does not (6). Experiments using specific PKC inhibitors suggest that PKC ζ regulates actin rearrangement and H. pylori engulfment (6) and that phagocytosis of nonopsonized H. pylori by macrophages may occur via a novel PKC ζ-regulated pathway. The ability of nonopsonized H. pylori to resist macrophage killing may be attributable to features of this PKC ζ-mediated phagocytic process. Opsonized H. pylori is phagocytosed by a PKC ζ-independent process, which is likely to involve conventional pathways (6).
One mechanism by which H. pylori impairs the antimicrobial activity of macrophages involves expression of catalase. In comparison to a wild-type catalase-positive H. pylori strain, an isogenic, catalase-deficient strain was more susceptible to macrophage-mediated killing (23). Another mechanism by which H. pylori resists macrophage killing is by blocking the production of nitric oxide. This effect is mediated by H. pylori arginase, which competes with nitric oxide synthase for arginine (83). In addition to resisting killing by macrophages, in vitro experiments indicate that H. pylori can induce macrophage apoptosis (41, 44, 82). H. pylori-induced apoptosis of macrophages may result in impaired innate and adaptive immune responses.
Interactions of H. pylori with Dendritic Cells
In response to H. pylori, monocyte-derived human DCs express costimulatory molecules and major histocompatibility complex class II proteins (92, 115), which results in increased efficiency of antigen presentation. H. pylori also stimulates dendritic cell expression of multiple cytokines, including IL-6, IL-8, IL-10, and IL-12 (88, 115, 240). Similar to several other bacterial pathogens, H. pylori can bind to DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), a DC-specific lectin (15, 27). The expression of cytokines by DCs in response to H. pylori is modulated by interactions between H. pylori LPS Le antigens (Lex, Ley, Lea, or Leb) and DC-SIGN (27). Dendritic cells incubated with Le antigen-negative H. pylori strains express more IL-6 and less IL-10 than do dendritic cells incubated with Le antigen-positive H. pylori (27). Given that IL-10 is known to down-regulate inflammatory responses, the interactions between H. pylori LPS Le antigens and DC-SIGN may contribute to suppression of inflammation.
Interactions of H. pylori with B Lymphocytes
H. pylori is reported to have several inhibitory effects on B lymphocytes (152, 234). In one study, H. pylori VacA interfered with the prelysosomal processing of tetanus toxin in Epstein-Barr virus-transformed B cells, and the ability of these cells to stimulate human CD4+ T cells was impaired in the presence of VacA (152). VacA inhibited the Ii-dependent pathway of antigen presentation mediated by newly synthesized MHC class II molecules but did not affect the pathway dependent on recycling MHC class II (152). Expression of CagA in B cells is reported to inhibit interleukin-3-dependent B-cell proliferation by inhibiting JAK-STAT signaling, which may result in inefficient antibody production and reduced cytokine expression (234).
Interactions of H. pylori with T Lymphocytes
In vitro experiments indicate that live H. pylori or H. pylori products can interfere with multiple functions of T lymphocytes (37, 77, 78, 219, 256) (Fig. 4). One report indicated that H. pylori can have proapoptotic effects on T cells (242), but most of the observed effects occur in the absence of cell death. Coincubation of H. pylori with T cells results in diminished expression of IL-2 and IL-2 receptor (CD25), inhibition of activation-induced proliferation, and cell cycle arrest (37, 77, 78, 219, 256).
FIG. 4.
Effects of H. pylori on T lymphocytes. Multiple H. pylori factors can suppress T-cell activity. VacA inhibits NFAT activity in T cells, leading to diminished IL-2 production, and also inhibits T-cell proliferation (37, 77, 219). Arginase inhibits T-cell receptor (TCR) signaling (256). An unidentified low-molecular-weight protein has been reported to inhibit T-cell proliferation by blocking cell cycle progression (78).
The effects of H. pylori on T cells are mediated by several different bacterial factors, one of which is VacA. VacA interferes with the activity of nuclear factor of activated T cells (NFAT), a transcription factor that regulates immune response genes, in Jurkat T cells, resulting in inhibition of IL-2 expression and G1/S cell cycle arrest (37, 77). The effects of VacA on Jurkat cells may be mediated by blocking calcium influx, thereby interfering with the activity of the calcium-dependent phosphatase calcineurin, which is required for NFAT activation (37, 77). VacA also activates intracellular signaling in T cells through mitogen-activated protein kinases MKK3/6 and p38 and the Rac-specific nucleotide exchange factor Vav, thereby causing disorganized actin polymerization (37). Studies of VacA effects on primary human CD4+ T cells indicate that VacA can also inhibit T-cell proliferation through a process that is not dependent on inhibition of NFAT (219).
H. pylori arginase also contributes to inhibition of T-cell proliferation. One study reported that incubation of T cells with a wild-type H. pylori strain, but not an arginase mutant strain, caused decreased expression of the CD3ζ chain of the T-cell receptor (256). In addition to VacA and arginase, an uncharacterized low-molecular-weight protein of H. pylori has been reported to inhibit proliferation of T lymphocytes (78). This low-molecular-weight H. pylori factor is reported to block cell cycle progression at the G1 phase.
CONCLUDING REMARKS
H. pylori persistently colonizes the human stomach despite development of a humoral and cellular immune response. Animal models have been very useful in identifying H. pylori factors that are required for colonization of the stomach and for elucidating the roles of various host factors in the development of gastric mucosal inflammation. A protective immune response to H. pylori can be elicited in animal models by immunization, but the immune effector components that mediate protection remain incompletely defined. H. pylori can alter the function of many different types of immune cells in vitro. These in vitro experiments provide important insights into the molecular mechanisms by which H. pylori resists immune clearance. However, further studies are needed to determine which in vitro interactions are most relevant in vivo. Future studies, directed toward understanding interactions between H. pylori and immune cells in vivo, are expected to lead to important new insights into the mechanisms of H. pylori persistence and also may lead to the development of novel therapeutic approaches for eradication of H. pylori.
Acknowledgments
We regret that many relevant publications have not been cited here due to space limitations. We are grateful for stimulating discussions with members of the Cover, Peek, and Wilson laboratories.
This work was supported by NIH grants R01 AI39657, R01 DK53623, and T32 AI 07474 and by the Department of Veterans Affairs.
REFERENCES
- 1.Akhiani, A. A. 2005. The role of type-specific antibodies in colonization and infection by Helicobacter pylori. Curr. Opin. Infect. Dis. 18:223-227. [DOI] [PubMed] [Google Scholar]
- 2.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 3.Akhiani, A. A., K. Schon, L. E. Franzen, J. Pappo, and N. Lycke. 2004. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 172:5024-5033. [DOI] [PubMed] [Google Scholar]
- 4.Akhiani, A. A., K. Schon, and N. Lycke. 2004. Vaccine-induced immunity against Helicobacter pylori infection is impaired in IL-18-deficient mice. J. Immunol. 173:3348-3356. [DOI] [PubMed] [Google Scholar]
- 5.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 6.Allen, L. A., and J. A. Allgood. 2002. Atypical protein kinase C-zeta is essential for delayed phagocytosis of Helicobacter pylori. Curr. Biol. 12:1762-1766. [DOI] [PubMed] [Google Scholar]
- 7.Allen, L. A., B. R. Beecher, J. T. Lynch, O. V. Rohner, and L. M. Wittine. 2005. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 174:3658-3667. [DOI] [PubMed] [Google Scholar]
- 8.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amedei, A., M. P. Bergman, B. J. Appelmelk, A. Azzurri, M. Benagiano, C. Tamburini, R. van der Zee, J. L. Telford, C. M. Vandenbroucke-Grauls, M. M. D'Elios, and G. Del Prete. 2003. Molecular mimicry between Helicobacter pylori antigens and H+,K+-adenosine triphosphatase in human gastric autoimmunity. J. Exp. Med. 198:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amieva, M. R., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell. Microbiol. 4:677-690. [DOI] [PubMed] [Google Scholar]
- 11.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA 102:9247-9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appelmelk, B. J., Y. Q. An, M. Geerts, B. G. Thijs, H. A. de Boer, D. M. MacLaren, J. de Graaff, and J. H. Nuijens. 1994. Lactoferrin is a lipid A-binding protein. Infect. Immun. 62:2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appelmelk, B. J., S. L. Martin, M. A. Monteiro, C. A. Clayton, A. A. McColm, P. Zheng, T. Verboom, J. J. Maaskant, D. H. van den Eijnden, C. H. Hokke, M. B. Perry, C. M. Vandenbroucke-Grauls, and J. G. Kusters. 1999. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in α3-fucosyltransferase genes. Infect. Immun. 67:5361-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelmelk, B. J., I. van Die, S. J. van Vliet, C. M. Vandenbroucke-Grauls, T. B. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 16.Aras, R. A., W. Fischer, G. I. Perez-Perez, M. Crosatti, T. Ando, R. Haas, and M. J. Blaser. 2003. Plasticity of repetitive DNA sequences within a bacterial (type IV) secretion system component. J. Exp. Med. 198:1349-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archimandritis, A., S. Sougioultzis, P. G. Foukas, M. Tzivras, P. Davaris, and H. M. Moutsopoulos. 2000. Expression of HLA-DR, costimulatory molecules B7-1, B7-2, intercellular adhesion molecule-1 (ICAM-1) and Fas ligand (FasL) on gastric epithelial cells in Helicobacter pylori gastritis; influence of H. pylori eradication. Clin. Exp. Immunol. 119:464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asahi, M., T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aspinall, G. O., and M. A. Monteiro. 1996. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry 35:2498-2504. [DOI] [PubMed] [Google Scholar]
- 20.Ayraud, S., B. Janvier, and J. L. Fauchere. 2002. Experimental colonization of mice by fresh clinical isolates of Helicobacter pylori is not influenced by the cagA status and the vacA genotype. FEMS Immunol. Med. Microbiol. 34:169-172. [DOI] [PubMed] [Google Scholar]
- 21.Backhed, F., B. Rokbi, E. Torstensson, Y. Zhao, C. Nilsson, D. Seguin, S. Normark, A. M. Buchan, and A. Richter-Dahlfors. 2003. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J. Infect. Dis. 187:829-836. [DOI] [PubMed] [Google Scholar]
- 22.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori infection have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 23.Basu, M., S. J. Czinn, and T. G. Blanchard. 2004. Absence of catalase reduces long-term survival of Helicobacter pylori in macrophage phagosomes. Helicobacter 9:211-216. [DOI] [PubMed] [Google Scholar]
- 24.Bauer, F., K. Schweimer, E. Kluver, J. R. Conejo-Garcia, W. G. Forssmann, P. Rosch, K. Adermann, and H. Sticht. 2001. Structure determination of human and murine beta-defensins reveals structural conservation in the absence of significant sequence similarity. Protein Sci. 10:2470-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beales, I., J. Calam, L. Post, S. Srinivasan, T. Yamada, and J. DelValle. 1997. Effect of transforming growth factor alpha and interleukin 8 on somatostatin release from canine fundic D cells. Gastroenterology 112:136-143. [DOI] [PubMed] [Google Scholar]
- 26.Berg, D. J., N. A. Lynch, R. G. Lynch, and D. M. Lauricella. 1998. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am. J. Pathol. 152:1377-1386. [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman, M. P., A. Engering, H. H. Smits, S. J. van Vliet, A. A. van Bodegraven, H. P. Wirth, M. L. Kapsenberg, C. M. J. E. Vanderbroucke-Grauls, Y. van Kooyk, and B. J. Appelmelk. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berstad, A. E., M. Kilian, K. N. Valnes, and P. Brandtzaeg. 1999. Increased mucosal production of monomeric IgA1 but no IgA1 protease activity in Helicobacter pylori gastritis. Am. J. Pathol. 155:1097-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betten, A., J. Bylund, T. Cristophe, F. Boulay, A. Romero, K. Hellstrand, and C. Dahlgren. 2001. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J. Clin. Investig. 108:1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanchard, T. G., S. J. Czinn, R. Maurer, W. D. Thomas, G. Soman, and J. G. Nedrud. 1995. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect. Immun. 63:1394-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard, T. G., F. Yu, C. L. Hsieh, and R. W. Redline. 2003. Severe inflammation and reduced bacteria load in murine Helicobacter infection caused by lack of phagocyte oxidase activity. J. Infect. Dis. 187:1609-1615. [DOI] [PubMed] [Google Scholar]
- 33.Blaser, M. J. 2005. The biology of cag in the Helicobacter pylori-human interaction. Gastroenterology 128:1512-1515. [DOI] [PubMed] [Google Scholar]
- 34.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bliss, C. M., Jr., D. T. Golenbock, S. Keates, J. K. Linevsky, and C. P. Kelly. 1998. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect. Immun. 66:5357-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogstedt, A. K., S. Nava, T. Wadstrom, and L. Hammarstrom. 1996. Helicobacter pylori infections in IgA deficiency: lack of role for the secretory immune system. Clin. Exp. Immunol. 105:202-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boncristiano, M., S. R. Paccani, S. Barone, C. Ulivieri, L. Patrussi, D. Ilver, A. Amedei, M. M. D'Elios, J. L. Telford, and C. T. Baldari. 2003. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J. Exp. Med. 198:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-κB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 102:9300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breton, A., and A. Descoteaux. 2000. Protein kinase C-alpha participates in FcγR-mediated phagocytosis in macrophages. Biochem. Biophys. Res. Commun. 276:472-476. [DOI] [PubMed] [Google Scholar]
- 40.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaturvedi, R., Y. Cheng, M. Asim, F. I. Bussiere, H. Xu, A. P. Gobert, A. Hacker, R. A. Casero, Jr., and K. T. Wilson. 2004. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J. Biol. Chem. 279:40161-40173. [DOI] [PubMed] [Google Scholar]
- 42.Chen, W., D. Shu, and V. S. Chadwick. 1999. Helicobacter pylori infection in interleukin-4-deficient and transgenic mice. Scand. J. Gastroenterol. 34:987-992. [DOI] [PubMed] [Google Scholar]
- 43.Chen, W., D. Shu, and V. S. Chadwick. 2001. Helicobacter pylori infection: mechanism of colonization and functional dyspepsia. Reduced colonization of gastric mucosa by Helicobacter pylori in mice deficient in interleukin-10. J. Gastroenterol. Hepatol. 16:377-383. [DOI] [PubMed] [Google Scholar]
- 44.Cheng, Y., R. Chaturvedi, M. Asim, F. I. Bussiere, H. Xu, R. A. Casero, Jr., and K. T. Wilson. 2005. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J. Biol. Chem. 280:22492-22496. [DOI] [PubMed] [Google Scholar]
- 45.Correa, P. 1992. Human gastric carcinogenesis: a multistep and multifactorial process. Cancer Res. 52:6735-6740. [PubMed] [Google Scholar]
- 46.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3:320-332. [DOI] [PubMed] [Google Scholar]
- 47.Crabtree, J. E., T. M. Shallcross, R. V. Heatley, and J. I. Wyatt. 1991.. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czinn, S. J., A. Cai, and J. G. Nedrud. 1993. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11:637-642. [DOI] [PubMed] [Google Scholar]
- 49.de Bernard, M., A. Cappon, L. Pancotto, P. Ruggiero, J. Rivera, G. Del Giudice, and C. Montecucco. 2005. The Helicobacter pylori VacA cytotoxin activates RBL-2H3 cells by inducing cytosolic calcium oscillations. Cell. Microbiol. 7:191-198. [DOI] [PubMed] [Google Scholar]
- 50.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 51.D'Elios, M. M., M. P. Bergman, A. Amedei, B. J. Appelmelk, and G. Del Prete. 2004. Helicobacter pylori and gastric autoimmunity. Microbes Infect. 6:1395-1401. [DOI] [PubMed] [Google Scholar]
- 52.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 53.Deml, L., M. Aigner, J. Decker, A. Eckhardt, C. Schutz, P. R. Mittl, S. Barabas, S. Denk, G. Knoll, N. Lehn, and W. Schneider-Brachert. 2005. Characterization of the Helicobacter pylori cysteine-rich protein A as a T-helper cell type 1 polarizing agent. Infect. Immun. 73:4732-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Tommaso, A., Z. Xiang, M. Bugnoli, P. Pileri, N. Figura, P. F. Bayeli, R. Rappuoli, S. Abrignani, and M. T. De Magistris. 1995. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect. Immun. 63:1102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney system. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 56.Dooley, C. P., H. Cohen, P. L. Fitzgibbons, M. Bauer, M. D. Appleman, G. I. Perez-Perez, and M. J. Blaser. 1989. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N. Engl. J. Med. 321:1562-1566. [DOI] [PubMed] [Google Scholar]
- 57.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, J. Del Valle, M. Yang, H. P. Wirth, G. I. Perez-Perez, and M. J. Blaser. 1999. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology 116:90-96. [DOI] [PubMed] [Google Scholar]
- 58.Dubois, A., N. Fiala, L. M. Heman-Ackah, E. S. Drazek, A. Tarnawski, W. N. Fishbein, G. I. Perez-Perez, and M. J. Blaser. 1994. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology 106:1405-1417. [DOI] [PubMed] [Google Scholar]
- 59.Eaton, K. A., and S. Krakowka. 1994. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect. Immun. 62:3604-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 61.Eaton, K. A., S. R. Ringler, and S. J. Danon. 1999. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect. Immun. 67:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 64:2445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Omar, E. M. 2001. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut 48:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 65.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2001. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature 412:99. [DOI] [PubMed] [Google Scholar]
- 66.El-Omar, E. M., C. S. Rabkin, M. D. Gammon, T. L. Vaughan, H. A. Risch, J. B. Schoenberg, J. L. Stanford, S. T. Mayne, J. Goedert, W. J. Blot, J. F. Fraumeni, Jr., and W. H. Chow. 2003. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124:1193-1201. [DOI] [PubMed] [Google Scholar]
- 67.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans, D. J., Jr., D. G. Evans, T. Takemura, H. Nakano, H. C. Lampert, D. Y. Graham, D. N. Granger, and P. R. Kvietys. 1995. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect. Immun. 63:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrero, R. L., J. M. Thiberge, and A. Labigne. 1997. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology 113:185-194. [DOI] [PubMed] [Google Scholar]
- 70.Figueiredo, C., J. C. Machado, P. Pharoah, R. Seruca, S. Sousa, R. Carvalho, A. F. Capelinha, W. Quint, C. Caldas, L. J. van Doorn, F. Carneiro, and M. Sobrinho-Simoes. 2002. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J. Natl. Cancer Inst. 94:1680-1687. [DOI] [PubMed] [Google Scholar]
- 71.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 72.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces Helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 73.Franco, A. T., D. A. Israel, M. K. Washington, U. Krishna, J. G. Fox, A. B. Rogers, A. S. Neish, L. Collier-Hyams, G. I. Perez-Perez, M. Hatakeyama, R. Whitehead, K. Gaus, D. P. O'Brien, J. Romero-Gallo, and R. M. Peek, Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 102:10646-10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frye, M., J. Bargon, B. Lembcke, T. O. Wagner, and R. Gropp. 2000. Differential expression of human alpha- and beta-defensins mRNA in gastrointestinal epithelia. Eur. J. Clin. Investig. 30:695-701. [DOI] [PubMed] [Google Scholar]
- 75.Garhart, C. A., F. P. Heinzel, S. J. Czinn, and J. G. Nedrud. 2003. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect. Immun. 71:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garhart, C. A., J. G. Nedrud, F. P. Heinzel, N. E. Sigmund, and S. J. Czinn. 2003. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect. Immun. 71:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 78.Gerhard, M., C. Schmees, P. Voland, N. Endres, M. Sander, W. Reindl, R. Rad, M. Oelsner, T. Decker, M. Mempel, L. Hengst, and C. Prinz. 2005. A secreted low-molecular-weight protein from Helicobacter pylori induces cell-cycle arrest of T cells. Gastroenterology 128:1327-1339. [DOI] [PubMed] [Google Scholar]
- 79.Gewirtz, A. T., Y. Yu, U. S. Krishna, D. A. Israel, S. L. Lyons, and R. M. Peek, Jr. 2004. Helicobacter pylori flagellin evades Toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 189:1914-1920. [DOI] [PubMed] [Google Scholar]
- 80.Gifford, J. L., H. N. Hunter, and H. J. Vogel. 2005. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 62:2588-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gobert, A. P., J. C. Bambou, C. Werts, V. Balloy, M. Chignard, A. P. Moran, and R. L. Ferrero. 2004. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a Toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J. Biol. Chem. 279:245-250. [DOI] [PubMed] [Google Scholar]
- 82.Gobert, A. P., Y. Cheng, J. Y. Wang, J. L. Boucher, R. K. Iyer, S. D. Cederbaum, R. A. Casero, Jr., J. C. Newton, and K. T. Wilson. 2002. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J. Immunol. 168:4692-4700. [DOI] [PubMed] [Google Scholar]
- 83.Gobert, A. P., D. J. McGee, M. Akhtar, G. L. Mendz, J. C. Newton, Y. Cheng, H. L. Mobley, and K. T. Wilson. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl. Acad. Sci. USA 98:13844-13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 85.Graham, D. Y., L. C. Alpert, J. L. Smith, and H. H. Yoshimura. 1988. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am. J. Gastroenterol. 83:974-980. [PubMed] [Google Scholar]
- 86.Graham, D. Y., A. R. Opekun, M. S. Osata, H. M. T. El-Zimaity, C. K. Lee, Y. Yamaoka, W. A. Qureshi, M. Cadoz, and T. P. Monath. 2004. Challenge model for Helicobacter pylori infection in human volunteers. Gut 53:1235-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guillemin, K., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 99:15136-15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guiney, D. G., P. Hasegawa, and S. P. Cole. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo, B. P., and J. J. Mekalanos. 2002. Rapid genetic analysis of Helicobacter pylori gastric mucosal colonization in suckling mice. Proc. Natl. Acad. Sci. USA 99:8354-8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guruge, J. L., P. G. Falk, R. G. Lorenz, M. Dans, H. P. Wirth, M. J. Blaser, D. E. Berg, and J. I. Gordon. 1998. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 95:3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haeberle, H. A., M. Kubin, K. B. Bamford, R. Garofalo, D. Y. Graham, F. El-Zaatari, R. Karttunen, S. E. Crowe, V. E. Reyes, and P. B. Ernst. 1997. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 65:4229-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hafsi, N., P. Voland, S. Schwendy, R. Rad, W. Reindl, M. Gerhard, and C. Prinz. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 173:1249-1257. [DOI] [PubMed] [Google Scholar]
- 93.Harris, P. R., P. B. Ernst, S. Kawabata, H. Kiyono, M. F. Graham, and P. D. Smith. 1998. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J. Infect. Dis. 178:1516-1520. [DOI] [PubMed] [Google Scholar]
- 94.Hase, K., M. Murakami, M. Iimura, S. P. Cole, Y. Horibe, T. Ohtake, M. Obonyo, R. L. Gallo, L. Eckmann, and M. F. Kagnoff. 2003. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125:1613-1625. [DOI] [PubMed] [Google Scholar]
- 95.Hatakeyama, M. 2004. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4:688-694. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi, S., T. Sugiyama, K. Yokota, H. Isogai, E. Isogai, K. Oguma, M. Asaka, N. Fujii, and Y. Hirai. 1998. Analysis of immunoglobulin A antibodies to Helicobacter pylori in serum and gastric juice in relation to mucosal inflammation. Clin. Diagn. Lab. Immunol. 5:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hazell, S. L., A. Lee, L. Brady, and W. Hennessy. 1986. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J. Infect. Dis. 153:658-663. [DOI] [PubMed] [Google Scholar]
- 98.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 99.Hoffman, P. S., N. Vats, D. Hutchison, J. Butler, K. Chisholm, G. Sisson, A. Raudonikiene, J. S. Marshall, and S. J. Veldhuyzen van Zanten. 2003. Development of an interleukin-12-deficient mouse model that is permissive for colonization by a motile KE26695 strain of Helicobacter pylori. Infect. Immun. 71:2534-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Honda, S., T. Fujioka, M. Tokieda, R. Satoh, A. Nishizono, and M. Nasu. 1998. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 58:4255-4259. [PubMed] [Google Scholar]
- 101.Hwang, I. R., T. Kodama, S. Kikuchi, K. Sakai, L. E. Peterson, D. Y. Graham, and Y. Yamaoka. 2002. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1β production in Helicobacter pylori infection. Gastroenterology 123:1793-1803. [DOI] [PubMed] [Google Scholar]
- 102.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 103.Ishihara, S., M. A. Rumi, Y. Kadowaki, C. F. Ortega-Cava, T. Yuki, N. Yoshino, Y. Miyaoka, H. Kazumori, N. Ishimura, Y. Amano, and Y. Kinoshita. 2004. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J. Immunol. 173:1406-1416. [DOI] [PubMed] [Google Scholar]
- 104.Ismail, H. F., P. Fick, J. Zhang, R. G. Lynch, and D. J. Berg. 2003. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 170:3782-3789. [DOI] [PubMed] [Google Scholar]
- 105.Israel, D. A., N. Salama, C. N. Arnold, S. F. Moss, T. Ando, H. P. Wirth, K. T. Tham, M. Camorlinga, M. J. Blaser, S. Falkow, and R. M. Peek, Jr. 2001. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J. Clin. Investig. 107:611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeremy, A. H., Y. Du, M. F. Dixon, P. A. Robinson, and J. E. Crabtree. 2006. Protection against Helicobacter pylori infection in the Mongolian gerbil after prophylactic vaccination. Microbes Infect. 8:340-346. [DOI] [PubMed] [Google Scholar]
- 107.Jones, N. L., A. S. Day, H. Jennings, P. T. Shannon, E. Galindo-Mata, and P. M. Sherman. 2002. Enhanced disease severity in Helicobacter pylori-infected mice deficient in Fas signaling. Infect. Immun. 70:2591-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jonsson, K., B. P. Guo, H. J. Monstein, J. J. Mekalanos, and G. Kronvall. 2004. Molecular cloning and characterization of two Helicobacter pylori genes coding for plasminogen-binding proteins. Proc. Natl. Acad. Sci. USA 101:1852-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamradt, A. E., M. Greiner, P. Ghiara, and S. H. Kaufmann. 2000. Helicobacter pylori infection in wild-type and cytokine-deficient C57BL/6 and BALB/c mouse mutants. Microb. Infect. 2:593-597. [DOI] [PubMed] [Google Scholar]
- 110.Karttunen, R., T. Karttunen, H. P. Ekre, and T. T. MacDonald. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kavermann, H., B. P. Burns, K. Angermuller, S. Odenbreit, W. Fischer, K. Melchers, and R. Haas. 2003. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J. Exp. Med. 197:813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawakubo, M., Y. Ito, Y. Okimura, M. Kobayashi, K. Sukara, S. Kasama, M. N. Fukuda, M. Fukuda, T. Katsuyama, and J. Nakayama. 2004. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003-1006. [DOI] [PubMed] [Google Scholar]
- 113.Kelso, A., A. B. Troutt, E. Maraskovsky, N. M. Gough, L. Morris, M. H. Pech, and J. A. Thomson. 1991. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol. Rev. 123:85-114. [DOI] [PubMed] [Google Scholar]
- 114.Khamri, W., A. P. Moran, M. L. Worku, Q. N. Karim, M. M. Walker, H. Annuk, J. A. Ferris, B. J. Appelmelk, P. Eggleton, K. B. Reid, and M. R. Thursz. 2005. Variations in Helicobacter pylori lipopolysaccharide to evade the innate immune component surfactant protein D. Infect. Immun. 73:7677-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kranzer, K., A. Eckhardt, M. Aigner, G. Knoll, L. Deml, C. Speth, N. Lehn, M. Rehli, and W. Schneider-Brachert. 2004. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect. Immun. 72:4416-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuipers, E. J., D. A. Israel, J. G. Kusters, M. M. Gerrits, J. Weel, A. van Der Endeqq, R. W. van Der Hulstqq, H. P. Wirth, J. Hook-Nikanne, S. A. Thompson, and M. J. Blaser. 2000. Quasispecies development of Helicobacter pylori observed in paired isolates obtained years apart from the same host. J. Infect. Dis. 181:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuipers, E. J., G. I. Perez-Perez, S. G. Meuwissen, and M. J. Blaser. 1995. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J. Natl. Cancer Inst. 87:1777-1780. [DOI] [PubMed] [Google Scholar]
- 118.Kumar, V. 2004. Homeostatic control of immunity by TCR peptide-specific Tregs. J. Clin. Investig. 114:1222-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwok, T., S. Backert, H. Schwarz, J. Berger, and T. F. Meyer. 2002. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect. Immun. 70:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larsen, E. C., J. A. DiGennaro, N. Saito, S. Mehta, D. J. Loegering, J. E. Mazurkiewicz, and M. R. Lennartz. 2000. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J. Immunol. 165:2809-2817. [DOI] [PubMed] [Google Scholar]
- 121.Lee, A. 2000. Animal models of gastroduodenal ulcer disease. Baillieres Best Pract. Res. Clin. Gastroenterol. 14:75-96. [DOI] [PubMed] [Google Scholar]
- 122.Lee, C. K., R. Weltzin, W. D. Thomas, Jr., H. Kleanthous, T. H. Ermak, G. Soman, J. E. Hill, S. K. Ackerman, and T. P. Monath. 1995. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J. Infect. Dis. 172:161-172. [DOI] [PubMed] [Google Scholar]
- 123.Lee, S. K., A. Stack, E. Katzowitsch, S. I. Aizawa, S. Suerbaum, and C. Josenhans. 2003. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 5:1345-1356. [DOI] [PubMed] [Google Scholar]
- 124.Lepper, P. M., M. Triantafilou, C. Schumann, E. M. Schneider, and K. Triantafilou. 2005. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell. Microbiol. 7:519-528. [DOI] [PubMed] [Google Scholar]
- 125.Li, S. D., D. Kersulyte, I. J. Lindley, B. Neelam, D. E. Berg, and J. E. Crabtree. 1999. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect. Immun. 67:3893-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loughlin, M. F., F. M. Barnard, D. Jenkins, G. J. Sharples, and P. J. Jenks. 2003. Helicobacter pylori mutants defective in RuvC Holliday junction resolvase display reduced macrophage survival and spontaneous clearance from the murine gastric mucosa. Infect. Immun. 71:2022-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu, H., P. I. Hsu, D. Y. Graham, and Y. Yamaoka. 2005. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology 128:833-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lucas, B., D. Bumann, A. Walduck, J. Koesling, L. Develioglu, T. F. Meyer, and T. Aebischer. 2001. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lundgren, A., E. Stromberg, A. Sjoling, C. Lindholm, K. Enarsson, A. Edebo, E. Johnsson, E. Suri-Payer, P. Larsson, A. Rudin, A. M. Svennerholm, and B. S. Lundin. 2005. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 73:523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lundgren, A., E. Suri-Payer, K. Enarsson, A. M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lundgren, A., C. Trollmo, A. Edebo, A. M. Svennerholm, and B. S. Lundin. 2005. Helicobacter pylori-specific CD4+ T cells home to and accumulate in the human Helicobacter pylori-infected gastric mucosa. Infect. Immun. 73:5612-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luqmani, Y. A., T. A. Campbell, C. Bennett, R. C. Coombes, and I. M. Paterson. 1991. Expression of lactoferrin in human stomach. Int. J. Cancer 49:684-687. [DOI] [PubMed] [Google Scholar]
- 134.Machado, J. C., C. Figueiredo, P. Canedo, P. Pharoah, R. Carvalho, S. Nabais, C. Castro Alves, M. L. Campos, L. J. Van Doorn, C. Caldas, R. Seruca, F. Carneiro, and M. Sobrinho-Simoes. 2003. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology 125:364-371. [DOI] [PubMed] [Google Scholar]
- 135.Machado, J. C., P. Pharoah, S. Sousa, R. Carvalho, C. Oliveira, C. Figueiredo, A. Amorim, R. Seruca, C. Caldas, F. Carneiro, and M. Sobrinho-Simoes. 2001. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology 121:823-829. [DOI] [PubMed] [Google Scholar]
- 136.Maeda, S., M. Akanuma, Y. Mitsuno, Y. Hirata, K. Ogura, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 276:44856-44864. [DOI] [PubMed] [Google Scholar]
- 137.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mahler, M., C. Janke, S. Wagner, and H. J. Hedrich. 2002. Differential susceptibility of inbred mouse strains to Helicobacter pylori infection. Scand. J. Gastroenterol. 37:267-278. [DOI] [PubMed] [Google Scholar]
- 139.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mai, U. E., G. I. Perez-Perez, L. M. Wahl, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1991. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J. Clin. Investig. 87:894-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mandell, L., A. P. Moran, A. Cocchiarella, J. Houghton, N. Taylor, J. G. Fox, T. C. Wang, and E. A. Kurt-Jones. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect. Immun. 72:6446-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 143.Marshall, B. J., J. A. Armstrong, D. B. McGechie, and R. J. Glancy. 1985. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med. J. Aust. 142:436-439. [DOI] [PubMed] [Google Scholar]
- 144.Marshall, B. J., L. J. Barrett, C. Prakash, R. W. McCallum, and R. L. Guerrant. 1990. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology 99:697-702. [DOI] [PubMed] [Google Scholar]
- 145.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 146.Mattsson, A., H. Lonroth, M. Quiding-Jarbrink, and A. M. Svennerholm. 1998. Induction of B cell responses in the stomach of Helicobacter pylori-infected subjects after oral cholera vaccination. J. Clin. Investig. 102:51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattsson, A., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, I. Ahlstedt, and A. Svennerholm. 1998. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect. Immun. 66:2705-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matz-Rensing, K., E. Kunz, C. Kraft, D. Lorenzen, S. Suerbaum, and F. J. Kaup. 2001. Experimental Helicobacter pylori infection of rhesus macaques (Macaca mulatta). Int. J. Med. Microbiol. 291:33-43. [DOI] [PubMed] [Google Scholar]
- 149.McGee, D. J., M. L. Langford, E. L. Watson, J. E. Carter, Y. T. Chen, and K. M. Ottemann. 2005. Colonization and inflammation deficiencies in Mongolian gerbils infected by Helicobacter pylori chemotaxis mutants. Infect. Immun. 73:1820-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Minoura, T., S. Kato, S. Otsu, M. Kodama, T. Fujioka, K. Iinuma, and A. Nishizono. 2005. Influence of age and duration of infection on bacterial load and immune responses to Helicobacter pylori infection in a murine model. Clin. Exp. Immunol. 139:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. J. Czinn. 1997. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 152.Molinari, M., M. Salio, C. Galli, N. Norais, R. Rappuoli, A. Lanzavecchia, and C. Montecucco. 1998. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 154.Monteiro, M. A., K. H. Chan, D. A. Rasko, D. E. Taylor, P. Y. Zheng, B. J. Appelmelk, H. P. Wirth, M. Yang, M. J. Blaser, S. O. Hynes, A. P. Moran, and M. B. Perry. 1998. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. J. Biol. Chem. 273:11533-11543. [DOI] [PubMed] [Google Scholar]
- 155.Montemurro, P., G. Barbuti, W. G. Dundon, G. Del Giudice, R. Rappuoli, M. Colucci, P. De Rinaldis, C. Montecucco, N. Semeraro, and E. Papini. 2001. Helicobacter pylori neutrophil-activating protein stimulates tissue factor and plasminogen activator inhibitor-2 production by human blood mononuclear cells. J. Infect. Dis. 183:1055-1062. [DOI] [PubMed] [Google Scholar]
- 156.Montemurro, P., H. Nishioka, W. G. Dundon, M. de Bernard, G. Del Giudice, R. Rappuoli, and C. Montecucco. 2002. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur. J. Immunol. 32:671-676. [DOI] [PubMed] [Google Scholar]
- 157.Moran, A. P., and G. O. Aspinall. 1998. Unique structural and biological features of Helicobacter pylori lipopolysaccharides. Prog. Clin. Biol. Res. 397:37-49. [PubMed] [Google Scholar]
- 158.Moran, A. P., W. Khamri, M. M. Walker, and M. R. Thursz. 2005. Role of surfactant protein D (SP-D) in innate immunity in the gastric mucosa: evidence of interaction with Helicobacter pylori lipopolysaccharide. J. Endotoxin Res. 11:357-362. [DOI] [PubMed] [Google Scholar]
- 159.Morris, A., and G. Nicholson. 1987. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am. J. Gastroenterol. 82:192-199. [PubMed] [Google Scholar]
- 160.Morris, A. J., M. R. Ali, G. I. Nicholson, et al. 1991. Long-term follow up of voluntary ingestion of Helicobacter pylori. Ann. Intern. Med. 114:662-663. [DOI] [PubMed] [Google Scholar]
- 161.Mueller, A., D. S. Merrell, J. Grimm, and S. Falkow. 2004. Profiling of microdissected gastric epithelial cells reveals a cell type-specific response to Helicobacter pylori infection. Gastroenterology 127:1446-1462. [DOI] [PubMed] [Google Scholar]
- 162.Muotiala, A., I. M. Helander, L. Pyhala, T. U. Kosunen, and A. P. Moran. 1992. Low biological activity of Helicobacter pylori lipopolysaccharide. Infect. Immun. 60:1714-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murphy, P. M. 1997. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin. Hematol. 34:311-318. [PubMed] [Google Scholar]
- 164.Murray, E., W. Khamri, M. M. Walker, P. Eggleton, A. P. Moran, J. A. Ferris, S. Knapp, Q. N. Karim, M. Worku, P. Strong, K. B. Reid, and M. R. Thursz. 2002. Expression of surfactant protein D in the human gastric mucosa and during Helicobacter pylori infection. Infect. Immun. 70:1481-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Neutra, M. R., E. Pringault, and J. P. Kraehenbuhl. 1996. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 14:275-300. [DOI] [PubMed] [Google Scholar]
- 166.Nilsson, C., A. Sillen, L. Eriksson, M. L. Strand, H. Enroth, S. Normark, P. Falk, and L. Engstrand. 2003. Correlation between cag pathogenicity island composition and Helicobacter pylori-associated gastroduodenal disease. Infect. Immun. 71:6573-6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishi, T., K. Okazaki, K. Kawasaki, T. Fukui, H. Tamaki, M. Matsuura, M. Asada, T. Watanabe, K. Uchida, N. Watanabe, H. Nakase, M. Ohana, H. Hiai, and T. Chiba. 2003. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect. Immun. 71:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nurgalieva, Z. Z., M. E. Conner, A. R. Opekun, C. Q. Zheng, S. N. Elliott, P. B. Ernst, M. Osato, M. K. Estes, and D. Y. Graham. 2005. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infect. Immun. 73:2999-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Obonyo, M., D. G. Guiney, J. Harwood, J. Fierer, and S. P. Cole. 2002. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect. Immun. 70:3295-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell. Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 171.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 172.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 192:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oh, J. D., S. M. Karam, and J. I. Gordon. 2005. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl. Acad. Sci. USA 102:5186-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.O'Rourke, J. L., and A. Lee. 2003. Animal models of Helicobacter pylori infection and disease. Microbes Infect. 5:741-748. [DOI] [PubMed] [Google Scholar]
- 175.Orsi, N. 2004. The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17:189-196. [DOI] [PubMed] [Google Scholar]
- 176.Papini, E., B. Satin, N. Norais, M. de Bernard, J. L. Telford, R. Rappuoli, and C. Montecucco. 1998. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Investig. 102:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Parsonnet, J. 1998. Helicobacter pylori. Infect. Dis. Clin. N. Am. 12:185-197. [DOI] [PubMed] [Google Scholar]
- 179.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 180.Perez-Perez, G. I., B. M. Dworkin, J. E. Chodos, and M. J. Blaser. 1988. Campylobacter pylori antibodies in humans. Ann. Intern. Med. 109:11-17. [DOI] [PubMed] [Google Scholar]
- 181.Perez-Perez, G. I., V. L. Shepherd, J. D. Morrow, and M. J. Blaser. 1995. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect. Immun. 63:1183-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peterson, R. A., II, T. Hoepf, and K. A. Eaton. 2003. Adoptive transfer of splenocytes in SCID mice implicates CD4+ T cells in apoptosis and epithelial proliferation associated with Helicobacter pylori-induced gastritis. Comp. Med. 53:498-509. [PubMed] [Google Scholar]
- 183.Peterson, W. L. 1991. Helicobacter pylori and peptic ulcer disease. N. Engl. J. Med. 324:1043-1048. [DOI] [PubMed] [Google Scholar]
- 184.Quiding-Jarbrink, M., B. S. Lundin, H. Lonroth, and A. M. Svennerholm. 2001. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin. Exp. Immunol. 123:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Quigley, E. M., and L. A. Turnberg. 1987. pH of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology 92:1876-1884. [DOI] [PubMed] [Google Scholar]
- 186.Rad, R., M. Gerhard, R. Lang, M. Schoniger, T. Rosch, W. Schepp, I. Becker, H. Wagner, and C. Prinz. 2002. The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. J. Immunol. 168:3033-3041. [DOI] [PubMed] [Google Scholar]
- 187.Rad, R., C. Prinz, B. Neu, M. Neuhofer, M. Zeitner, P. Voland, I. Becker, W. Schepp, and M. Gerhard. 2003. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J. Infect. Dis. 188:272-281. [DOI] [PubMed] [Google Scholar]
- 188.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ramarao, N., S. D. Gray-Owen, and T. F. Meyer. 2000. Helicobacter pylori induces but survives the extracellular release of oxygen radicals from professional phagocytes using its catalase activity. Mol. Microbiol. 38:103-113. [DOI] [PubMed] [Google Scholar]
- 190.Ramarao, N., and T. F. Meyer. 2001. Helicobacter pylori resists phagocytosis by macrophages: quantitative assessment by confocal microscopy and fluorescence-activated cell sorting. Infect. Immun. 69:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ramsey, E. J., K. V. Carey, W. L. Peterson, J. J. Jackson, F. K. Murphy, N. W. Read, K. B. Taylor, J. S. Trier, and J. S. Fordtran. 1979. Epidemic gastritis with hypochlorhydria. Gastroenterology 76:1449-1457. [PubMed] [Google Scholar]
- 192.Rieder, G., J. L. Merchant, and R. Haas. 2005. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128:1229-1242. [DOI] [PubMed] [Google Scholar]
- 193.Rittig, M. G., B. Shaw, D. P. Letley, R. J. Thomas, R. H. Argent, and J. C. Atherton. 2003. Helicobacter pylori-induced homotypic phagosome fusion in human monocytes is independent of the bacterial vacA and cag status. Cell. Microbiol. 5:887-899. [DOI] [PubMed] [Google Scholar]
- 194.Rocha, G. A., D. M. Queiroz, E. N. Mendes, A. J. Barbosa, G. F. Lima Junior, and C. A. Oliveira. 1991. Helicobacter pylori acute gastritis: histological, endoscopical, clinical, and therapeutic features. Am. J. Gastroenterol. 86:1592-1595. [PubMed] [Google Scholar]
- 195.Rossi, G., P. Ruggiero, S. Peppoloni, L. Pancotto, D. Fortuna, L. Lauretti, G. Volpini, S. Mancianti, M. Corazza, E. Taccini, F. Di Pisa, R. Rappuoli, and G. Del Giudice. 2004. Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy. Infect. Immun. 72:3252-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sakagami, T., M. Dixon, J. O'Rourke, R. Howlett, F. Alderuccio, J. Vella, T. Shimoyama, and A. Lee. 1996. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut 39:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Satin, B., G. Del Giudice, V. Della Bianca, S. Dusi, C. Laudanna, F. Tonello, D. Kelleher, R. Rappuoli, C. Montecucco, and F. Rossi. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191:1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saunders, N. J., J. F. Peden, D. W. Hood, and E. R. Moxon. 1998. Simple sequence repeats in the Helicobacter pylori genome. Mol. Microbiol. 27:1091-1098. [DOI] [PubMed] [Google Scholar]
- 199.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schibli, D. J., P. M. Hwang, and H. J. Vogel. 1999. Structure of the antimicrobial peptide tritrpticin bound to micelles: a distinct membrane-bound peptide fold. Biochemistry 38:16749-16755. [DOI] [PubMed] [Google Scholar]
- 201.Schmausser, B., M. Andrulis, S. Endrich, S. K. Lee, C. Josenhans, H. K. Muller-Hermelink, and M. Eck. 2004. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin. Exp. Immunol. 136:521-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schmausser, B., C. Josenhans, S. Endrich, S. Suerbaum, C. Sitaru, M. Andrulis, S. Brandlein, P. Rieckmann, H. K. Muller-Hermelink, and M. Eck. 2004. Downregulation of CXCR1 and CXCR2 expression on human neutrophils by Helicobacter pylori: a new pathomechanism in H. pylori infection? Infect. Immun. 72:6773-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schreiber, S., M. Konradt, C. Groll, P. Scheid, G. Hanauer, H. O. Werling, C. Josenhans, and S. Suerbaum. 2004. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl. Acad. Sci. USA 101:5024-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Segal, E. D., C. Lange, A. Covacci, L. S. Tompkins, and S. Falkow. 1997. Induction of host signal transduction pathways by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 94:7595-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 207.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 208.Sobala, G. M., J. E. Crabtree, M. F. Dixon, C. J. Schorah, J. D. Taylor, B. J. Rathbone, R. V. Heatley, and A. T. Axon. 1991. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32:1415-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Solnick, J. V., D. R. Canfield, S. Yang, and J. Parsonnet. 1999. Rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific-pathogen-free monkeys. Lab. Anim. Sci. 49:197-201. [PubMed] [Google Scholar]
- 210.Solnick, J. V., L. M. Hansen, N. R. Salama, J. K. Boonjakuakul, and M. Syvanen. 2004. Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc. Natl. Acad. Sci. USA 101:2106-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sommer, F., G. Faller, M. Rollinghoff, T. Kirchner, T. W. Mak, and M. Lohoff. 2001. Lack of gastritis and of an adaptive immune response in interferon regulatory factor-1-deficient mice infected with Helicobacter pylori. Eur. J. Immunol. 31:396-402. [DOI] [PubMed] [Google Scholar]
- 213.Soto, G., C. T. Bautista, D. E. Roth, R. H. Gilman, B. Velapatino, M. Ogura, G. Dailide, M. Razuri, R. Meza, U. Katz, T. P. Monath, D. E. Berg, and D. N. Taylor. 2003. Helicobacter pylori reinfection is common in Peruvian adults after antibiotic eradication therapy. J. Infect. Dis. 188:1263-1275. [DOI] [PubMed] [Google Scholar]
- 214.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 97:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Strober, W., B. Kelsall, I. Fuss, T. Marth, B. Ludviksson, R. Ehrhardt, and M. Neurath. 1997. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol. Today 18:61-64. [DOI] [PubMed] [Google Scholar]
- 216.Su, B., P. J. Ceponis, S. Lebel, H. Huynh, and P. M. Sherman. 2003. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 71:3496-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 218.Sun, X. L., H. M. Baker, S. C. Shewry, G. B. Jameson, and E. N. Baker. 1999. Structure of recombinant human lactoferrin expressed in Aspergillus awamori. Acta Crystallogr. D 55:403-407. [DOI] [PubMed] [Google Scholar]
- 219.Sundrud, M. S., V. J. Torres, D. Unutmaz, and T. L. Cover. 2004. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. USA 101:7727-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Supajatura, V., H. Ushio, A. Wada, K. Yahiro, K. Okumura, H. Ogawa, T. Hirayama, and C. Ra. 2002. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J. Immunol. 168:2603-2607. [DOI] [PubMed] [Google Scholar]
- 221.Sutton, P., J. Wilson, T. Kosaka, I. Wolowczuk, and A. Lee. 2000. Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol. Cell Biol. 78:28-30. [DOI] [PubMed] [Google Scholar]
- 222.Suzuki, T., K. Kato, S. Ohara, K. Noguchi, H. Sekine, H. Nagura, and T. Shimosegawa. 2002. Localization of antigen-presenting cells in Helicobacter pylori-infected gastric mucosa. Pathol. Int. 52:265-271. [DOI] [PubMed] [Google Scholar]
- 223.Takeda, K., and S. Akira. 2003. Toll receptors and pathogen resistance. Cell. Microbiol. 5:143-153. [DOI] [PubMed] [Google Scholar]
- 224.Takenaka, R., K. Yokota, K. Ayada, M. Mizuno, Y. Zhao, Y. Fujinami, S. N. Lin, T. Toyokawa, H. Okada, Y. Shiratori, and K. Oguma. 2004. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology 150:3913-3922. [DOI] [PubMed] [Google Scholar]
- 225.Talley, N. J., J. E. Ormand, C. A. Frie, and A. R. Zinsmeister. 1992. Stability of pH gradients in vivo across the stomach in Helicobacter pylori gastritis, dyspepsia, and health. Am. J. Gastroenterol. 87:590-594. [PubMed] [Google Scholar]
- 226.Taylor, D. N., and M. J. Blaser. 1991. The epidemiology of Helicobacter pylori infection. Epidemiol. Rev. 13:42-59. [DOI] [PubMed] [Google Scholar]
- 227.Teneberg, S., M. Jurstrand, K. A. Karlsson, and D. Danielsson. 2000. Inhibition of nonopsonic Helicobacter pylori-induced activation of human neutrophils by sialylated oligosaccharides. Glycobiology 10:1171-1181. [DOI] [PubMed] [Google Scholar]
- 228.Terres, A. M., and J. M. Pajares. 1998. An increased number of follicles containing activated CD69+ helper T cells and proliferating CD71+ B cells are found in H. pylori-infected gastric mucosa. Am. J. Gastroenterol. 93:579-583. [DOI] [PubMed] [Google Scholar]
- 229.Thalmaier, U., N. Lehn, K. Pfeffer, M. Stolte, M. Vieth, and W. Schneider-Brachert. 2002. Role of tumor necrosis factor alpha in Helicobacter pylori gastritis in tumor necrosis factor receptor 1-deficient mice. Infect. Immun. 70:3149-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tham, K. T., R. M. Peek, Jr., J. C. Atherton, T. L. Cover, G. I. Perez-Perez, Y. Shyr, and M. J. Blaser. 2001. Helicobacter pylori genotypes, host factors, and gastric mucosal histopathology in peptic ulcer disease. Hum. Pathol. 32:264-273. [DOI] [PubMed] [Google Scholar]
- 231.Thompson, L. J., S. J. Danon, J. E. Wilson, J. L. O'Rourke, N. R. Salama, S. Falkow, H. Mitchell, and A. Lee. 2004. Chronic Helicobacter pylori infection with Sydney strain 1 and a newly identified mouse-adapted strain (Sydney strain 2000) in C57BL/6 and BALB/c mice. Infect. Immun. 72:4668-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Togawa, S., T. Joh, M. Itoh, N. Katsuda, H. Ito, K. Matsuo, K. Tajima, and N. Hamajima. 2005. Interleukin-2 gene polymorphisms associated with increased risk of gastric atrophy from Helicobacter pylori infection. Helicobacter 10:172-178. [DOI] [PubMed] [Google Scholar]
- 233.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 234.Umehara, S., H. Higashi, N. Ohnishi, M. Asaka, and M. Hatakeyama. 2003. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene 22:8337-8342. [DOI] [PubMed] [Google Scholar]
- 235.Unemo, M., M. Aspholm-Hurtig, D. Ilver, J. Bergstrom, T. Boren, D. Danielsson, and S. Teneberg. 2005. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J. Biol. Chem. 280:15390-15397. [DOI] [PubMed] [Google Scholar]
- 236.van Doorn, L. J., C. Figueiredo, R. Sanna, A. Plaisier, P. Schneeberger, W. de Boer, and W. Quint. 1998. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 115:58-66. [DOI] [PubMed] [Google Scholar]
- 237.van Doorn, N. E., E. P. van Rees, F. Namavar, and J. de Graaff. 1998. Local cellular immune response in the acute phase of gastritis in mice induced chemically and by Helicobacter pylori. J. Med. Microbiol. 47:863-870. [DOI] [PubMed] [Google Scholar]
- 238.Velin, D., D. Bachmann, H. Bouzourene, and P. Michetti. 2005. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology 129:142-155. [DOI] [PubMed] [Google Scholar]
- 239.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 240.Voland, P., N. Hafsi, M. Zeitner, S. Laforsch, H. Wagner, and C. Prinz. 2003. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect. Immun. 71:3837-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang, G., Z. Ge, D. A. Rasko, and D. E. Taylor. 2000. Lewis antigens in Helicobacter pylori: biosynthesis and phase variation. Mol. Microbiol. 36:1187-1196. [DOI] [PubMed] [Google Scholar]
- 242.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 243.Warren, J. R. 2000. Gastric pathology associated with Helicobacter pylori. Gastroenterol. Clin. N. Am. 29:705-751. [DOI] [PubMed] [Google Scholar]
- 244.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]
- 245.Whary, M. T., and J. G. Fox. 2004. Natural and experimental Helicobacter infections. Comp. Med. 54:128-158. [PubMed] [Google Scholar]
- 246.Whitney, A. E., J. Guarner, L. Hutwagner, and B. D. Gold. 2000. Helicobacter pylori gastritis in children and adults: comparative histopathologic study. Ann. Diagn. Pathol. 4:279-285. [DOI] [PubMed] [Google Scholar]
- 247.Wirth, H. P., M. H. Beins, M. Yang, K. T. Tham, and M. J. Blaser. 1998. Experimental infection of Mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect. Immun. 66:4856-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu, M. S., C. Y. Wu, C. J. Chen, M. T. Lin, C. T. Shun, and J. T. Lin. 2003. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int. J. Cancer 104:617-623. [DOI] [PubMed] [Google Scholar]
- 249.Xiang, Z., S. Censini, P. F. Bayeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 63:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yamamoto, J., S. Watanabe, M. Hirose, T. Osada, C. Ra, and N. Sato. 1999. Role of mast cells as a trigger of inflammation in Helicobacter pylori infection. J. Physiol. Pharmacol. 50:17-23. [PubMed] [Google Scholar]
- 251.Yamamoto, T., M. Kita, T. Ohno, Y. Iwakura, K. Sekikawa, and J. Imanishi. 2004. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol. Immunol. 48:647-654. [DOI] [PubMed] [Google Scholar]
- 252.Yamaoka, Y., S. Kikuchi, H. M. el-Zimaity, O. Gutierrez, M. S. Osato, and D. Y. Graham. 2002. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology 123:414-424. [DOI] [PubMed] [Google Scholar]
- 253.Yamauchi, K., M. Tomita, T. J. Giehl, and R. T. Ellison III. 1993. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 61:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yasunaga, Y., Y. Shinomura, S. Kanayama, Y. Higashimoto, M. Yabu, Y. Miyazaki, Y. Murayama, H. Nishibayashi, S. Kitamura, and Y. Matsuzawa. 1997. Mucosal interleukin-1 beta production and acid secretion in enlarged fold gastritis. Aliment. Pharmacol. Ther. 11:801-809. [DOI] [PubMed] [Google Scholar]
- 255.Ye, G., C. Barrera, X. Fan, W. K. Gourley, S. E. Crowe, P. B. Ernst, and V. E. Reyes. 1997. Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J. Clin. Investig. 99:1628-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zabaleta, J., D. J. McGee, A. H. Zea, C. P. Hernandez, P. C. Rodriguez, R. A. Sierra, P. Correa, and A. C. Ochoa. 2004. Helicobacter pylori arginase inhibits T cell proliferation and reduces the expression of the TCR zeta-chain (CD3ζ). J. Immunol. 173:586-593. [DOI] [PubMed] [Google Scholar]
- 257.Zavros, Y., S. Rathinavelu, J. Y. Kao, A. Todisco, J. Del Valle, J. V. Weinstock, M. J. Low, and J. L. Merchant. 2003. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc. Natl. Acad. Sci. USA 100:12944-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zheng, P. Y., and N. L. Jones. 2003. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 5:25-40. [DOI] [PubMed] [Google Scholar]