Abstract
Focal changes in the cerebral metabolic rate of glucose utilization (CMRglc) are small (10–40%) during sensory activation in awake humans, as well as in awake rodents and primates (20–50%). They are significantly larger (50–250%) in sensory activation studies of anesthetized rats and cats. Our data, in agreement with literature values, show that in the resting anesthetized state values of CMRglc are lower than in the resting nonanesthetized state whereas the final state values, reached upon activation, are similar for the anesthetized and nonanesthetized animals. The lower resting anesthetized state values of CMRglc explain why the increments upon activation from anesthesia are larger than when starting from the nonanesthetized conditions. Recent 13C NMR measurements in our laboratory have established a quantitative relationship between the energetics of glucose oxidation, CMRglc (oxidative), and the flux of the glutamate/γ-aminobutyric acid/glutamine neurotransmitter cycle, Vcycle. In both the resting awake value of CMRglc(oxidative), and its increment upon stimulation, a large majority (≈80%) of the brain energy consumption is devoted to Vcycle. In the differencing methods of functional imaging, it is assumed that the incremental change in the measured signal represents the modular activity that supports the functional response. However, the same amount of activity must be present during the response to stimulation, irrespective of the initial basal state of the cortex. Thus, whereas the incremental signals of ΔCMRglc can localize neurotransmitter activity, the magnitude of such activity during the response is represented by the total localized CMRglc, not the increment.
Modern imaging methods detect regional changes in functional brain activity by assuming coupling between brain glucose metabolism and the underlying neuronal activity. The principle that the rate of glucose metabolism in the brain reflects functional activity was first expressed by Roy and Sherrington in 1890 (1). Since that time, there has been great progress in understanding the neuronal processes that support brain function such as action potentials and neurotransmitter action (2–4), but understanding of the coupling between these cellular processes and glucose metabolism has been limited (5). Only recently has there been evidence for a stoichiometric relationship between glucose oxidation and functional neuronal processes, in particular, to neurotransmitter fluxes (6).
For a variety of technical reasons, functional metabolic imaging measures increments in regional brain glucose metabolism in response to peripheral stimulation.§ During sensory stimulation these increments generally are small in awake humans (10) and animals (11), with a maximum increase of about 40% for intense stimulations (12, 13). Increments of about 10% are more usual during physiological visual stimuli (14, 15) or cognitive tasks (16). The focus on incremental glucose metabolism has led to the prevailing view in the field that the increment represents the total energy required for functional activity (9). Although rarely stated explicitly, this view is evidenced by the common interpretation of the magnitude of the increment as being correlated with the amount of functional activity of the region (17). This interpretation of the increment in glucose metabolism has been justified by the agreement between lesion data (18) and functional images of incremental brain activity (19, 20). However, the interpretation that incremental glucose metabolism reflects neuronal activity has been questioned recently by findings of decreases in regional glucose metabolism in cognitive tasks (9).
The ability to relate glucose metabolism to functional activity in a quantitative manner had been limited by the lack of methods for measuring the activity of the specific neuronal processes that subserve function. Recently we have used in vivo 13C magnetic resonance spectroscopy (MRS) to measure, in the cortex of the human (21) and rat (22), the release of neuronal glutamate and its uptake into surrounding astrocytes. The rate of this process, referred to as the glutamate/γ-aminobutyric acid (GABA)/glutamine neurotransmitter cycle (Vcycle), was found in the rat to increase linearly in a 1:1 stoichiometry with glucose oxidation above pentobarbital-induced isoelectric conditions (6). In the mildly anesthetized rat, the rate of the Vcycle is approximately 80% of the rate of total glucose metabolism. Preliminary studies indicate that in the awake nonstimulated human a similar high rate of the Vcycle is present in occipital/parietal cortex (23). The measured stoichiometry supports recent proposals from cellular studies of a coupling between glutamate neurotransmitter cycling and glucose metabolism (24). We recently have explored implications of this high baseline neurotransmitter activity for the interpretation of functional imaging (25). It was concluded that because the incremental glucose metabolism during stimulation and the large brain activity at rest both represent similar neurotransmitter activity the assignment of the increment to the total neuronal activity required for performing the function was uncertain.
In this paper we further examine the relation between incremental brain glucose metabolism and functional activity by reanalyzing the results of sensory stimulation studies of animals under different depths of anesthesia. With several anesthetics, the increases in electrical and glucose metabolism in response to sensory stimulation occur in the same regions as in the awake animal even though these anesthetics substantially reduce basal brain glucose metabolism (5, 7). If the increment in glucose metabolism supports the neuronal activity associated with the function then the magnitude of the increment should be independent of the basal metabolic rate. Alternatively, if the functional neuronal activity requires a particular amount of glucose metabolism, rather than a particular increment, then the size of the increment should inversely correlate with the basal metabolic rate, being larger from anesthesia. Focal values of CMRglc, along with the evoked electrical single-unit response, were obtained from the literature for somatosensory and whisker barrel stimulation of anesthetized and nonanesthetized animals. The consequences of the findings for the current understanding of neuronal activity and neuroenergetics are discussed.
METHODS
Focal cortical values of CMRglc and evoked unit response were obtained from the literature for somatosensory stimuli as shown in Tables 1 and 2. Parameters of each sensory stimulus, along with the anesthesia information, are listed in the tables.
Table 1.
Data on CMRglc (see Fig. 1A)
| Rat*
|
Monkey†
|
||||||
|---|---|---|---|---|---|---|---|
| A | B1 | C | B2 | D | E | F | |
| Location | Sensorimotor cortex | Sensorimotor cortex | Barrel cortex | Sensorimotor cortex | Barrel cortex | Barrel cortex | Sensorimotor cortex |
| Anesthetic | α-chloralose (80 mg/kg)‡ | α-chloralose (80 mg/kg)‡ | α-chloralose (40 mg/kg) | α-chloralose (80 mg/kg) | 1.5% halothane | 7% halothane | None (awake) |
| Stimulus | Forepaw (3 Hz, 0.3 ms) | Forepaw (3 Hz, 0.3 ms) | Whisker (1 Hz) | Forepaw (3 Hz, 0.3 ms) | Whisker (2–3 Hz) | Whisker (3–5 Hz) | Forepaw (3 Hz) |
| Activated state | 1.05 ± 0.28 | 1.32 ± 0.33 | 1.09 ± 0.17 | 1.04 ± 0.27 | 1.05 ± 0.15 | 1.52 ± 0.18 | 0.53 ± 0.07 |
| Resting awake state | 0.87 ± 0.05 | 0.87 ± 0.05 | 0.93 ± 0.06 | 0.87 ± 0.05 | 0.93 ± 0.06 | 0.93 ± 0.06 | 0.44 ± 0.03 |
| Resting anesthetized state | 0.21 ± 0.03 | 0.25 ± 0.04 | 0.47 ± 0.09 | 0.52 ± 0.18 | 0.64 ± 0.08 | 0.70 ± 0.12 | — |
| Reference | 31, 32 | 29 | 26 | 30 | 27 | 39 | 11 |
Table 2.
Data on evoked unit response in rat (see Fig. 1B)
| G | H1 | H2 | H2 | |
|---|---|---|---|---|
| Location | Barrel cortex | Sensorimotor cortex | Sensorimotor cortex | Sensorimotor cortex |
| Anesthetic | Urethane (1.5 g/kg) | Pentobarbital (40 mg/kg) | Pentobarbital (40 mg/kg) | None (awake) |
| Stimulus | Whisker (1 Hz) | Hindpaw | Forepaw | Forepaw |
| Activated state | 2.10 ± 0.09 | 1.80 ± 0.10 | 1.82 ± 0.10 | 2.20 ± 0.15 |
| Resting awake state | 1.60 ± 0.07 | 1.60 ± 0.07 | 1.60 ± 0.07 | 1.60 ± 0.07 |
| Resting anesthetized state | 0.20 ± 0.05 | 0.70 ± 0.12 | 1.02 ± 0.11 | — |
| Reference | 34 | 36 | 35 | 35 |
Resting awake state values were obtained from ref. 35. All values are in units of spikes/stimulation.
CMRglc.
The CMRglc data (in Table 1) were obtained from studies of anesthetized rats and awake monkeys. Most of the rat data were obtained by autoradiography (26–30). Data from two studies of rats were obtained by 1H[13C] MRS (31, 32) while the monkeys were studied by PET (11). Long stimulations were used (> 40 min for autoradiography and MRS, and > 10 min for PET). The resting awake state values for rat and monkey, for specific regions of the brain, were obtained from refs. 33 and 7, respectively. The CMRglc value obtained by autoradiography and PET represent the total cerebral glucose metabolic rate, CMRglc(total), which is the sum of an aerobic component, CMRglc(oxidative), and an anaerobic component, CMRglc(nonoxidative). Previous 1H[13C] MRS studies on anesthetized rats have shown that CMRglc (oxidative)≈0.9CMRglc(total) during both rest (31) and sustained stimulation (32).
Evoked Unit Response.
The evoked electrical unit response data (in Table 2) all were obtained from studies of rats (34–36). Small glass microelectrodes (1- to 3-μm tip size) were used to measure single-unit electrical recordings from the cortex in the vicinity of layer IV. Electrical recordings were measured for about 500–700 ms after stimulus. The stimulus duration was 20–25 ms. The control epoch was defined as 300–500 ms poststimulus, and the activated epoch was defined as 7–25 ms poststimulus. The stimulus was repeated 20–50 times, and the poststimulus histograms of the electrical response were stored. The intensities of the evoked unit responses in these histograms were calculated as: number of spikes in each epoch times 1,000/(number of stimuli) × (number of ms in epoch). The resting awake and anesthetized state values, for specific regions of the brain, were obtained from ref. 35 as determined from the evoked unit response when the stimulus was situated outside the region from where the maximum evoked responses in the cortex were measured.
RESULTS
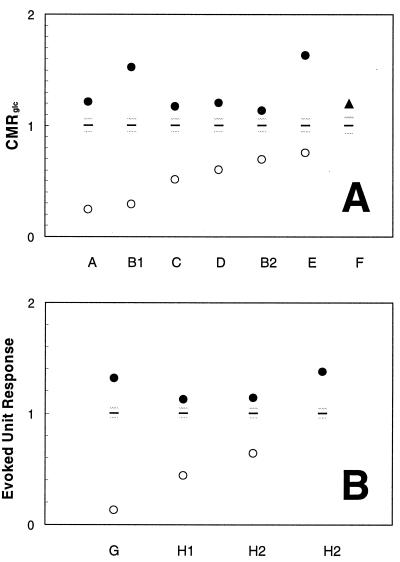
Resting awake state values for the specific brain areas reported in Tables 1 and 2 were used to normalize the resting anesthetized state and activated state values, as shown in Fig. 1. This approach allows the comparison of different regional in vivo data from rats and monkeys on the same vertical scale. In all cases, the activated state reached from the resting anesthetized state was higher than the resting awake state, and also beyond the SDs of the resting awake state plotted in Fig. 1. As shown, the increases upon activation from anesthesia are very large. The range of changes from the resting anesthetized state to the awake state for the CMRglc and the evoked unit response data (in unitless scale) are 0.65 ± 0.24 to 1.38 ± 0.28 and 0.40 ± 0.26 to 1.19 ± 0.10, respectively. A Student’s t test analysis (with two samples assuming unequal variances and one tail) shows that the P values for the changes in CMRglc and evoked unit response mentioned above are 0.000005 and 0.010000, respectively. In comparison, the respective changes from the resting awake state for the CMRglc and evoked unit response data (in unitless scale) are 1.00 ± 0.06 to 1.20 ± 0.16 and 1.00 ± 0.04 to 1.38 ± 0.09, respectively, which are also statistically significant.
Figure 1.
Data from Tables 1 and 2, respectively, were used to normalize the resting anesthetized state and activated state values of CMRglc (A) and evoked unit response (B) from rats, cats, and monkeys on the same vertical scale. The mean ± SD values of each resting awake state depicted possible variations in the awake state (black horizontal line represents mean and the gray horizontal lines represent the SD). The activated state reached from the resting anesthetized state is higher than the resting awake state, explaining why the increments upon activation from anesthesia are larger than when starting from the awake resting state. For the anesthetized animal the increment with stimulation raises the local activity to a level that is similar to that reached upon stimulation of the nonanesthetized awake state, which supports the suggestion that a particular magnitude of activity is a required response to a sensory stimulus and not a particular increment. Refer to Tables 1 and 2 for references.
Regardless of the initial basal state, i.e., whether the animal is anesthetized or awake, the relative activated state values measured by the CMRglc and evoked unit response are similar (i.e., 1.38 ± 0.28 vs. 1.20 ± 0.16 and 1.19 ± 0.10 vs. 1.38 ± 0.09, respectively). To determine whether there is any correlation between the values of the resting anesthetized state and the activated state, a linear regression analysis was carried out with absolute values of the resting anesthetized state vs. the activated state for the CMRglc and evoked unit response data. For the CMRglc data a very weak positive correlation was found (R2 = 0.07). The regression analysis of evoked unit response data was carried out with only three points, and a negative correlation was observed (R2 = 0.81).
DISCUSSION
Our survey of the literature on functional data from anesthetized and awake animals demonstrates that the increased activity caused by sensory stimulation raises CMRglc to values above the awake resting state (see Fig. 1A). Regional activity of the stimulated state is independent of whether the animal is anesthetized or awake (32) although activity in the rest of the brain is characteristic of awake or anesthetized states (31). This activity leads to larger regional increments in CMRglc upon stimulation of anesthetized animals because unstimulated activity under anesthesia is lower. The results show that the response to a sensory stimulation requires a particular level of brain activity (as measured by CMRglc), not a particular increment. Some implications of this finding for the interpretation of PET and fMRI are developed below.
A limitation in interpreting the results of studies of anesthetized animals is that the effects of anesthetic on neuronal activity in the stimulated state are not completely characterized. The primary assumption in the analysis is that during stimulation the neuronal activity induced in the region is, to a first order, independent of the anesthetic used (5, 7). The energy provided by glucose metabolism supports a multitude of cellular activities related to function during a particular stimulation. Histologically, this focal complexity spans many cortical layers, usually layers I–IV, each layer having different cellular morphology and connectivities (37, 38). Electrophysiologically, this localized complexity can be understood by the varied firing rates of single neurons during increased brain activity (39). Histological evidence supporting the assumption about equal responses is that for the anesthetics used in the studies reviewed (40) the mapping of sensory stimuli with or without anesthetics revealed the same neuroanatomy of activation (5, 7). From a standpoint of bulk electrophysiological activity the response to stimulation is also similar under anesthesia based on the independence of the evoked electrical response to the level of anesthesia as shown in Fig. 1B.
The resting state, as defined here and in the literature, is the rate of regional glucose metabolism without any peripheral stimulation. For the resting awake state values of CMRglc determined by autoradiographic methods, the ambient conditions for the experiment are significant because uncontrolled stimuli are likely to be present. Careful measures have been taken to avoid such influences upon the autoradiographic data (33, 41). On the other hand, anesthetized conditions keep the animal insensitive to such external influences. As a consequence of unavoidable stimuli, the values of CMRglc reported for the resting awake state are most likely higher than in the total absence of stimulation. However despite this possible overestimate, in all cases the value of CMRglc during activation was higher than in the resting awake state (Fig. 1A). Because it has been shown that the receptive fields delineated at the laminar level of the cortex with either tactile or electrical stimulation are indistinguishable (36), the use of tactile and electrical stimuli in the studies surveyed here are essentially stimuli of the same nature.
Estimate of the Neurotransmitter Cycle Flux in the Resting and Activated States from CMRglc.
A previous limitation on the usage of CMRglc (or coupled neurophysiological parameters such as CBF) is that they measure the total energy requirements of the brain as opposed to measuring the energy requirements of specific functional neuronal activity. However, this limitation has been removed as we recently have shown, by 13C MRS in the rat, that the rate of a specific neuronal activity, the Vcycle, may be calculated from measurements of CMRglc (6). Glutamate is the main excitatory neurotransmitter (42) and GABA the main inhibitory neurotransmitter (43) in mammalian cortex. Together glutamatergic and GABAergic cells account for more than 90% of cortical neurons (44). In the Vcycle, glutamate or GABA released by the neurons is taken up by the surrounding glial processes and converted into glutamine. With appropriate metabolic modeling, the rate of this pathway may be calculated from 13C MRS measurements of glutamine synthesis (22). We have shown in the rat cortex that above isoelectric conditions the rate of the glutamate/glutamine cycle increases in an approximate 1:1 stoichiometry with oxidative glucose metabolism (6). Under mildly anaesthetized conditions, the rate of the glutamate/glutamine cycle is approximately 85% of the rate of oxidative glucose metabolism [CMRglc(oxidative)].
These results provide a calibration, in the rat cortex, between CMRglc(oxidative) and the specific functional neuronal activity represented by the rate of the Vcycle. The rat somatosensory cortex data may be converted to Vcycle by the experimental relationship (in units of μmol/g per min) of
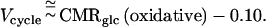 |
1 |
Because the intercept expression is small, even when compared with the lowest activated state in Tables 1 and 2, essentially the same level of neurotransmitter cycling is achieved upon activation, independent of the different activities in the nonstimulated state.
Implications for the Interpretation of Functional Imaging.
The significance of the results (from anesthetized animals) for understanding brain activity becomes clear when we consider the protocol of the usual functional imaging experiments, with PET and fMRI, and its interpretation (9). Although PET (and autoradiographic) measurements can determine neuroenergetic parameters directly, fMRI requires additional information for the quantitation (45, 46). The underlying assumption of both of these functional imaging methodologies is that the regions involved in performing a sensory or cognitive function are located by an increase in their neuroenergetic requirements. In a simplified example of such an experimental design, a baseline image taken in the absence of a stimulation is subtracted, voxel by voxel, from one taken during sensory stimulation (47). The difference image is tested for statistical significance and is displayed to localize the brain activity associated with the stimulation (48). Although far more complex image processing paradigms are used in the field (49–51), they all depend on the ability to localize brain function based on increases in local energy requirements as reflected by CMRglc or coupled neurophysiological parameters.
Although not explicitly stated, the interpretation of many functional imaging experiments has depended on the more restrictive assumption that the increment in CMRglc (or coupled neurophysiological parameters) is proportional to the neuronal activity required by the task (9). Any study (52) in which differences in signal intensity are used to distinguish the degree in which a region is recruited by a task or “how strongly the brain areas react” (17) depends on this assumption. In a recent paper (25) we addressed the impact of the finding of a high level of glutamate/GABA/glutamine neurotransmitter cycling in the nonstimulated brain. Increments in neurotransmitter cycling were calculated from reported increments in CMRglc with sensory and cognitive stimulations. In the presence of sensory stimulation the neurotransmitter activity, Vcycle, is about 20–30% higher than in the basal awake state. If the difference in the imaging signal, ΔS, is identified with the magnitude of neuronal activity supporting the function, then the baseline neuronal activity is implicitly assumed not to be associated with the function. The neglect of the basal CMRglc has been generally accepted because of the belief that most of the energy in this condition did not reflect functional neuronal activity (47, 48). However, our relationship between CMRglc and Vcycle indicates that the baseline awake value of this specific neuronal activity is quite substantial and in fact is larger than the incremental activity.
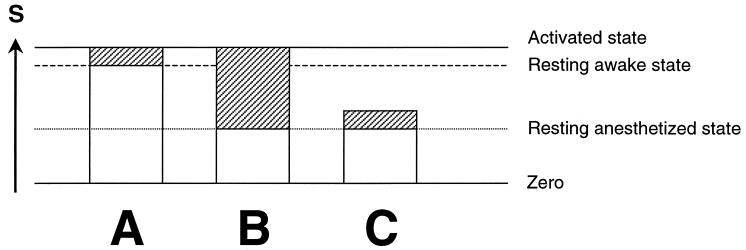
If the increment in CMRglc measures the energy required for supporting brain function then the incremental activity should be independent of the baseline. Starting from the resting anesthetized state, ΔS should be the same as when stimulated from the resting awake state (in Fig. 2, compare A and C). If, on the other hand, the total neuronal activity taking place in a region was needed to perform the task, then the final state during the task should be the same, regardless of the initial state, and the incremental signal, ΔS, during the task will be larger under anesthesia (in Fig. 2, compare A and B). These two possibilities are presented schematically in Fig. 2. The animal experiments summarized in Tables 1 and 2 and Fig. 1 show that the incremental signal under anesthesia is significantly larger than when starting from the awake resting state. During stimulation, S is observed to rise to approximately the same absolute level above the resting awake state, independent of the initial state. These results support a view that nearly all the neuronal activity in a region is required for a task, and that this activity is much larger than found in a particular increment.
Figure 2.
Schematic representation of possible increase in activity upon stimulation for an animal that is nonanesthetized (A) and anesthetized (B and C). The incremental functional imaging signal, ΔS, obtained by differencing, is represented by the shaded rectangles and is normally used to reveal the focally activated regions. The remaining neuronal activity, which is represented by white rectangles, is ignored by the differencing method. For the anesthetized animal, the incremental activity shown by the shaded areas would be larger for B than for C. If the incremental activity needed to perform the task were modular, and therefore independent of the initial activity, then the increment should be the same for the resting anesthetized or resting awake states (compare A and C). If, on the other hand, the final level rather than the increment was needed to support the activity upon responding to stimulation, the incremental signal from anesthesia would be much larger than in the response from the awake state. The data summarized in Tables 1 and 2 and Fig. 1 show that the incremental signal under anesthesia is actually larger, and that during stimulation, S always rises to approximately the same absolute level independent of the initial basal state. These results support a view in which a particular magnitude of neuronal activity is required for a task (B), not a particular increment (C).
The finding that substantially more neuronal activity is involved in supporting function has implications for the interpretations of functional imaging experiments. Most significantly the ability to calculate the degree of involvement of a region in a process based on the magnitude of the increment must be used with caution. In sensorimotor cortex it appears, based on the present data, that most of the neuronal activity in the region is co-opted for supporting the perceptual process. However, there is no evidence supporting the ability to assign the entire or incremental neuronal activity to one function in cognitive tasks. Even in sensory regions there is substantial neuronal activity (and glucose consumption) in the nonstimulated state because of internal processes.
The high level of neuronal activity in the absence of stimulation provides an explanation for recent reports of negative signals in functional imaging experiments (53). On the implicit assumption of cognitive psychology that brain activity exists only in response to a stimulus, the basal CMRglc has been considered to be dissociated from meaningful neuronal activity, serving essentially a housekeeping function. On this basis, a reduction in baseline activity during stimulation is not expected. Raichle (9), facing such experimental decreases, suggested that “the recognition of such changes probably represents an important contribution of functional brain imaging to our understanding of cortical function and should stimulate increased interest in the manner in which brain resources are allocated on a large systems level during task performance.” We suggest that the negative signals may be explained by the present analysis. If the entire magnitude of neuronal activity, not just the increment, is required to support a function, then the negative signal indicates that the functional processes occurring in the baseline state required more activity than during the task. How the total magnitude is composed of individual neuronal contributions remains to be understood. However, the negative functional imaging signal does not require a novel explanation from this perspective. Rather it supports the importance of total regional activity for brain function.
SUMMARY
Our survey of the literature on functional data from anesthetized and awake animals demonstrates that the increased activity caused by sensory stimulation raises neurophysiological measures of activity, such as CMRglc and evoked unit response, to values above the awake resting state (Fig. 1). This increment raises the local activity to a level that is similar to that reached upon stimulation of the unanesthetized awake state. On the other hand, activity in the anesthetized resting state is significantly lower than in the awake resting state, which leads to larger increases in the neurophysiological parameters upon stimulation of anesthetized animals. The results support the suggestion that a particular magnitude of activity is required during the response to a sensory stimulus and not just a particular increment.
Acknowledgments
We acknowledge helpful discussions with Drs. Kevin L. Behar, Edward J. Novotny, and Ognen A.C. Petroff. This work was supported by National Institutes of Health Grants DK-27121 (R.G.S.), NS-32126 (D.L.R.), and NS-37203 (F.H.) and National Science Foundation Grant DBI-9730892 (F.H.).
ABBREVIATIONS
- CMRglc
cerebral metabolic rate for glucose utilization
- GABA
γ-aminobutyric acid
- Vcycle
glutamate/GABA/glutamine neurotransmitter cycle
- CBF
cerebral blood flow
- fMRI
functional MRI
- MRS
magnetic resonance spectroscopy
- PET
positron emission tomography
Footnotes
In many positron emission tomography (PET) studies cerebral blood flow (CBF) is measured because it is easier than the well-established PET measurements of cerebral metabolic rates of glucose (CMRglc) or oxygen (CMRO2). Likewise, most functional MRI (fMRI) experiments use an image contrast that is sensitive to changes in CBF. The high correlation between CBF and CMRglc (7, 8) has been used to justify the interpretation that the measured changes of CBF, in both PET and fMRI, reflect brain functional activity (9). Because of the recent evidence for coupling between CMRglc and neurotransmitter activity (6), we focus here on CMRglc data.
References
- 1.Roy C S, Sherrington C S. J Physiol (London) 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie J M. J Physiol. 1967;188:309–329. doi: 10.1113/jphysiol.1967.sp008141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creutzfeldt O D. In: Brain Work: The Coupling of Function Metabolism and Blood Flow in the Brain. Ingvar D H, Lassen N A, editors. Munksgaard, Copenhagen: Alfred Benzon Foundation; 1975. , Alfred Benzon Symposium 7, pp. 21–46. [Google Scholar]
- 4.Nicholls D G. J Neurochem. 1989;52:331–341. doi: 10.1111/j.1471-4159.1989.tb09126.x. [DOI] [PubMed] [Google Scholar]
- 5.Sokoloff L. Dev Neurosci. 1993;15:194–206. doi: 10.1159/000111335. [DOI] [PubMed] [Google Scholar]
- 6.Sibson N R, Dhankhar A, Mason G F, Rothman D L, Behar K L, Shulman R G. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokoloff L. J Cereb Blood Flow Metab. 1981;1:7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- 8.Raichle M E. In: Handbook of Physiology: The Nervous System V. Plum F, editor. Washington, DC: Am. Physiol. Soc.; 1987. pp. 633–674. [Google Scholar]
- 9.Raichle M E. Proc Natl Acad Sci USA. 1998;95:576–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsberg M D, Chang J Y, Kelley R E, Yoshii F, Barker W W, Ingenito G, Boothe T E. Ann Neurol. 1988;23:152–160. doi: 10.1002/ana.410230208. [DOI] [PubMed] [Google Scholar]
- 11.Tsukada H, Kakiuchi T, Shizuno H, Nakanishi S, Ouchi Y. Brain Res. 1997;749:10–17. doi: 10.1016/s0006-8993(96)01028-1. [DOI] [PubMed] [Google Scholar]
- 12.Hallett M, Dubinsky R M, Zeffiro T, Bierner S M. J Neuroimaging. 1995;4:1–5. doi: 10.1111/jon1994411. [DOI] [PubMed] [Google Scholar]
- 13.Wei L, Lin S Z, Tajima A, Nakata H, Acuff V, Patlak C, Pettigrew K, Fenstermacher J. Hypertension. 1992;20:501–510. doi: 10.1161/01.hyp.20.4.501. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg J H, Reivich M, Alavi A, Hand P, Rosenquist A, Rintelmann W, Stein A, Tusa R, Dann R, Christman D, et al. Science. 1981;212:678–680. doi: 10.1126/science.6971492. [DOI] [PubMed] [Google Scholar]
- 15.Miyaoka, M., Shinohara, M., Batipps, M., Pettigrew, K. D., Kennedy, C. & Sokoloff, L. (1979) Acta Neurol. Scand.60, Suppl. 72, 16–17.
- 16.Posner M I, Petersen S E, Fox P T, Raichle M E. Nature (London) 1988;140:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 17.Posner M I, Raichle M E. Proc Natl Acad Sci USA. 1998;95:763–764. doi: 10.1073/pnas.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milner B. In: The Frontal Granular Cortex and Behavior. Warren J M, Akert K, editors. New York: McGraw-Hill; 1964. pp. 65–85. [Google Scholar]
- 19.Frith C D, Friston K, Liddle P F, Frackowiak R S J. Proc R Soc London. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 20.Hyder F, Phelps E A, Wiggins C J, Labar K S, Blamire A M, Shulman R G. Proc Natl Acad Sci USA. 1997;94:6989–6994. doi: 10.1073/pnas.94.13.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruetter R, Novotny E J, Boulware S D, Mason G F, Rothman D L, Shulman G I, Prichard J W, Shulman R G. J Neurochem. 1994;63:1377–1385. doi: 10.1046/j.1471-4159.1994.63041377.x. [DOI] [PubMed] [Google Scholar]
- 22.Sibson N R, Dhankhar A, Mason G F, Behar K L, Rothman D L, Shulman R G. Proc Natl Acad Sci USA. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason G F, Gruetter R, Rothman D L, Behar K L, Shulman R G, Novotny E J. J Cereb Blood Flow Metab. 1995;15:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- 24.Tsacopoulos M, Magistretti P J. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shulman R G, Rothman D L. Proc Natl Acad Sci USA. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cholet N, Seylaz J, Lacombe P, Bonvento G. J Cereb Blood Flow Metab. 1997;17:1191–1201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg M D, Dietrich W D, Busto R. Neurology. 1987;37:11–19. doi: 10.1212/wnl.37.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Kossut M, Hand P J, Greenberg J, Hand C L. J Neurophysiol. 1988;60:829–852. doi: 10.1152/jn.1988.60.2.829. [DOI] [PubMed] [Google Scholar]
- 29.Ueki M, Linn F, Hossman K A. J Cereb Blood Flow Metab. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- 30.Ueki M, Mies G, Hossman K A. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 31.Hyder F, Chase J R, Behar K L, Mason G F, Siddeek M, Rothman D L, Shulman R G. Proc Natl Acad Sci USA. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyder F, Rothman D L, Mason G F, Rangarajan A, Behar K L, Shulman R G. J Cereb Blood Flow Metab. 1997;17:1040–1047. doi: 10.1097/00004647-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Savaki H E, Desban M, Glowinski J, Besson M J. J Comp Neurol. 1983;213:36–45. doi: 10.1002/cne.902130104. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong-James M, Fox K. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- 35.Chapin J K, Lin C-S. J Comp Neurol. 1984;229:199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- 36.Chapin J K. Exp Brain Res. 1986;62:549–559. doi: 10.1007/BF00236033. [DOI] [PubMed] [Google Scholar]
- 37.Krieg W J S. J Comp Neurol. 1946;84:221–275. doi: 10.1002/cne.900840205. [DOI] [PubMed] [Google Scholar]
- 38.Krieg W J S. J Comp Neurol. 1946;84:275–324. doi: 10.1002/cne.900840302. [DOI] [PubMed] [Google Scholar]
- 39.Welker C. Brain Res. 1971;26:259–275. [PubMed] [Google Scholar]
- 40.Siesjo B K. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- 41.Dermon C R, Pizarro P, Georgopoulos P, Savaki H E. J Neurosci. 1990;10:2861–2878. doi: 10.1523/JNEUROSCI.10-09-02861.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erecinska M, Silver I A. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 43.Krnjevic K. J Mind Behav. 1987;8:537–547. [Google Scholar]
- 44.Shepherd G M. Neurobiology. 3rd Ed. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 45.Kim S G, Ugurbil K. Magn Reson Med. 1997;38:59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- 46.Hyder F, Shulman R G, Rothman D L. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- 47.Mintun M A, Fox P T, Raichle M E. J Cereb Blood Flow Metab. 1989;9:96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- 48.Fox P T. J Cereb Blood Flow Metab. 1991;11:A79–A82. doi: 10.1038/jcbfm.1991.41. [DOI] [PubMed] [Google Scholar]
- 49.Friston K J, Frith C D, Liddle P F, Frackowiak R S. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 50.Friston K J, Holmes A P, Poline J B, Grasby P J, Williams S C, Frackowiak R S, Turner R. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 51.Friston K J, Frith C D, Fletcher P, Liddle P F, Frackowiak R S. Cereb Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- 52.Raichle M E, Fiez J A, MacLeod A M K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 53.Ojemann J G, Buckner R L, Akbudak E, Snyder A Z, Ollinger J M, McKinstry R C, Rosen B R, Petersen S E, Raichle M E, Conturo T E. Hum Brain Mapp. 1998;6:203–215. doi: 10.1002/(SICI)1097-0193(1998)6:4<203::AID-HBM2>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]