Abstract
hnRNP A1 is a nucleocytoplasmic shuttling protein that is involved in many aspects of mRNA metabolism. We have previously shown that activation of the p38 stress-signaling pathway in mammalian cells results in both hyperphosphorylation and cytoplasmic accumulation of hnRNP A1, affecting alternative splicing regulation in vivo. Here we show that the stress-induced cytoplasmic accumulation of hnRNP A1 occurs in discrete phase-dense particles, the cytoplasmic stress granules (SGs). Interestingly, mRNA-binding activity is required for both phosphorylation of hnRNP A1 and localization to SGs. We also show that these effects are mediated by the Mnk1/2 protein kinases that act downstream of p38. Finally, depletion of hnRNP A1 affects the recovery of cells from stress, suggesting a physiologically significant role for hnRNP A1 in the stress response. Our data are consistent with a model whereby hnRNP A1 recruitment to SGs involves Mnk1/2-dependent phosphorylation of mRNA-bound hnRNP A1.
mRNAs are associated with a plethora of RNA-binding proteins forming messenger ribonucleoprotein complexes (mRNPs). Among the most abundant components of mRNPs are proteins in the heterogenous nuclear ribonucleoprotein (hnRNP) family, which in humans consists of at least 20 different polypeptides (15, 46). Among them, hnRNP A1 is a very abundant nuclear protein that is involved in alternative splicing regulation both in vitro and in vivo by antagonizing the activity of the SR family of proteins (8, 40, 66). Only recently, it was proposed that hnRNP A/B proteins may also have a positive role in generic splicing by modulating the conformation of mammalian pre-mRNAs (39).
Although it is nuclear at steady state, hnRNP A1 shuttles continuously between the nucleus and the cytoplasm, a property common to a subset of hnRNPs (47). Thus, hnRNP A1 is also implicated in postsplicing activities, such as mRNA export (27) and cap-dependent and internal ribosome entry site-mediated translation (5, 57). In addition, a role for hnRNP A1 in regulating mRNA stability has been suggested from data that support its ability to bind to AUUUA-rich sequences (AREs) (23). The shuttling activity of hnRNP A1 has been proposed to play a role in cell proliferation, survival, and differentiation of normal and transformed cells because it is required for normal myelopoiesis and BCR/ABL leukemogenesis (25).
Mammalian cells have evolved a variety of mechanisms to facilitate cellular recovery from environmental stressors. Stress granules (SGs) are cytoplasmic domains that harbor translationally arrested mRNAs that accumulate in cells exposed to a broad range of stresses, such as oxidative, genotoxic, hyperosmotic, or heat shock. The key event leading to the formation of SGs is the stress-induced phosphorylation of the translation initiation factor eIF2α. Both osmotic shock (OSM) and arsenite (ARS) treatment have been shown to induce phosphorylation of eIF2α to comparable levels (38). This phosphorylation event inhibits mRNA translation by depleting the eIF2-GTP-tRNAiMet ternary complex and triggers assembly of the stalled mRNAs together with bound proteins (32). Several components of the SGs have been identified, including the related RNA-binding proteins TIA-1 and TIAR, poly(A) binding protein (PABP), and translation initiation factors such as eIF3, eIF4E, and eIF4G (for a review, see reference 31). The RNA-binding protein TIA-1 contains three RNA recognition motifs (RRMs) at the N terminus and a glutamine-rich, prion-related domain at the C terminus. Identification of TIA-1 mRNA targets showed that this protein binds to a U-rich motif localizing preferentially to the 3′-untranslated regions of target genes (37). Proteins involved in regulating mRNA stability, such as HuR and tristetraprolin, and a great majority of poly(A)+ RNAs are also recruited to the SGs (reviewed in reference 31). G3BP, a phosphorylation-dependent endoribonuclease that interacts with RasGAP, is also recruited to SGs in cells exposed to arsenite (58).
Processing bodies (P bodies or PBs) are distinct cytoplasmic sites of 5′-3′ mRNA degradation that contain the decapping enzymes Dcp1 and Dcp2, the exonuclease Xrn1, the deadenylase hCcr4, the GW182 RNA-binding protein, and the Lsm1-7 proteins (10, 17, 26; for recent reviews, see references 18 and 63). Several components, such as eIF4E and tristetraprolin, are present in both PBs and SGs. In contrast, eIF3, G3BP, eIF4G, and PABP-1 are localized exclusively in SGs, whereas Dcp1 and -2 are restricted to PBs (34). This led to the proposal that mRNAs released from disassembled polysomes are sorted and remodeled at SGs, from which selected transcripts are delivered to PBs for degradation (34). Interestingly, some mRNAs within SGs escape degradation and are stored in an abortive translation initiation complex, which makes them available for rapid reinitiation in the eventual recovery from stress.
Both c-Jun N-terminal kinases and p38 mitogen-activated protein (MAP) kinase are components of signaling cascades that are activated in response to a number of extracellular stresses and that control the transcriptional and posttranscriptional events that determine the functional response to stress (reviewed in references 12 and 35). The activation of signaling pathways has been shown to control the activity and affect the subcellular distribution of RNA-binding proteins. For instance, a rise in the intracellular calcium concentration results in the cytoplasmic accumulation of the SR-related protein Tra2-β1 in primary neuronal cultures (11). Furthermore, phosphorylation of the polypyrimidine tract binding protein (PTB, or hnRNP I) by protein kinase A causes its cytoplasmic accumulation (65; for reviews, see references 52 and 55). We have previously shown that activation of the p38 MKK3/6/p38 stress-signaling pathway in mammalian cells results in both hyperphosphorylation and cytoplasmic accumulation of hnRNP A1 and affects alternative splicing regulation (59). More recently, we mapped the stress-induced phosphorylation sites in hnRNP A1 to a stretch of serines located adjacent to the M9 motif, which mediates bidirectional transport of hnRNP A1. This phosphorylation event abrogates interactions between hnRNP A1 and its import receptor, transportin, resulting in its cytoplasmic accumulation (1).
Here we show that the stress-induced cytoplasmic accumulation of hnRNP A1 occurs in the cytoplasmic SGs with similar kinetics to those of TIA-1. We found that this stress-induced hyperphosphorylation is required for the cytoplasmic accumulation of hnRNP A1 but not for subsequent recruitment to the SGs. In contrast, binding of hnRNP A1 to poly(A)+ mRNA in the cytoplasm is required for both localization to SGs and hyperphosphorylation in response to stress. Our data are consistent with a model whereby hnRNP A1 exits the nucleus bound to mRNA. Once in the cytoplasm and upon exposure to stress stimuli, mRNA-bound hnRNP A1 is phosphorylated by the Mnk1/2 protein kinases, causing the recruitment of hnRNP A1 and its associated mRNA to the SGs. The physiological significance of hnRNP A1 in the stress response is underscored by the observation that cells lacking hnRNP A1 exhibit decreased viability rates, both during stress and following release from stress conditions.
MATERIALS AND METHODS
Plasmid construction.
Green fluorescent protein (GFP)-G3BP was kindly provided by J. Tazi. The genes for GFP-TIA-1 and hnRNP A1-GFP were amplified by PCR with specific primers and cloned into the EcoRI/BamHI sites of plasmids pEGFP-C1 and pEGFP-N1 (Clontech), respectively. For PABP-1-GFP, human PABP-1 cDNA was cloned into the BglII and SalI sites of vector pEGFP-N1 (Clontech). T7-hnRNP A1 has been described previously (7); T7-G274A was generated from T7-hnRNP A1 by introducing the point mutation with specific oligonucleotides. For T7-F2-G274A, the same mutation was introduced into plasmid F2 (1). To create the T7-UP1 and T7-M(RRM1,2)-G274A plasmids, the corresponding fragments were amplified from vectors pET9d-UP1 and pET9d-M(RRM1,2) (41), respectively, and cloned into the XbaI/BamHI sites of vector pCGT. The G274A mutation was then created as described above. To generate T7-eIF4E, T7-MNK1, T7-MNK2 (corresponding to the splice variants Mnk1a and Mnk2a), and T7-MK2, the respective human cDNAs were cloned into the XbaI/BamHI sites of vector pCGT. Mutants T7-MNK1-AA (T197A and T202A), T7-MNK2-AA (T209A and T214A), and MK2-K76R have been described previously (61) and were created by Pfu polymerase-mediated mutagenesis. Primer sequences are available upon request.
Cell culture and transfections.
HeLa and 3T3 cells were grown under standard conditions and transfected with DNA plasmids by using Lipofectamine 2000 (Invitrogen). For small interfering RNA (siRNA) transfections, siRNA-annealed oligonucleotide duplexes were purchased from Ambion (for Mnk1, Mapkap kinase 2 [MK2], and PRAK) and Dharmacon (for Mnk2 and Trn1) and transfected using siPORT NeoFX (Ambion) following the provider's instructions. Knockdown of hnRNP A1 was achieved by transfection of the pSUPER plasmid (Oligoengine), encoding a small hairpin RNA targeting the sequence 5′-AGCAAGAGATGGCTAGTGC-3′ (RNAi A1-1) or 5′-TGAGAGATCCAAACACCAA-3′ (RNAi A1-2), as described previously (24). RNA interference (RNAi)-insensitive constructs were prepared from the original plasmids by introducing three coding-silent changes into the RNAi A1-1 sequence.
Immunofluorescence and antibodies.
Cells were fixed and permeabilized for immunofluorescence assays 36 to 48 h after transfection. Before fixation, cells were either left untreated or stressed with arsenite (0.5 mM, 1 h) or sorbitol (0.6 M, 2.5 h). Fixation and immunostaining were performed as described previously (1). A rabbit antibody against hDcp1a (a kind gift of Bertrand Séraphin, CGM, Gif-sur-Yvette, France) was used at a dilution of 1/1,000. Goat anti-TIA-1 (sc-1751) and rabbit anti-PRAK (sc-25419) antibodies were obtained from Santa Cruz Biotechnology, Inc. A rabbit anti-MK2 antibody was obtained from Cell Signaling Technology. Rabbit anti-MNK1 and anti-MNK2 serums were kindly provided by R. Fukunaga (Osaka University) and used at a dilution of 1/1,000. A monoclonal anti-hnRNP A1 antibody (4B10) was purchased from Abcam, and a monoclonal anti-T7-tag antibody was purchased from Novagen.
Fluorescence recovery after photobleaching (FRAP) analysis.
All data were obtained on the stage of a Zeiss LSM 510 confocal microscope, using the 488-nm line of a 30-mW argon-ion laser with a pinhole adjustment resulting in a 1-absorbance-unit optical slice. A circular region of interest (ROI) with a diameter of 2 μm containing one stress granule was recorded five times for 5 s each with a laser power of 0.6 mW at the maximum scan speed (pixel time, 1.28 μs). The ROI was then bleached with 60 scan iterations at 12 mW. Fluorescence recovery was measured every 3 s for 80 s. An additional ROI in the cytoplasm of another cell was monitored in parallel to detect fluorescence fluctuations independent of bleaching. Average fluorescence intensities within ROIs were measured under the same conditions for each data set and exported into Microsoft Excel. For each fusion protein, this procedure was repeated 15 times with different cells, and the mean intensity value for each time point was calculated.
Poly(A) binding capture assay.
Cytoplasmic extracts were obtained as described previously (1), and a poly(A) binding capture assay was performed as reported previously (49). Poly(A) binding proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting. The loaded amounts from the oligo(dT)-captured samples were normalized by the amounts of T7-tagged proteins present in the input samples.
Immunoprecipitation-Western blotting.
T7-tagged hnRNP A1 or mutant proteins from stressed or control cells were immunoprecipitated from total extracts by incubation for 1 h at 4°C with anti-T7-tag antibody linked to agarose beads (Novagen). After extensive washing, the bound fraction was eluted by boiling in SDS-PAGE sample buffer. Equivalent amounts of immunoprecipitated hnRNP A1 proteins were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with one of the following antibodies: anti-T7 tag (1:1,000) from Novagen and antiphosphoserine (1:100) from Abcam (Cambridge, United Kingdom).
In vitro kinase assay.
The kinase assay for MNKs was done essentially as described before (16), with the following modifications. Cells in 10-cm plates were transfected with wild-type or T7-MNK1-AA or T7-MNK2-AA mutant protein, and at 24 h posttransfection, cells were treated with 0.6 M sorbitol to activate the kinases. Following cell lysis, the kinases were immunoprecipitated with 20 μl of anti-T7-tag antibody-containing agarose (Novagen) and added to T7-hnRNP A1 or T7-eIF4E (obtained from immunoprecipitated HeLa cells in a similar way) and 0.5 μCi of [γ-32P]ATP in a total volume of 50 μl. The mixture was then incubated for 30 min at 37°C and analyzed by SDS-PAGE.
Viability assays.
HeLa cells were grown in white opaque 96-well plates and transfected with either vector pSUPER-A1-1 or pSUPER-A1-2, control vector, or both pSUPER-A1-1 and the RNAi-insensitive constructs. At 48 hours posttransfection, cells were stressed with 0.6 M sorbitol for 2.5 h and were left to recover in normal medium. At each time point indicated, cellular viability was assessed by adding an equal volume of CellTiterGlo (Promega). The experiment determines the relative number of cells, assayed by measuring the amount of ATP in those cells in a luciferase/luciferin-based assay. The luminescence generated is proportional to the viable cell number. The mixture was placed on an orbital shaker at room temperature for 10 min before the plates were read with a Wallac 1420 Victor3 multidetector (Perkin-Elmer). The assay was performed in triplicate, and the results shown are the averages from three independent experiments.
RESULTS
hnRNP A1 localizes to cytoplasmic SGs under stress conditions.
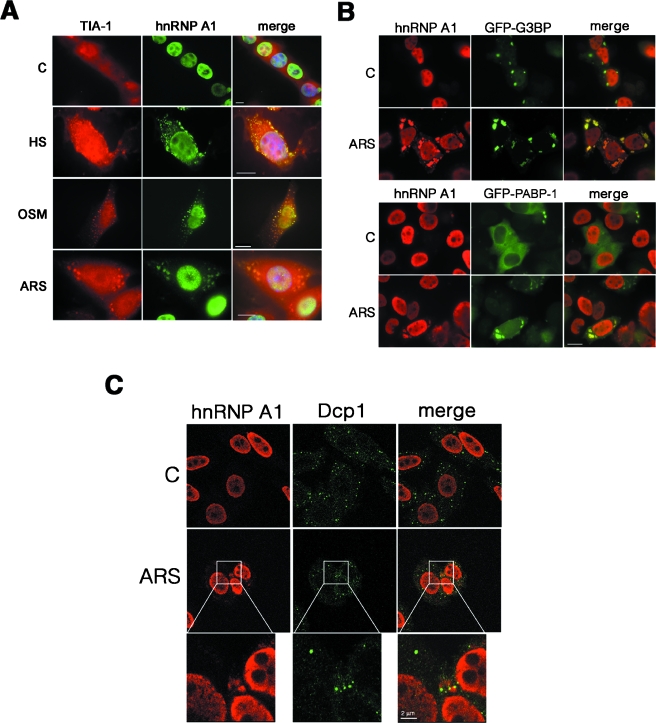
We have previously shown that exposure of different mammalian cells to OSM induces a detectable accumulation of the otherwise mainly nuclear protein hnRNP A1 in the cytoplasm (1, 59). Here we show that upon exposure to different stress stimuli, endogenous hnRNP A1 protein displays a punctate pattern in the cytoplasm, colocalizing with the RNA-binding protein TIA-1 (a bona fide component of cytoplasmic stress granules) (Fig. 1A). This relocalization of hnRNP A1 occurred in virtually 100% of the cells upon OSM and in approximately 40 to 50% of the cells under heat shock or oxidative (ARS) stress and was seen in both murine 3T3 cells (Fig. 1A) and human HeLa cells (see below). To further confirm the relocalization of hnRNP A1, GFP fusions of two other SG markers, the RNA-binding protein G3BP and the poly(A) binding protein PABP-1, were also used (Fig. 1B). Although overexpression of G3BP induces the assembly of some SGs in nonstressed cells (as already reported [58]), endogenous hnRNP A1 remains nuclear unless the stress stimulus is present (Fig. 1B, upper panels). Interestingly, hnRNP A1 does not relocalize to other described cytoplasmic mRNA foci, such as P bodies, upon stress, as seen by the lack of colocalization with the decapping enzyme Dcp1, which is a marker of PBs (Fig. 1C).
FIG. 1.
hnRNP A1 localizes to stress granules in stress-activated cells. (A) NIH 3T3 cells cultured on glass coverslips were left untreated (C) or were exposed to heat shock (HS; 42°C for 1 h), high-osmolarity medium (OSM; Dulbecco's modified Eagle's medium plus 600 mM sorbitol for 2.5 h), or oxidative stress (ARS; 0.5 mM arsenite for 1 h). The cells were fixed and immunostained with anti-TIA-1 and anti-hnRNP A1 antibodies to detect the endogenous proteins. (B) hnRNP A1 colocalizes with the stress granule markers G3BP and PABP-1 upon oxidative stress in HeLa cells. Cells were either left untreated (C) or exposed to oxidative stress (ARS) for 1 h, and endogenous hnRNP A1 was stained with the 4B10 antibody. To visualize G3BP or PABP-1, GFP fusions were transfected into cells 24 h before analysis. Bars = 10 μm. (C) HeLa cells were either left untreated (C) or stressed for 1 h with 0.5 mM arsenite (ARS). After fixation, endogenous hnRNP A1 and Dcp1 (a marker of P bodies) were detected by confocal microscopy. For the panels showing stressed cells, the indicated insets were reproduced as amplified replicate views of the same fields, showing Dcp1, hnRNP A1, and the merged view.
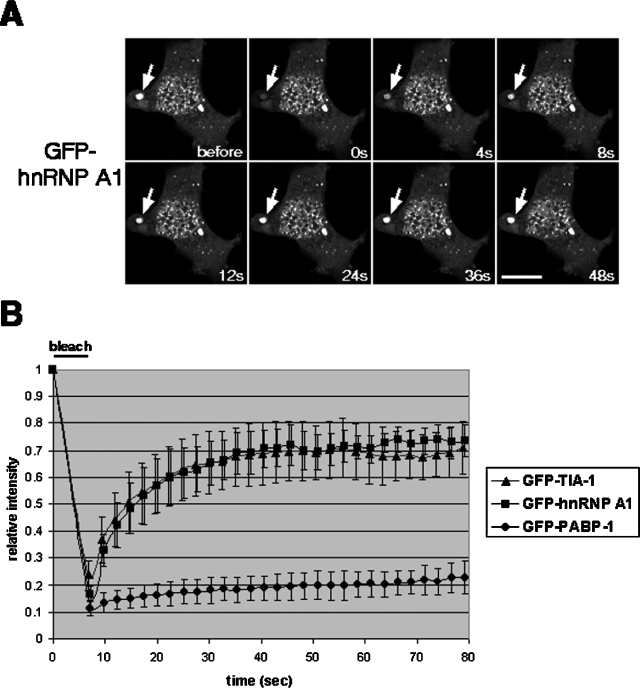
Next, we analyzed the kinetics of hnRNP A1 localization to SGs by using FRAP. For this technique, we used an epitope-tagged version of hnRNP A1, hnRNP A1-GFP, which has been shown to behave the same as endogenous hnRNP A1 in its subcellular localization and nucleocytoplasmic shuttling (30, 36). We found that in arsenite-treated cells, GFP-tagged hnRNP A1 moved in and out of SGs very rapidly, with very similar kinetics to those of GFP-TIA-1 (Fig. 2A and B). The recovery of fluorescence for hnRNP A1-GFP reached its maximum about 24 s after photobleaching, and at that time >70% of the fluorescent signal was restored. These values were almost identical for the GFP-TIA-1 fusion (Fig. 2B) (33). In contrast, a GFP-tagged poly(A) binding protein (PABP-1) exhibited much slower movement and less complete recovery than either hnRNP A1 or TIA-1 (Fig. 2B).
FIG. 2.
Dynamic properties of SG components under oxidative stress. (A) HeLa cells were transfected with a plasmid encoding hnRNP A1-GFP, GFP-TIA-1, or PABP-1-GFP. The images in panel A correspond to hnRNP A1-GFP, with arrows indicating the analyzed stress granule. Images were recorded just before photobleaching (before) and every 4 s during the course of recovery. (B) For each time point, the fluorescence intensity within the region of interest was measured, analyzed as described in Materials and Methods, and plotted in the graph relative to the intensity before bleaching. Bar = 10 μm.
RNA-binding activity is required for phosphorylation and localization of hnRNP A1 to SGs.
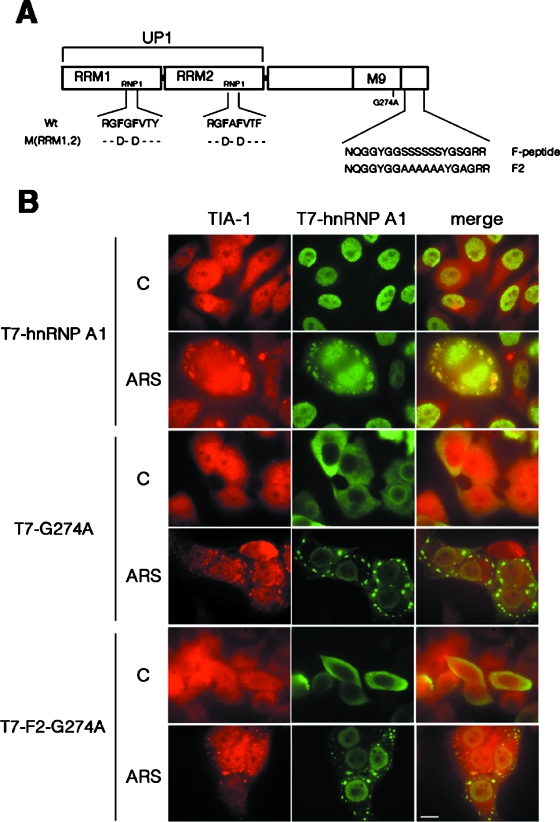
Next, we analyzed the functional domains that are required for the localization of hnRNP A1 to the SGs upon stress stimuli. Figure 3A depicts the domain structure of hnRNP A1 as well as several constructs encoding mutant proteins. hnRNP A1 has a modular structure consisting of two closely related tandem RRMs that determine RNA-binding specificity followed by a C-terminal glycine-rich domain (3). The C terminus also harbors the M9 motif (amino acids 268 to 305), a sequence of 38 amino acid residues required for interaction with the import receptor, transportin, which is directly involved in the nuclear import and export of hnRNP A1 (43, 54, 62). HeLa cells were transiently transfected with a T7 epitope-tagged wild-type or mutant hnRNP A1 construct (Fig. 3B). Interestingly, a cytoplasmic form of hnRNP A1 generated by mutation of the M9 motif (G274A) was still targeted to SGs upon ARS treatment, suggesting that a nuclear history is not required for localization to SGs. We next asked whether stress-induced phosphorylation of hnRNP A1 is required for its localization to SGs. Previously, we demonstrated that hnRNP A1 is phosphorylated within the C-terminal F peptide and that mutation of serine residues to alanine within this peptide inhibits cytoplasmic accumulation of hnRNP A1 under stress (1). In this study, we combined the F2 mutant, which prevented stress-induced phosphorylation, with the G274A mutant (Fig. 3A, F2-G274A mutant). Thus, we obtained a cytoplasmic hnRNP A1 protein that is not phosphorylated upon stress. This mutant protein still relocalized to the SGs upon oxidative stress (Fig. 3B), demonstrating that phosphorylation of the F peptide causes cytoplasmic accumulation but is not required for targeting of hnRNP A1 to SGs.
FIG. 3.
Neither a nuclear history nor phosphorylation of the F peptide per se is required for hnRNP A1 recruitment to SGs. (A) Cartoon depicting the functional domains of hnRNP A1 and the mutant proteins used in this work. Within the RRMs, the Phe residues in the RNP1 submotifs that were mutated to Asp residues [M(RRM1,2) construct] are highlighted. The UP1 fragment contains both RRMs but lacks the C-terminal glycine-rich domain and the M9 sequence required for bidirectional transport of the hnRNP A1 protein. The G274A point mutation blocks nuclear import activity and thus retains the protein in the cytoplasm (43). The F2 mutant protein cannot be hyperphosphorylated upon exposure to stress (1). (B) HeLa cells were transfected with T7-tagged versions of either wild-type hnRNP A1, the G274A point mutant, or the F2-G274A double mutant. At 36 to 48 h posttransfection, cells were either left untreated (C) or stressed with 0.5 mM arsenite for 1 h (ARS) and stained with antibodies to detect endogenous TIA-1 and transfected T7-tagged proteins. Bar = 10 μm.
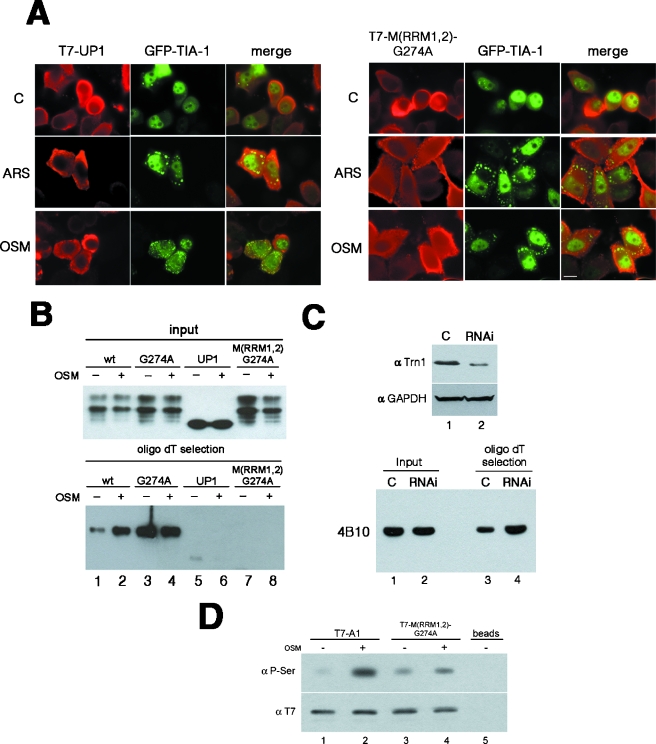
In order to study the requirement for RNA binding, we analyzed the recruitment of RNA binding-deficient forms of hnRNP A1 to the SGs. UP1, a proteolytic fragment of hRNP A1 that contains both RRMs but lacks the C-terminal Gly-rich domain, was not recruited to TIA-1 containing SGs upon stress but remained diffusely distributed throughout the cytoplasm (Fig. 4A, T7-UP1 panels). It has been established that two conserved Phe residues in the RNP-1 submotif of each of the two RRMs of hnRNP A1 mediate specific RNA-protein interactions and are essential for modulating alternative splicing (41). Surprisingly, a mutant protein with these Phe residues replaced by Asp remains nuclear even upon treatment with arsenite or sorbitol (data not shown). Since the M9 domain essential for the shuttling properties of the protein remains intact in this mutant, the failure to be relocalized upon stress might indicate a defect in phosphorylation, which is the major cause for stress-induced cytoplasmic accumulation of hnRNP A1 (see below). We next combined these mutations in both RRMs of hnRNP A1 with the G274A mutation in the M9 domain in order to obtain a cytoplasmic hnRNP A1 variant that does not bind to RNA [Fig. 4A, T7-M(RRM1,2)-G274A panels]. Again, we observed that this hnRNP A1 mutant was not recruited to the SGs, despite its cytoplasmic accumulation. Both UP1 and the RRM1,2 mutant proteins have previously been described as deficient in RNA binding in vitro (41). In order to confirm this observation in vivo, we performed in vivo cross-linking and oligo(dT)-mediated selection of cytoplasmic poly(A) mRNA-binding proteins. Analysis of the captured proteins by Western blotting showed that neither UP1 nor the cytoplasmic RRM mutant M(RRM1,2)-G274A bound to poly(A) mRNA in vivo, whereas both the wild-type hnRNP A1 protein and the cytoplasmic G274A mutant were efficiently cross-linked to poly(A) mRNA (Fig. 4B, lower panel). These experiments clearly showed that RNA binding is required for SG recruitment. Interestingly, the RNA-binding domains of G3BP, another SG component, also mediate its recruitment to SGs in COS cells (58). Remarkably, the poly(A) capture assay also showed that wild-type hnRNP A1 binds much more efficiently to poly(A) mRNA after stress treatment (Fig. 4B, lower panel, compare lanes 1 and 2). In addition, the mutant with a point mutation in the M9 domain (G274A) bound to poly(A) very efficiently and equally well irrespective of stress treatment. Taken together, these data point to an increased binding of hnRNP A1 to poly(A) mRNA following stress, probably due to the abrogation of the interaction with transportin. In order to address this, we analyzed the binding of hnRNP A1 to poly(A)+ RNA in cells depleted of transportin. We obtained very efficient depletion of Trn by RNAi (Fig. 4C, upper panel) and were able to show that binding of hnRNP A1 to poly(A)+ RNA is much more efficient in these cells (Fig. 4C, lower panel). This experiment strongly suggests that the interaction of Trn with hnRNP A1 promotes the release of hnRNP A1 from mRNA in an analogous manner to that shown for Mtr10p, the import receptor for Npl3p in Saccharomyces cerevisiae (22, 64). Finally, we observed that epitope-tagged wild-type hnRNP A1 showed increased phosphorylation upon stress, as described for endogenous hnRNP A1, but notably, the RNA binding-deficient M(RRM1,2)-G274A variant protein showed reduced phosphorylation (Fig. 4C, compare lane 2 with lane 4). This strongly suggests that the stress-induced phosphorylation of hnRNP A1 requires mRNA-bound protein, with the mRNP being the platform for the reaction.
FIG. 4.
RNA binding is required for both recruitment of hnRNP A1 to SGs and stress-induced hyperphosphorylation. (A) HeLa cells were transfected with GFP-TIA-1, together with T7-tagged versions of the RNA binding-defective mutants (UP1) or M(RRM1,2)-G274A (a cytoplasmic protein also mutated in the RRMs). Cells were then stressed with arsenite (ARS) or sorbitol (OSM) and fixed for immunostaining with an anti-T7-tag antibody. Bar = 10 μm. (B) The ability of the hnRNP A1 mutants to bind to poly(A) mRNA in vivo was tested by means of UV cross-linking and oligo(dT)-mediated poly(A) mRNP capture. Cytoplasmic extracts from UV-cross-linked control (−) or osmotically stressed (+) cells were incubated with oligo(dT)-cellulose beads under denaturing conditions, and captured proteins were analyzed by Western blotting, with the loaded amounts being normalized previously to the equal presence of each protein in the input cytoplasmic extracts. (C) Depletion of Trn results in increased binding of hnRNP A1 to poly(A)+ RNA. (Top) RNAi-mediated knockdown of Trn. Cells were treated with either siRNA oligonucleotides against Trn (RNAi lane) or control oligonucleotides (C lane) and analyzed by Western blotting with antibodies against Trn or glyceraldehyde-3-phosphate dehydrogenase. (Bottom) UV cross-linking and oligo(dT)-mediated poly(A) mRNP capture assay with cells depleted of Trn (RNAi lanes) or control cells (C lanes). The presence of hnRNP A1 in the captured samples was assessed by Western blotting with the 4B10 antibody. The loaded amounts were previously normalized to the equal presence of hnRNP A1 in each cytoplasmic extract (input samples). (D) Stress-dependent hyperphosphorylation on Ser is diminished in the RNA binding-defective mutant M(RRM1,2)-G274A. Total extracts were prepared from cells transfected with either T7-hnRNP A1 (lanes 1 and 2) or T7-M(RRM1,2)-G274A (lanes 3 and 4) and either left untreated (−) or stressed with sorbitol for 2.5 h (+). The transfected proteins were immunoprecipitated with an anti-T7-tag antibody, analyzed by SDS-PAGE, and Western blotted, first with a monoclonal anti-P-Ser antibody (upper panel) and then with an anti-T7-tag antibody (lower panel). Lane 5, control immunoprecipitation from nontransfected cells.
Mnk1/2 phosphorylates hnRNP A1 under stress stimuli.
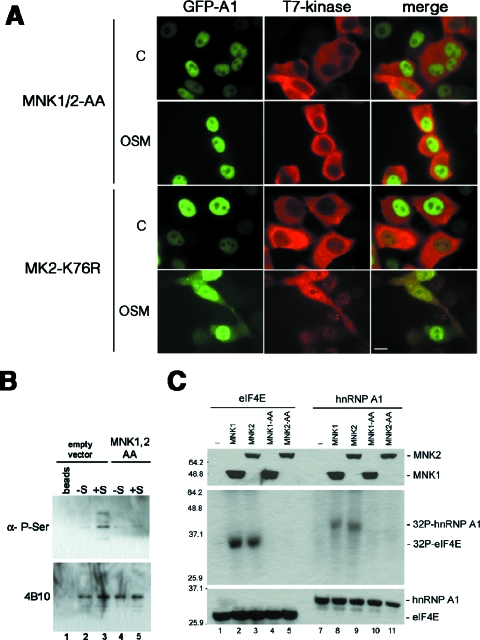
Activation of the p38 kinase in mammalian cells is sufficient to promote both hyperphosphorylation and cytoplasmic accumulation of hnRNP A1; however, hnRNP A1 is not a direct substrate for this kinase (59). For this reason, we analyzed the potential roles of several protein kinases acting downstream of p38. HeLa cells were transfected with expression plasmids encoding T7-tagged versions of wild-type versions or dominant-negative mutants of Mnk1/2 (60), MK2 (56), and PRAK (44) (Fig. 5A and data not shown). The transfected cells were exposed to OSM, and the subcellular localization of GFP-tagged hnRNP A1 was determined. Expression of the dominant-negative mutants of both Mnk1 and -2 abrogated the effect of OSM by blocking the accumulation of hnRNP A1 in the cytoplasm (Fig. 5A, MNK1/2-AA panels), whereas the expression of wild-type Mnk1/2 had no effect (data not shown). This strongly suggests that these protein kinases are responsible for hnRNP A1's altered subcellular distribution upon stress. In contrast, the expression of dominant-negative MK2 had no effect (Fig. 5A, MK2-K76R panels). It has been shown that immunoprecipitated hnRNP A1 from stressed cells shows increased phosphorylation, as revealed by using a phosphoserine antibody (1). We confirmed this result and also showed that dominant-negative versions of Mnk1/2 are able to block the increased phosphorylation of hnRNP A1 in stressed cells (Fig. 5B). Moreover, wild-type Mnk1 and Mnk2 purified from stressed cells, but not their dominant-negative counterparts, were able to phosphorylate eIF4E, as expected, but more interestingly, also phosphorylated hnRNP A1 in vitro (Fig. 5C).
FIG. 5.
Mnk1/2 proteins mediate stress-induced hyperphosphorylation and cytoplasmic accumulation of hnRNP A1. (A) hnRNP A1-GFP was cotransfected into HeLa cells with either T7-tagged MNK1/2-AA or T7-tagged MK2-K76R. Cells were then either left untreated (control panels) or stressed with 0.6 M sorbitol for 2.5 h (OSM) before being fixed and immunostained. Bar = 10 μm. (B) Total extracts were prepared from control (empty vector)-transfected or T7-MNK1/2-AA-transfected (MNK1/2-AA) cells and left untreated (−S) or stressed with sorbitol for 2.5 h (+S). Endogenous hnRNP A1 was immunoprecipitated and Western blotted, first with a monoclonal anti-P-Ser antibody (upper panel) and then with 4B10 (lower panel). Lane 1, control immunoprecipitation with protein A beads alone. (C) In vitro kinase assay. Both T7-tagged MNK1 and MNK2 proteins (and their respective dominant-negative mutants, MNK1-AA and MNK2-AA) were immunoprecipitated from stressed HeLa cells and incubated together with either T7-eIF4E (lanes 2 to 5) or T7-hnRNP A1 (lanes 7 to 10) in the presence of [γ-32P]ATP. The resulting labeled proteins were then analyzed by SDS-PAGE (middle panel). An aliquot of every reaction was also analyzed by Western blotting to assess the presence of the immunoprecipitated proteins (top and bottom panels). Lanes 1 and 6, control reactions in the absence of Mnk1/2 proteins.
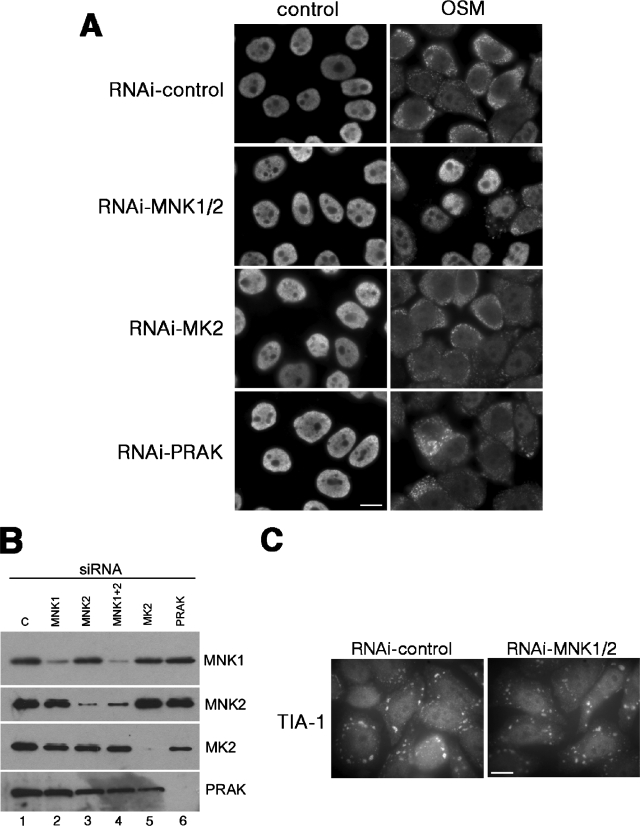
To further confirm the role of Mnk1/2 protein kinases in the stress-induced phosphorylation of hnRNP A1, we showed that the depletion of Mnk1/2 by RNAi almost completely abolished the cytoplasmic accumulation of hnRNP A1 upon stress, whereas RNAi-mediated depletion of MK2 or PRAK had no effect (Fig. 6A). The level of depletion achieved for each protein was assessed by Western blotting (Fig. 6B). Interestingly, the depletion of Mnk1/2 had no effect on the formation of TIA-1-containing stress granules (Fig. 6C). From this series of experiments, we concluded that in HeLa cells stress signaling activates the Mnk1/2 protein kinases, which phosphorylate mRNA-bound hnRNP A1 in the F peptide, driving its cytoplasmic accumulation.
FIG. 6.
Depletion of Mnk1/2 by RNAi blocks relocalization of hnRNP A1 to SGs upon OSM. (A) HeLa cells were transfected with a siRNA targeting either Mnk1 and -2, MK2, or PRAK, as indicated, or with control siRNAs (RNAi-control). After 48 h, cells were either left untreated (control) or stressed with 0.6 M sorbitol for 2.5 h (OSM). The cells were fixed and immunostained with the 4B10 antibody to detect endogenous hnRNP A1 protein. (B) Western blot analysis of the transfected cells shows specific depletion of the targeted proteins. (C) RNAi-mediated depletion of Mnk1/2 does not prevent the assembly of TIA-1-containing stress granules. HeLa cells were treated with either control siRNAs or siRNAs against Mnk1/2, stressed with arsenite, and immunostained to detect endogenous TIA-1 protein. Bars = 10 μm.
hnRNP A1 increases cell viability following stress.
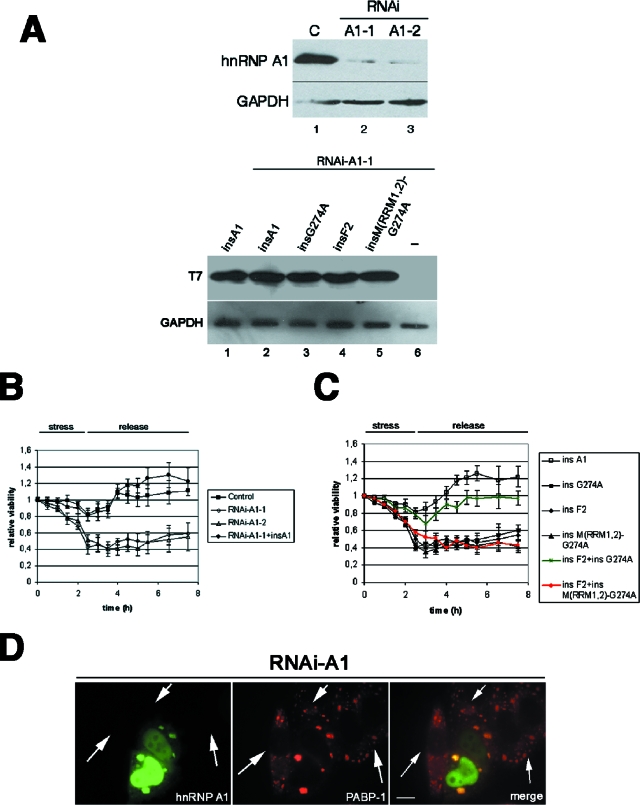
In order to test for a role of hnRNP A1 in the stress response, we compared the recovery from stress of wild-type cells with that of cells depleted of hnRNP A1 by using RNAi. HeLa cells were stressed with OSM for 2.5 h, the stress stimulus was removed, and the amount of ATP in the cells (as a measure of the viable cell number) was monitored during and after stress release. As shown in Fig. 7B, wild-type cells recovered well after a few hours. In contrast, even though down-regulation of hnRNP A1 had no measurable effect on the viability of cells prior to the induction of stress, cells lacking hnRNP A1 lost viability during stress and recovered poorly after release. The results obtained by targeting hnRNP A1 for RNAi with two different sequences were similar (sequences A1-1 and A1-2 in Fig. 7A and B), indicating a specific effect of the knockdown of hnRNP A1. Also, depletion of the second-step splicing factor SRrp53 (9) resulted in cells with rates of recovery similar to those of control cells (data not shown). Thus, we conclude that RNAi-mediated down-regulation of hnRNP A1 reduces cell viability after stress release. To convincingly demonstrate that this poor recovery was due to the lack of hnRNP A1, we complemented HeLa cells that underwent RNAi for hnRNP A1 with a T7-tagged construct encoding a mutated human hnRNP A1 that is insensitive to RNAi (Fig. 7A, insA1). As shown in Fig. 7B, overexpression of insA1 rescued the viability of cells depleted of endogenous hnRNP A1. We next asked whether targeting of the protein to the SGs was relevant for its role in stress survival. To address this issue, we prepared RNAi-insensitive mutant versions of the cytoplasmic G274A protein, the nuclear F2 protein, and the cytoplasmic but non-RNA-binding M(RRM1,2)-G274A protein. None of these mutants on its own could rescue the depletion of endogenous hnRNP A1, as measured in viability assays, but remarkably, the combination of the nuclear F2 mutant and the cytoplasmic G274A mutant resembled the rescue of the wild-type protein (Fig. 7C, compare insA1 to the combination of insF2 plus insG274A [in green]). In contrast, the F2 mutant together with the stress granule-defective M(RRM1,2)-G274A mutant (Fig. 7C, red line) showed no effect. This is a clear indication that the presence of hnRNP A1 in the SGs plays a role in cell viability under stress. Furthermore, this experiment strongly suggests that nuclear and cytoplasmic functions of hnRNP A1 are required for its role in the stress response. Interestingly, formation of the SGs does not seem to be compromised in hnRNP A1-depleted cells (Fig. 7D). Further studies will be required to assess the level at which hnRNP A1 functions within the SGs.
FIG. 7.
Depletion of hnRNP A1 results in reduced cell viability during and after stress release. (A) Expression of endogenous hnRNP A1, but not that of RNAi-insensitive transfected hnRNP A1 proteins, is significantly reduced by means of RNAi. (Top) Western blot showing depleted expression of endogenous hnRNP A1 in cells transfected with pSUPER-A1-1 or pSUPER-A1-2 vector (lanes 2 and 3) compared to that in control vector-transfected cells (lane 1) and glyceraldehyde-3-phosphate dehydrogenase expression. (Bottom) Western blot showing similar levels of expression of transfected T7-tagged RNAi-insensitive hnRNP A1 mutants in RNAi-treated cells (lanes 2 to 5), in contrast to control cells (lane 1). (B) HeLa cells were transfected with either control plasmid (▪), pSUPER-A1-1 (⋄), pSUPER-A1-2 (▵), or pSUPER-A1-1 together with T7-insA1 (•). Forty-eight hours after transfection, cells were stressed with 0.6 M sorbitol for 2.5 h, followed by replacement of the medium back to physiological conditions. Cellular viability was assessed for each time point. (C) SG-defective mutants of hnRNP A1 are unable to rescue cellular viability. HeLa cells were cotransfected with pSUPER-A1-1 (to deplete the endogenous protein) together with each of the RNAi-insensitive mutants shown in the figure. At 48 h posttransfection, cells were stressed with sorbitol, and their viability was measured as described above. (D) RNAi-mediated depletion of hnRNP A1 does not prevent assembly of the SGs. HeLa cells were transfected with vector pSUPER-A1-1, and 48 h later, cells were stressed with arsenite, fixed, and immunostained to detect endogenous hnRNP A1 and PABP-1. The arrows point to cells depleted of hnRNP A1. Bar = 10 μm.
In summary, stress stimuli induce the Mnk1/2-mediated phosphorylation of hnRNP A1, which results in increased mRNA-binding activity and recruitment of hnRNP A1-mRNA complexes to the SGs with a concomitant effect on stress survival.
DISCUSSION
It is well known that stress stimuli have a profound impact on nuclear RNA processing. For instance, pre-mRNA splicing is repressed upon heat shock (4), possibly through activation of the general splicing repressor SRp38 by dephosphorylation (51). Furthermore, both heat shock and chemical stresses induce the formation of transient nuclear structures, called nuclear stress bodies, in human cells (14, 29, 42; reviewed in reference 2). Differential recruitment of SR proteins to the nuclear stress bodies has been proposed to affect pre-mRNA splicing of specific transcripts (13). In contrast, other nuclear pre-mRNA processing factors, such as hnRNP A1, become localized to the cytoplasm, suggesting novel stress-induced functions. Here we show that upon stress stimuli, hnRNP A1 accumulates in cytoplasmic SGs. These cytoplasmic bodies harbor many different proteins and mRNAs, including the RNA-binding protein TIA-1, which is also involved in alternative splicing regulation (19, 21, 28). SGs may act as sites of mRNA triage from which mRNAs targeted for decay are exported to the PBs, which are the actual sites of mRNA decay. Thus, it would be expected that protein components that are exclusive to SGs, such as translation initiation factors eIF3 and eIF4G, PABP-1, and the RNase G3BP, must be removed from mRNAs that are exported to the PBs. Our data suggest that hnRNP A1 falls into this category, since it is present in SGs but absent from PBs, suggesting that it has to be removed from mRNAs either in the SGs or during the transport of hnRNP A1-bound mRNAs to the PBs (Fig. 1). Another possibility is that hnRNP A1 binding to particular mRNAs might prevent or delay the transit of those mRNPs from SGs to P bodies, thus controlling the turnover or translatability of its target mRNAs. FRAP experiments indicated that TIA-1 shuttles very rapidly in and out of SGs, with a residence time of seconds (Fig. 2) (33). We found almost identical properties for the recruitment of hnRNP A1 to SGs. We also found that neither a nuclear history nor stress-induced phosphorylation of hnRNP A1 in the F peptide sequence is indeed necessary for recruitment to SGs (Fig. 3).
Our previous work mapped the sites of stress-induced phosphorylation in hnRNP A1 to the C-terminal domain, between amino acids 301 and 318, a segment termed the F peptide (1). In this study, we set out to identify the protein kinase(s) responsible for the stress-induced phosphorylation of hnRNP A1. We had already shown that the phosphorylation event was dependent on the activation of the p38 kinase pathway. Activation of the Erk and p38 signaling pathways results in the phosphorylation and activation of the MAP kinase interacting kinase Mnk1, among others, stimulating in vitro kinase activity on their natural substrate, the cytoplasmic cap-binding protein eIF4E (60). In contrast, the related kinase Mnk2 has high basal activity (50). We present three lines of evidence to demonstrate that the Mnk1/2 proteins are the kinases responsible for the stress-induced phosphorylation of hnRNP A1. Firstly, the reduction of Mnk1/2 activity by means of RNAi or by using dominant-negative mutant proteins abolished hnRNP A1 cytoplasmic accumulation upon OSM (Fig. 5 and 6). Secondly, dominant-negative mutants of Mnk1/2 can block the increased phosphorylation of hnRNP A1, as shown in immunoprecipitation-Western blot assays. We also present evidence showing that both Mnk1 and Mnk2 do indeed phosphorylate hnRNP A1 in vitro, as recently shown (6). Interestingly, it was shown only recently that hnRNP A1 is phosphorylated by the Mnk kinases in response to T-cell activation. Phosphorylation of hnRNP A1 by the Mnk kinases negatively regulates binding of hnRNP A1 to the ARE of tumor necrosis factor alpha (TNF-α) mRNA (6). This work identified two phosphorylation sites in hnRNP A1; one corresponds to Ser192, and the second one corresponds to Ser310 to -312, which overlap the stress-induced phosphorylation site that we mapped previously. Several important differences in hnRNP A1 phosphorylation and activity seem to exist between T-cell activation and the stress response. First, during T-cell activation there is no apparent relocalization of hnRNP A1 to the cytoplasm, as is the case upon exposure to stress stimuli. Moreover, in response to stress stimuli, only one phosphorylation site in hnRNP A1, termed the F peptide, was found (1). This site is identical to one of the sites found upon T-cell activation (6). Furthermore, whereas T-cell activation results in decreased binding of hnRNP A1 to the TNF-α ARE, we found that stress stimuli do indeed promote increased binding of hnRNP A1 to poly(A) mRNA (Fig. 4).
We have shown previously that stress-mediated phosphorylation of hnRNP A1 abrogates the interaction with its import receptor, transportin, resulting in hnRNP A1 accumulation in the cytoplasm (1). Here we show that this decreased interaction of Trn with hnRNP A1 results in increased binding of cytoplasmic hnRNP A1 to poly(A) mRNA (Fig. 4 and 8). This suggests that Trn actively promotes the release of hnRNP A1 from mRNA in an analogous manner to that shown for the import receptor Mtr10p, which is involved in the release of the yeast SR-like protein Npl3p from polysome-associated mRNAs (64). Importantly, we provide data that support an mRNA-mediated hyperphosphorylation event in hnRNP A1 by the Mnk1/2 protein kinases, which are interacting partners of the scaffold protein eIF4G within mRNPs (48, 50). Thus, Mnk1/2 might phosphorylate hnRNP A1 only when bound to the same mRNA molecule. This hypothesis is supported by data showing that stress-induced phosphorylation of the cytoplasmic, RNA-binding-defective M(RRM1,2)-G274A mutant (Fig. 4C) is dramatically reduced relative to that of the wild-type protein. In that sense, stress-dependent phosphorylation of hnRNP A1 and the subsequent decrease in hnRNP A1-Trn interaction can be envisaged as a means to promote a longer residence time for the protein within particular mRNPs, thus involving it in the altered cytoplasmic control of mRNA metabolism that occurs in stressed cells.
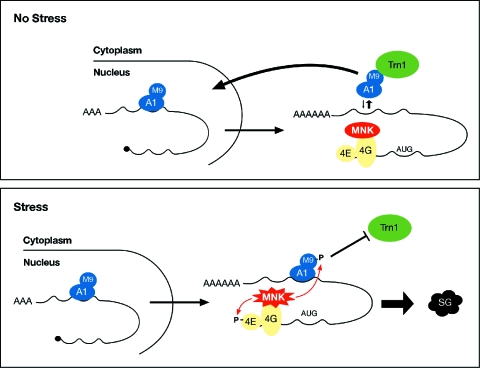
FIG. 8.
Model for recruitment of hnRNP A1 to SGs following stress-induced Mnk1/2 activation. In nonstressed cells (upper diagram), hnRNP A1 is released from mRNAs in the cytoplasm through interaction with the import receptor transportin (Trn1) and driven back to the nucleus. Upon exposure to stress (lower diagram), Mnk1/2-mediated phosphorylation of poly(A) mRNA-bound hnRNP A1 downstream of the M9 domain abrogates the interaction with Trn1, thereby increasing the binding of hnRNP A1 to mRNA and allowing its recruitment to the SGs.
Is there a specific role for hnRNP A1 or, more generally, for hnRNPs in the stress response? Stress has been shown to modify the subcellular localization of several other RNA-binding proteins aside from hnRNP A1, including the second-step splicing factor Slu7 (53) and HuR (20). It has previously been suggested that hnRNP A1 has an antiapoptotic effect in human cancer cells (45) and functions in cell proliferation (24). Here we describe a new role for hnRNP A1 in ensuring cell viability during the response to stress. Furthermore, we show that this function in stress survival relies (at least partially) on the recruitment of hnRNP A1 to the stress granules (Fig. 7).
In summary, we have identified hnRNP A1 as a novel component of the SGs. Our data are consistent with a model whereby stress-induced phosphorylation of hnRNP A1 prevents its interaction with transportin, resulting in increased cytoplasmic hnRNP A1 which remains bound to poly(A) mRNAs that are targeted to the SGs (Fig. 8). The absence of hnRNP A1 from PBs and its role in facilitating the recovery of cells from stress suggest that mRNA-bound hnRNP A1 has a protective role during the stress response. The identification of RNA targets upon exposure to stress will elucidate this interesting problem.
. . . . . .
Acknowledgments
We thank Rikiro Fukunaga (Osaka, Japan), Akila Mayeda (Miami, Fla.), Bertrand Seraphin (Paris, France) and Jamal Tazi (Montpellier, France) for their generous gifts of reagents. We also thank Inga Thomson (MRC HGU) for help with the FRAP experiments. We are grateful to Juan Valcarcel (Barcelona, Spain), Wendy Bickmore, and Jeremy Sanford (MRC HGU) for helpful comments on the manuscript.
This work was supported by the Medical Research Council (J.C.L. and J.F.C.) and a long-term postdoctoral fellowship from EMBO (S.G.).
REFERENCES
- 1.Allemand, E., S. Guil, M. Myers, J. Moscat, J. F. Caceres, and A. R. Krainer. 2005. Regulation of heterogenous nuclear ribonucleoprotein A1 transport by phosphorylation in cells stressed by osmotic shock. Proc. Natl. Acad. Sci. USA 102:3605-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biamonti, G. 2004. Nuclear stress bodies: a heterochromatin affair? Nat. Rev. Mol. Cell Biol. 5:493-498. [DOI] [PubMed] [Google Scholar]
- 3.Biamonti, G., M. Buvoli, M. T. Bassi, C. Morandi, F. Cobianchi, and S. Riva. 1989. Isolation of an active gene encoding human hnRNP protein A1. Evidence for alternative splicing. J. Mol. Biol. 207:491-503. [DOI] [PubMed] [Google Scholar]
- 4.Bond, U. 1988. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 7:3509-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnal, S., F. Pileur, C. Orsini, F. Parker, F. Pujol, A. C. Prats, and S. Vagner. 2005. Heterogeneous nuclear ribonucleoprotein A1 is a novel internal ribosome entry site trans-acting factor that modulates alternative initiation of translation of the fibroblast growth factor 2 mRNA. J. Biol. Chem. 280:4144-4153. [DOI] [PubMed] [Google Scholar]
- 6.Buxade, M., J. L. Parra, S. Rousseau, N. Shpiro, R. Marquez, N. Morrice, J. Bain, E. Espel, and C. G. Proud. 2005. The Mnks are novel components in the control of TNFalpha biosynthesis and phosphorylate and regulate hnRNP A1. Immunity 23:177-189. [DOI] [PubMed] [Google Scholar]
- 7.Caceres, J. F., T. Misteli, G. R. Screaton, D. L. Spector, and A. R. Krainer. 1997. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J. Cell Biol. 138:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caceres, J. F., S. Stamm, D. M. Helfman, and A. R. Krainer. 1994. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science 265:1706-1709. [DOI] [PubMed] [Google Scholar]
- 9.Cazalla, D., K. Newton, and J. F. Caceres. 2005. A novel SR-related protein is required for the second step of pre-mRNA splicing. Mol. Cell. Biol. 25:2969-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cougot, N., S. Babajko, and B. Seraphin. 2004. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 165:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daoud, R., G. Mies, A. Smialowska, L. Olah, K. A. Hossmann, and S. Stamm. 2002. Ischemia induces a translocation of the splicing factor tra2-beta 1 and changes alternative splicing patterns in the brain. J. Neurosci. 22:5889-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, R. J. 1994. MAPKs: new JNK expands the group. Trends Biochem. Sci. 19:470-473. [DOI] [PubMed] [Google Scholar]
- 13.Denegri, M., I. Chiodi, M. Corioni, F. Cobianchi, S. Riva, and G. Biamonti. 2001. Stress-induced nuclear bodies are sites of accumulation of pre-mRNA processing factors. Mol. Biol. Cell 12:3502-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denegri, M., D. Moralli, M. Rocchi, M. Biggiogera, E. Raimondi, F. Cobianchi, L. De Carli, S. Riva, and G. Biamonti. 2002. Human chromosomes 9, 12, and 15 contain the nucleation sites of stress-induced nuclear bodies. Mol. Biol. Cell 13:2069-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyfuss, G., V. N. Kim, and N. Kataoka. 2002. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 3:195-205. [DOI] [PubMed] [Google Scholar]
- 16.Duncan, R. F., H. Peterson, and A. Sevanian. 2005. Signal transduction pathways leading to increased eIF4E phosphorylation caused by oxidative stress. Free Radic. Biol. Med. 38:631-643. [DOI] [PubMed] [Google Scholar]
- 17.Eystathioy, T., A. Jakymiw, E. K. Chan, B. Seraphin, N. Cougot, and M. J. Fritzler. 2003. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA 9:1171-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillman, C., and J. Lykke-Andersen. 2005. RNA decapping inside and outside of processing bodies. Curr. Opin. Cell Biol. 17:326-331. [DOI] [PubMed] [Google Scholar]
- 19.Forch, P., O. Puig, N. Kedersha, C. Martinez, S. Granneman, B. Seraphin, P. Anderson, and J. Valcarcel. 2000. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol. Cell 6:1089-1098. [DOI] [PubMed] [Google Scholar]
- 20.Gallouzi, I. E., C. M. Brennan, M. G. Stenberg, M. S. Swanson, A. Eversole, N. Maizels, and J. A. Steitz. 2000. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc. Natl. Acad. Sci. USA 97:3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatto-Konczak, F., C. F. Bourgeois, C. Le Guiner, L. Kister, M. C. Gesnel, J. Stevenin, and R. Breathnach. 2000. The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol. Cell. Biol. 20:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert, W., C. W. Siebel, and C. Guthrie. 2001. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA 7:302-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton, B. J., C. M. Burns, R. C. Nichols, and W. F. Rigby. 1997. Modulation of AUUUA response element binding by heterogeneous nuclear ribonucleoprotein A1 in human T lymphocytes. The roles of cytoplasmic location, transcription, and phosphorylation. J. Biol. Chem. 272:28732-28741. [DOI] [PubMed] [Google Scholar]
- 24.He, Y., M. A. Brown, J. A. Rothnagel, N. A. Saunders, and R. Smith. 2005. Roles of heterogeneous nuclear ribonucleoproteins A and B in cell proliferation. J. Cell Sci. 118:3173-3183. [DOI] [PubMed] [Google Scholar]
- 25.Iervolino, A., G. Santilli, R. Trotta, C. Guerzoni, V. Cesi, A. Bergamaschi, C. Gambacorti-Passerini, B. Calabretta, and D. Perrotti. 2002. hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis. Mol. Cell. Biol. 22:2255-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingelfinger, D., D. J. Arndt-Jovin, R. Luhrmann, and T. Achsel. 2002. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 8:1489-1501. [PMC free article] [PubMed] [Google Scholar]
- 27.Izaurralde, E., A. Jarmolowski, C. Beisel, I. W. Mattaj, G. Dreyfuss, and U. Fischer. 1997. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J. Cell Biol. 137:27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izquierdo, J. M., N. Majos, S. Bonnal, C. Martinez, R. Castelo, R. Guigo, D. Bilbao, and J. Valcarcel. 2005. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 19:475-484. [DOI] [PubMed] [Google Scholar]
- 29.Jolly, C., A. Metz, J. Govin, M. Vigneron, B. M. Turner, S. Khochbin, and C. Vourc'h. 2004. Stress-induced transcription of satellite III repeats. J. Cell Biol. 164:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamath, R. V., D. J. Leary, and S. Huang. 2001. Nucleocytoplasmic shuttling of polypyrimidine tract-binding protein is uncoupled from RNA export. Mol. Biol. Cell 12:3808-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedersha, N., and P. Anderson. 2002. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30:963-969. [DOI] [PubMed] [Google Scholar]
- 32.Kedersha, N., S. Chen, N. Gilks, W. Li, I. J. Miller, J. Stahl, and P. Anderson. 2002. Evidence that ternary complex (eIF2-GTP-tRNA(i) (Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedersha, N., M. R. Cho, W. Li, P. W. Yacono, S. Chen, N. Gilks, D. E. Golan, and P. Anderson. 2000. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151:1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedersha, N., G. Stoecklin, M. Ayodele, P. Yacono, J. Lykke-Andersen, M. J. Fitzler, D. Scheuner, R. J. Kaufman, D. E. Golan, and P. Anderson. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169:871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 36.Lai, M. C., H. W. Kuo, W. C. Chang, and W. Y. Tarn. 2003. A novel splicing regulator shares a nuclear import pathway with SR proteins. EMBO J. 22:1359-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López de Silanes, I., S. Galban, J. L. Martindale, X. Yang, K. Mazan-Mamczarz, F. E. Indig, G. Falco, M. Zhan, and M. Gorospe. 2005. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol. Cell. Biol. 25:9520-9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, L., A. P. Han, and J. J. Chen. 2001. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 21:7971-7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Contreras, R., J. F. Fisette, F. U. Nasim, R. Madden, M. Cordeau, and B. Chabot. 2006. Intronic binding sites for hnRNP A/B and hnRNP F/H proteins stimulate pre-mRNA splicing. PLoS Biol. 4:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayeda, A., and A. R. Krainer. 1992. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell 68:365-375. [DOI] [PubMed] [Google Scholar]
- 41.Mayeda, A., S. H. Munroe, J. F. Caceres, and A. R. Krainer. 1994. Function of conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 13:5483-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metz, A., J. Soret, C. Vourc'h, J. Tazi, and C. Jolly. 2004. A key role for stress-induced satellite III transcripts in the relocalization of splicing factors into nuclear stress granules. J. Cell Sci. 117:4551-4558. [DOI] [PubMed] [Google Scholar]
- 43.Michael, W. M., M. Choi, and G. Dreyfuss. 1995. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell 83:415-422. [DOI] [PubMed] [Google Scholar]
- 44.New, L., Y. Jiang, M. Zhao, K. Liu, W. Zhu, L. J. Flood, Y. Kato, G. C. Parry, and J. Han. 1998. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 17:3372-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patry, C., L. Bouchard, P. Labrecque, D. Gendron, B. Lemieux, J. Toutant, E. Lapointe, R. Wellinger, and B. Chabot. 2003. Small interfering RNA-mediated reduction in heterogeneous nuclear ribonucleoparticle A1/A2 proteins induces apoptosis in human cancer cells but not in normal mortal cell lines. Cancer Res. 63:7679-7688. [PubMed] [Google Scholar]
- 46.Pinol-Roma, S., Y. D. Choi, M. J. Matunis, and G. Dreyfuss. 1988. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 2:215-227. [DOI] [PubMed] [Google Scholar]
- 47.Pinol-Roma, S., and G. Dreyfuss. 1992. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355:730-732. [DOI] [PubMed] [Google Scholar]
- 48.Pyronnet, S., H. Imataka, A. C. Gingras, R. Fukunaga, T. Hunter, and N. Sonenberg. 1999. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 18:270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanford, J. R., J. D. Ellis, D. Cazalla, and J. F. Caceres. 2005. Reversible phosphorylation differentially affects nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor. Proc. Natl. Acad. Sci. USA 102:15042-15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheper, G. C., N. A. Morrice, M. Kleijn, and C. G. Proud. 2001. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol. Cell. Biol. 21:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin, C., Y. Feng, and J. L. Manley. 2004. Dephosphorylated SRp38 acts as a splicing repressor in response to heat shock. Nature 427:553-558. [DOI] [PubMed] [Google Scholar]
- 52.Shin, C., and J. L. Manley. 2004. Cell signalling and the control of pre-mRNA splicing. Nat. Rev. Mol. Cell Biol. 5:727-738. [DOI] [PubMed] [Google Scholar]
- 53.Shomron, N., M. Alberstein, M. Reznik, and G. Ast. 2005. Stress alters the subcellular distribution of hSlu7 and thus modulates alternative splicing. J. Cell Sci. 118:1151-1159. [DOI] [PubMed] [Google Scholar]
- 54.Siomi, H., and G. Dreyfuss. 1995. A nuclear localization domain in the hnRNP A1 protein. J. Cell Biol. 129:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamm, S. 2002. Signals and their transduction pathways regulating alternative splicing: a new dimension of the human genome. Hum. Mol. Genet. 11:2409-2416. [DOI] [PubMed] [Google Scholar]
- 56.Stokoe, D., D. G. Campbell, S. Nakielny, H. Hidaka, S. J. Leevers, C. Marshall, and P. Cohen. 1992. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 11:3985-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svitkin, Y. V., L. P. Ovchinnikov, G. Dreyfuss, and N. Sonenberg. 1996. General RNA binding proteins render translation cap dependent. EMBO J. 15:7147-7155. [PMC free article] [PubMed] [Google Scholar]
- 58.Tourriere, H., K. Chebli, L. Zekri, B. Courselaud, J. M. Blanchard, E. Bertrand, and J. Tazi. 2003. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160:823-831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.van Der Houven Van Oordt, W., M. T. Diaz-Meco, J. Lozano, A. R. Krainer, J. Moscat, and J. F. Caceres. 2000. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waskiewicz, A. J., J. C. Johnson, B. Penn, M. Mahalingam, S. R. Kimball, and J. A. Cooper. 1999. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 19:1871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weighardt, F., G. Biamonti, and S. Riva. 1995. Nucleo-cytoplasmic distribution of human hnRNP proteins: a search for the targeting domains in hnRNP A1. J. Cell Sci. 108:545-555. [DOI] [PubMed] [Google Scholar]
- 63.Wilusz, C. J., and J. Wilusz. 2004. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 20:491-497. [DOI] [PubMed] [Google Scholar]
- 64.Windgassen, M., D. Sturm, I. J. Cajigas, C. I. Gonzalez, M. Seedorf, H. Bastians, and H. Krebber. 2004. Yeast shuttling SR proteins Npl3p, Gbp2p, and Hrb1p are part of the translating mRNPs, and Npl3p can function as a translational repressor. Mol. Cell. Biol. 24:10479-10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie, J., J. A. Lee, T. L. Kress, K. L. Mowry, and D. L. Black. 2003. Protein kinase A phosphorylation modulates transport of the polypyrimidine tract-binding protein. Proc. Natl. Acad. Sci. USA 100:8776-8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, X., M. R. Bani, S. J. Lu, S. Rowan, Y. Ben David, and B. Chabot. 1994. The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5′ splice site selection in vivo. Proc. Natl. Acad. Sci. USA 91:6924-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]