Abstract
The recently discovered human bocavirus (HBoV) is the first member of the family Parvoviridae, genus Bocavirus, to be potentially associated with human disease. Several studies have identified HBoV in respiratory specimens from children with acute respiratory disease, but the full spectrum of clinical disease and the epidemiology of HBoV infection remain unclear. The availability of rapid and reliable molecular diagnostics would therefore aid future studies of this novel virus. To address this, we developed two sensitive and specific real-time TaqMan PCR assays that target the HBoV NS1 and NP-1 genes. Both assays could reproducibly detect 10 copies of a recombinant DNA plasmid containing a partial region of the HBoV genome, with a dynamic range of 8 log units (101 to 108 copies). Eight blinded clinical specimen extracts positive for HBoV by an independent PCR assay were positive by both real-time assays. Among 1,178 NP swabs collected from hospitalized pneumonia patients in Sa Kaeo Province, Thailand, 53 (4.5%) were reproducibly positive for HBoV by one or both targets. Our data confirm the possible association of HBoV infection with pneumonia and demonstrate the utility of these real-time PCR assays for HBoV detection.
The parvoviruses of vertebrates are classified within the family Parvoviridae, subfamily Parvovirinae, and further into five genera, including Parvovirus, Erythrovirus, Dependovirus, Amdovirus, and Bocavirus (9). Until recently, parvovirus B19, within the genus Erythrovirus, was the only parvovirus recognized to cause human disease. This has changed with the discovery of a new parvovirus that has been identified in respiratory specimens from young Swedish children with lower respiratory tract infections (LRTIs) (1). Provisionally named human bocavirus (HBoV), due to its genetic similarities to other members of the Bocavirus genus, this virus has now been identified in respiratory specimens from children with upper and LRTI from Australia (8), Canada (2), France (6), and Japan (7). The prevalence of HBoV among children with respiratory disease reported in these studies ranges from 1.5 to 5.7%. However, the causal role of HBoV in LRTI, the full spectrum of clinical disease, and the epidemiology of HBoV infections still need to be defined.
To date, HBoV has not been successfully cultured, no serological tests are yet available, and only conventional PCR assays have been described. Real-time PCR, in contrast to conventional assays, offers rapid results, potentially increased sensitivity and specificity of detection, is less prone to false-positive results from amplicon contamination, and is more amenable for quantitative estimation of viral load (3). In this study, we developed real-time PCR assays targeting the HBoV NS1 and NP-1 genes and used these assays to test for HBoV in a large collection of respiratory specimens from hospitalized pneumonia patients in Thailand.
(Data from the manuscript were presented in part at the International Conference on Emerging Infectious Diseases, Atlanta, Ga., 19 to 22 March 2006.)
MATERIALS AND METHODS
Clinical specimens.
Respiratory specimens were collected from 1,178 hospitalized pneumonia patients identified through active population-based surveillance for pneumonia in Sa Kaeo Province, Thailand, between 1 September 2004 and 31 August 2005 (S. J. Olsen, Y. Laosiritaworn, S. Siasiriwattana, S. Chunsuttiwat, and S. F. Dowell, submitted for publication). The protocol underwent institutional ethical review and was approved by the Thailand Ministry of Health and the Centers for Disease Control and Prevention (CDC). Nasopharyngeal swabs were collected in chilled viral transport medium and frozen at −70°C within 48 h of collection. Two 300-μl aliquots of each specimen were then added to duplicate vials containing AL extraction buffer (QIAGEN, Valencia, CA) and shipped on dry ice to the CDC for PCR testing for respiratory pathogens. Upon receipt, total nucleic acid was extracted from one aliquot of each specimen by using the automated BioRobot MDx (QIAGEN) following the manufacturer's instructions. Nucleic acid eluates (∼80 μl) were supplemented with 100 μl of RNA storage buffer (Ambion, Austin, TX), split into two aliquots, and stored at −70°C until PCR testing.
DNA extracts of respiratory specimens from 12 patients that had previously tested positive or negative for HBoV by an independent PCR assay (developed at the Qpid Laboratory, SASVRC, Royal Children's Hospital, Queensland, Australia) (8) were shipped to the CDC on dry ice for confirmatory testing.
Primers and probes.
Conserved regions of the HBoV NS1 and NP-1 genes were identified from alignments of nucleotide sequences available from GenBank (for NS1, DQ206700-08, DQ000495-96, and DQ200648, and for NP-1, DQ000495-96, AB243566-72, DQ296618-35, DQ353695-99, DQ299885, DQ267760-75, DQ284856, DQ295844, and AM109958-66) using ClustalW (4). Primer and probe sets were designed to these conserved regions using Primer Express software, version 2.0.0 (Applied Biosystems, Foster City, CA). The best primer and probe sets selected by the software that showed no major nonspecific homologies by BLAST (Basic Local Alignment Search Tool) analysis were synthesized by the CDC Biotechnology Core Facility using standard phosphoramidite chemistry. TaqMan probes were labeled at the 5′ ends with the reporter molecule 6-carboxyfluorescein and at the 3′ ends with Black Hole Quencher 1 (Biosearch Technologies, Inc., Novato, CA). Optimal primer and probe concentrations were determined by checkerboard titrations against the HBoV plasmid (see below). Primer and probe sets that gave the highest amplification efficiencies at optimized conditions and with no detectable cross-reactions were chosen for further study (Table 1).
TABLE 1.
HBoV primers and probes used in real-time PCR assays
| Gene target | Primer/probea | Sequence (5′ to 3′) | Amplicon length (bp) | Positionb |
|---|---|---|---|---|
| NS1 | Primer, fwd | TGC AGA CAA CGC YTA GTT GTT T | 88 | 1554-1575 |
| Primer, rev | CTG TCC CGC CCA AGA TAC A | 1641-1623 | ||
| Probe | CCA GGA TTG GGT GGA ACC TGC AAA | 1598-1621 | ||
| NP-1 | Primer, fwd | AGA GGC TCG GGC TCA TAT CA | 81 | 2478-2497 |
| Primer, rev | CAC TTG GTC TGA GGT CTT CGA A | 2558-2537 | ||
| Probe | AGG AAC ACC CAA TCA RCC ACC TAT CGT CT | 2500-2528 |
Probes 5′ end labeled with 6-carboxyfluorescein and 3′ end labeled with Black Hole Quencher. fwd, forward; rev, reverse.
Nucleotide numbering based on HBoV strain st1 (accession no. DQ000495).
Plasmid standard construction.
A 1,298-bp DNA subgenomic fragment of HBoV was amplified from a positive nasal swab by using primers bracketing the real-time PCR target regions in the NS1 and NP-1 genes (forward, 5′-CCTGTGCTGTGTCCTGAACAAAC-3′, and reverse, 5′-CAATGCGAGTAGAGTGCCAGTAGAAC-3′). The product was cloned into plasmid vector pCRII-TOPO (Invitrogen, Carlsbad, CA) and sequenced for verification (GenBank accession no. DQ499604). The pCRII HBoV plasmid was purified using a QIAprep mini prep kit (QIAGEN) and quantified by UV spectroscopy (4.1 × 1010 copies/μl). To generate standard curves for quantitative determinations and to access amplification efficiency, replicate serial 10-fold dilutions of the pCRII HBoV plasmid were prepared in 10 mM Tris-EDTA buffer containing 100 μg/ml herring sperm DNA (Promega, Madison, WI) and stored at −20°C until use. To minimize potential for contamination, HBoV plasmid DNA was prepared in a separate laboratory.
HBoV real-time PCR assays.
The real-time PCR assays were performed using iQ Supermix (Bio-Rad, Hercules, CA) with each 25-μl reaction mixture containing 0.5 μM forward and reverse primers, 0.1 μM probe, and 5 μl of nucleic acid extract. Amplification was carried out in 96-well plates on an iCycler iQ real-time detection system (Bio-Rad). Thermocycling conditions consisted of 3 min at 95°C for activation of the iTaq DNA polymerase and 45 cycles of 15 s at 95°C and 1 min at 60°C. Each run included one synthetic template control and one no-template control for each target. Specimen extracts were also tested by real-time PCR for the human RNase P gene to monitor specimen quality and the presence of PCR inhibitors as previously described (5). A positive test for both NS1 and NP-1 targets or for a single target confirmed from a second extraction from a new sample aliquot was considered definitive evidence of HBoV infection.
Conventional HBoV PCR.
Real-time HBoV PCR assays were compared with a conventional PCR assay using NS1 gene primers HBoV01.2 and HBoV02.2 and protocol as previously described by Sloots et al. (8), with amplification performed on the GeneAmp PCR System 9700 (Applied Biosystems). The resulting 291-bp amplicon was identified by gel electrophoresis and ethidium bromide staining.
RESULTS
Real-time PCR amplification efficiency.
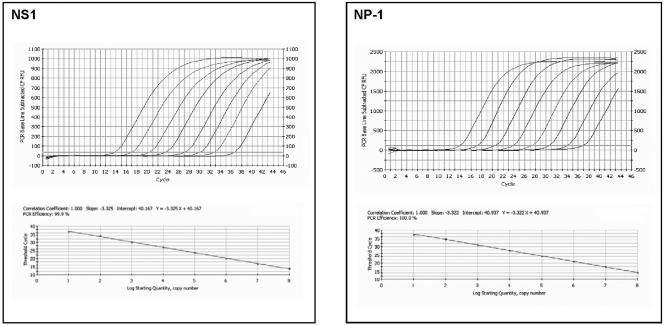
Optimized PCR assays targeting conserved regions of the NS1 and NP-1 genes were further evaluated for amplification efficiency and limit of target detection. PCR amplification of serial 10-fold dilutions of the pCRII HBoV plasmid achieved >95% efficiency with linear amplification over an 8-log dynamic range from 101 to 108 copies per reaction for both primer/probe sets (Fig. 1; Table 2). To assess the effect of exogenous nucleic acid extract on the amplification efficiency, 10-fold serial dilutions of the pCRII HBoV plasmid were prepared in pooled total nucleic acid extracted from seven HBoV-negative nose and throat swabs and sputum specimens collected from laboratory staff. Exogenous nucleic acid from respiratory specimens showed a minimal effect on the amplification efficiencies of both primer/probe sets (Table 2).
FIG. 1.
NS1 and NP-1 real-time PCR assays showing representative results obtained with serial 10-fold dilutions (101 to 108 copies per reaction) of the pCRII HBoV plasmid. The top panels show baseline subtractive curve fit views of the data with relative fluorescence units (RFU) plotted against cycle number. The default setting of 10 times the standard deviation of the RFU measured in all wells over the baseline cycles was used to calculate the CT for a positive reaction (horizontal line). The bottom panels show standard curve analysis of the DNA amplification plots with CT values plotted proportionately against the logarithm of the input copy number. The dynamic range of NS1 and NP-1 PCR assays spans 8 log units with slopes of −3.3 and an R2 value of 1.0.
TABLE 2.
Efficiency of HBoV real-time PCR assays
| Target and efficiency type | Mean CT valuesa at estimated pCRII HBoV plasmid DNA copy no.
|
Slopeb | Efficiency (%)c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | |||
| NS1 | ||||||||||
| Plasmid without extractd | 37.08 ± 0.70 | 33.68 ± 0.16 | 30.67 ± 0.06 | 27.53 ± 0.09 | 23.92 ± 0.16 | 20.68 ± 0.09 | 17.49 ± 0.13 | 13.98 ± 0.11 | −3.29 | 101.3 |
| Plasmid with extract | 37.74 ± 0.85 | 34.56 ± 0.74 | 30.96 ± 0.20 | 27.46 ± 0.09 | 24.24 ± 0.14 | 20.86 ± 0.14 | 17.50 ± 0.16 | 14.06 ± 0.09 | −3.39 | 97.3 |
| NP-1 | ||||||||||
| Plasmid without extract | 37.18 ± 0.32 | 34.86 ± 0.19 | 31.37 ± 0.15 | 27.77 ± 0.08 | 24.61 ± 0.12 | 21.21 ± 0.09 | 17.86 ± 0.06 | 14.63 ± 0.09 | −3.29 | 101.3 |
| Plasmid with extract | 38.87 ± 0.28 | 35.08 ± 0.15 | 31.61 ± 0.25 | 28.26 ± 0.19 | 25.16 ± 0.49 | 21.59 ± 0.04 | 18.16 ± 0.04 | 14.73 ± 0.09 | −3.41 | 96.3 |
Values shown are means of triplicate samples ± standard deviations.
Slope determined from the formula Y = Y intercept − slope log10. Slopes calculated from 101 to 108 copies per reaction.
Efficiency = [10(−1/slope)] − 1.
Reactions performed in presence of pooled total nucleic acid extract from seven human respiratory specimens.
Real-time PCR limit of detection.
To assess the limit of detection of the NS1 and NP-1 assays, 1, 5, and 10 copies of the pCRII HBoV plasmid per reaction were tested in 18 replicates. At 10 copies, 100% of the replicates were positive by both assays, at 5 copies, 10 (56%) NS1 and 11 (61%) NP-1 replicates were positive, and at 1 copy, 7 (39%) NS1 and 5 (28%) NP-1 replicates were positive.
Real-time PCR reproducibility.
To assess the intra- and interassay reproducibility, 10-fold serial dilutions of the pCRII HBoV plasmid from 101 to 108 copies per reaction were tested in triplicate on three different days. Over the linear range of the assays, the coefficient of variation of the mean cycle threshold (CT) values within runs ranged from 0.20 to 1.88% and 0.28 to 0.86% and from run to run ranged from 0.33 to 2.12% and 0.94 to 2.79% for the NS1 and NP-1 real-time PCR assays, respectively (Table 3).
TABLE 3.
Reproducibility of HBoV real-time PCR assays
| Target and reproducibility type | CV (%) for no. of pCRII HBoV plasmid DNA for indicated copies/reactiona
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | |
| NS1 | ||||||||
| Intraassayb | 1.88 | 0.46 | 0.2 | 0.34 | 0.68 | 0.45 | 0.73 | 0.77 |
| Interassay (%)c | 2.12 | 1.51 | 0.33 | 0.55 | 0.52 | 0.79 | 1.41 | 1.36 |
| NP-1 | ||||||||
| Intraassay (%) | 0.86 | 0.55 | 0.46 | 0.29 | 0.49 | 0.28 | 0.33 | 0.61 |
| Interassay (%) | 2.32 | 1.69 | 1.25 | 0.94 | 1.95 | 2.02 | 2.76 | 2.79 |
Tenfold serial dilutions of HBoV plasmid DNA; dilution series thawed on three different days and assays performed in triplicate for each dilution. CV, coefficient of variation.
Determined from three replicates within each assay.
Determined from three independent assays performed on different days.
Real-time PCR specificity.
The specificity of the real-time PCR assays was assessed by testing nucleic acid extracts of pooled respiratory specimens containing human and microbiological flora DNA as well as DNA from laboratory cultures or positive clinical specimens containing human pathogens that may colonize the respiratory tract, including human parvovirus B19, adenovirus, herpes simplex virus 1, Mycoplasma pneumoniae, and Chlamydia pneumoniae.
Because we anticipate incorporating the HBoV real-time PCR assays into a respiratory pathogen assay panel that includes a reverse transcription step, we also tested cDNA prepared from laboratory strains of RNA viruses, including human respiratory syncytial virus, parainfluenza viruses 1, 2, and 3, rhinovirus 1A, and influenza viruses A and B. No specific amplification was detected by either NS1 or NP-1 assay from any of the above samples.
Comparison of real-time and conventional PCR.
Tenfold serial dilutions of the pCRII HBoV plasmid were tested in parallel by the real-time PCR assays and a conventional PCR assay that amplifies a 279-bp region of the NS1 gene (8). The detection limits for both assays were identical. Of 12 blinded clinical specimen extracts previously tested by the conventional PCR, 7 positive and 5 negative for HBoV DNA were correctly identified by both real-time PCR assays (Table 4).
TABLE 4.
HBoV real-time PCR results from 12 blinded sample extractsa
| Sample ID | Reference PCR resultb | Real-time PCR result forc:
|
|
|---|---|---|---|
| NS1 | NP-1 | ||
| BRIS001B | Neg | Neg | Neg |
| BRIS002B | Pos | 12.9 | 13.3 |
| BRIS003B | Pos | 32.8 | 31.9 |
| BRIS004B | Neg | Neg | Neg |
| BRIS005B | Pos | 27.7 | 27.5 |
| BRIS006B | Pos | 37.0 | 37.6 |
| BRIS007B | Pos | 34.4 | 35.1 |
| BRIS008B | Pos | 19.1 | 19.5 |
| BRIS009B | Neg | Neg | Neg |
| BRIS010B | Pos | 35.7 | 36.7 |
| BRIS011B | Neg | Neg | Neg |
| BRIS012B | Neg | Neg | Neg |
ID, identification no. Neg, negative; pos, positive.
See reference 8 for details.
Results (CT values) from 1 μl of a 1:10 dilution of DNA extract tested.
Real-time PCR results from patients with pneumonia.
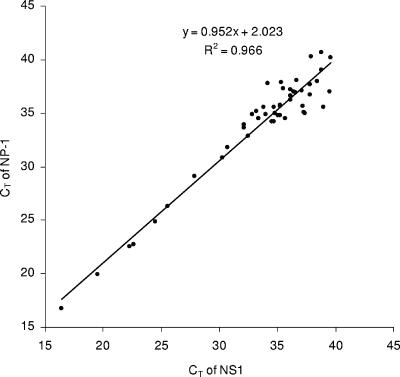
Of the 1,178 respiratory specimens from hospitalized patients with pneumonia in Sa Kaeo Province, Thailand, 32 were PCR positive for both NS1 and NP-1 targets and 39 were positive for only a single target (23 for NS1 and 16 for NP-1). On retesting from a second, previously unopened specimen aliquot, 14 of the 39 single-target positive specimens were positive for both targets, 2 were positive by another target only, and 5 were positive by the same target only. Eighteen samples positive for a single target on initial testing could not be confirmed from the second sample aliquot. The CT values obtained for NS1- and NP-1-positive specimens were highly correlated (R2 = 0.966) and ranged from 16.4 to 38.8 and from 16.7 to 40.7, respectively (Fig. 2). The CT values of all single-target positive specimens were near the assay detection limit (CT ≥ 37). Altogether, 53 (4.5%) of the patients had detectable HBoV DNA based on our positive test criteria.
FIG. 2.
CT values of NS1 plotted against NP-1 for 48 clinical specimens positive for both targets.
DISCUSSION
The objective of the study was to develop real-time PCR assays for HBoV that would be suitable for diagnostic applications. Given the limited sequence data currently available for the virus, and in anticipation of possible sequence variation among unrecognized HBoV strains, two discrete targets were selected from conserved regions of the virus nonstructural protein genes for assay development. The assays performed equivalently, with a reproducible detection limit of 10 target genome copies per reaction and linear amplification over a wide dynamic range suitable for quantitative applications.
The potential for contamination with HBoV DNA that could lead to false-positive results was a major concern in this study. This concern was heightened by our finding of a predominance of high CT values (low genome copy number) and a high proportion of single-target positives among the study specimens. This was partially addressed by using real-time PCR assays that benefit from a closed tube system that minimizes the potential for amplicon contamination and by requiring two independent assay targets (NS1 and NP-1) to be positive in the same sample aliquot or a single assay target to be positive from two independent sample extracts for the sample test result to be considered positive. In addition, we rigorously followed recommended laboratory practices that promote a DNA contamination-free environment (3), including strict separation of pre- and post-PCR activities and by conducting routine surveillance for contamination by ample use of negative PCR controls. Plasmid control DNA handling and PCR setup activities were done in separate rooms, and extractions of clinical specimens were performed prior to the introduction of any artificial HBoV DNA into our laboratory.
The prevalence of HBoV infection found among Thai patients hospitalized with pneumonia (4.5%) was similar to prevalence estimates previously reported for children with LRTI (1, 7, 8), although this likely represents an underestimate of the true number of positive specimens. As noted above, most of the HBoV PCR-positive patients had relatively low virus loads. We found no evidence from the RNase P extraction control (data not shown) of poor specimen quality or the presence of PCR inhibitors or that delays in timing of specimen collection from onset of symptoms could account for these findings. We therefore conclude that HBoV may often be present in respiratory specimens from patients with pneumonia at low levels, thus highlighting the importance of using sensitive PCR assays and testing replicates of two or more targets to ensure the detection of HBoV.
In conclusion, we have developed sensitive and specific real-time PCR assays with which we confirmed the presence of HBoV DNA in patients hospitalized with pneumonia. The availability of these assays and the pCRII HBoV plasmid control will facilitate further studies to better define the epidemiology of HBoV infection and its role in human disease.
Acknowledgments
We thank Brian Holloway and Karen McCaustland (CDC Biotechnology Core Facility Branch) for oligonucleotide synthesis and expert technical advice.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
REFERENCES
- 1.Allander, T., M. T. Tammi, M. Eriksson, A. Bjerkner, A. Tiveljung-Lindell, and B. Andersson. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 102:12891-12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastien, N., K. Brandt, K. Dust, D. Ward, and Y. Li. 2006. Human bocavirus infection, Canada. Emerg. Infect. Dis. 12:848-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustin, S. A., and T. Nolan. 2004. A-Z of quantitative PCR. International University Line, La Jolla, Calif.
- 4.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emery, S. L., D. D. Erdman, M. D. Bowen, B. R. Newton, J. M. Winchell, R. F. Meyer, S. Tong, B. T. Cook, B. P. Holloway, K. A. McCaustland, P. A. Rota, B. Bankamp, L. E. Lowe, T. G. Ksiazek, W. J. Bellini, and L. J. Anderson. 2004. Real-time reverse transcription-polymerase chain reaction assay for SARS-associated coronavirus. Emerg. Infect. Dis. 10:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foulongne, V., M. Rodiere, and M. Segondy. 2006. Human bocavirus in children. Emerg. Infect. Dis. 12:862-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma, X., R. Endo, N. Ishiguro, T. Ebihara, H. Ishiko, T. Ariga, and H. Kikuta. 2006. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J. Clin. Microbiol. 44:1132-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sloots, T. P., P. McErlean, D. J. Speicher, K. E. Arden, M. D. Nissen, and I. M. Mackay. 2006. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J. Clin. Virol. 35:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tattersall, P. 2005. Family Parvoviridae, p. 353-369. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: classification and nomenclature of viruses. Eighth report of the International Committee on the Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom.