Abstract
Cryptococcus neoformans is a frequent cause of meningoencephalitis in immunosuppressed individuals. To better understand the mechanisms of a protective immune response to C. neoformans, a long-term in vitro model of human immune control of cryptococcal infection was developed. Peripheral blood mononuclear cells (PBMC) prestimulated with heat-killed C. neoformans significantly restricted the growth of C. neoformans after a subsequent live infection compared to that with unstimulated PBMC. Live infection with encapsulated C. neoformans was controlled for as long as 10 days, while infection with acapsular organisms could sometimes be eradicated. During immune control, fungal cells were both intracellular and extracellular within aggregates of mononuclear phagocytes and lymphocytes. Optimal immune control depended on the presence of both CD4+ and CD8+ T cells. Immune control of cryptococcal growth was more effective following prestimulation with acapsular compared with encapsulated organisms. Prestimulation with acapsular organisms was associated with a significant and prolonged increase in interleukin-6 (IL-6) production compared with prestimulation with encapsulated C. neoformans. Addition of IL-6 and depletion of CD25+ T cells prior to prestimulation and infection with encapsulated organisms resulted in reductions in cryptococcal growth that reached borderline statistical significance. Depletion of CD25+ T cells significantly reduced cryptococcal growth in wells with unstimulated PBMC. The results demonstrate an association between high levels of IL-6 and resistance to infection and, through suppression of IL-6 release, an additional mechanism whereby the cryptococcal capsule subverts a protective immune response. Further work is required to clarify the mechanism of action of IL-6 in this setting and any interaction with regulatory T cells.
Cryptococcus neoformans is a frequent cause of meningoencephalitis in immunosuppressed individuals. In particular, over the past 2 decades, cryptococcosis has emerged as a very common opportunistic infection in patients with late-stage human immunodeficiency virus (HIV) infection, especially in Southern and East Africa and Southeast Asia (7, 8, 14). Unfortunately, treatment remains unsatisfactory (6, 17, 29), and new immunotherapeutic management strategies, based on an understanding of the mechanisms of protective human immunity to cryptococcal infection, are urgently needed.
Very valuable insights into the immune control of cryptococcal infection have been gained from studies with murine models (18, 22), although such studies always leave open the question of differences between mice and humans with regard to immunity to infection and cryptococcal infection in particular. In the human system, much in vitro work has characterized the interaction of isolated human immune cells with C. neoformans. In a complementary but contrasting approach, we have sought to build on the work of Levitz and colleagues (20) to develop a more complex, longer-term model of human immune control of cryptococcal infection using specifically stimulated human peripheral blood mononuclear cells (PBMC) with the aim of reflecting some of the complex interactions of mononuclear phagocytes and activated lymphocytes that appear to be important in a protective granulomatous response to cryptococcal infection in patients. Here we describe this model and the organization of aggregates of host cells formed in response to in vitro infection, and we analyze the cellular and cytokine components that are important in immune control of cryptococcal infection in the model.
MATERIALS AND METHODS
Reagents.
All reagents were from Sigma-Aldrich Ltd., United Kingdom, unless otherwise stated. The cell culture medium was RPMI 1640 supplemented with l-glutamine, penicillin, streptomycin, and 10% pooled human serum (PHS). Pooled human serum was obtained by combining sera from at least 10 healthy donors under ice-cold conditions to preserve complement activity and was stored in aliquots at −70°C until use. Cell cultures were carried out at 37°C in humidified air supplemented with 5% CO2.
Cryptococcus neoformans.
Serotype D strain B3501 and its acapsular isogenic CAP67 mutant were used in all experiments and were gifts from Eric Jacobson (Medical College of Virginia, Richmond). C. neoformans was grown on Sabouraud dextrose agar plates with chloramphenicol (Oxoid Ltd., United Kingdom), harvested after 4 days of culture, washed, counted, and resuspended at the desired concentration. For prestimulation of PBMC, fungi were heat killed at 60°C for 2 h, washed in RPMI, and stored at 4°C until use.
Prestimulation and infection of PBMC.
Human whole blood was obtained from donors by venipuncture and anticoagulated with pyrogen-free heparin (1,000 U/ml; Leo Laboratories Ltd., United Kingdom). PBMC were isolated by density gradient centrifugation with Histopaque solution, washed three times with cold Hanks balanced salt solution, resuspended in culture medium, and plated out in 96-well flat-bottom plates (Corning Costar) at 106 per well. After an hour at 37°C, PHS (10% total volume) and either 2 × 105 encapsulated (B3501) or acapsular (CAP67 mutant) heat-killed C. neoformans organisms or additional medium were added to a total volume of 200 μl per well. After 12 days of prestimulation, the supernatant was carefully removed from each well, and 10% PHS and 1 × 103 to 2 × 103 CFU of C. neoformans were added in fresh medium. For each experiment, the inoculum of live organisms was determined by dilution and spread plates on Sabouraud dextrose agar. Three-quarters of the culture medium was replaced on days 6 and 9 during prestimulation and every other day following infection with live organisms. In some experiments, additional aliquots of supernatant were also collected on days 1, 2, and 3 of prestimulation.
At the indicated time points after addition of live organisms, host cells were lysed with water, and C. neoformans CFU determined by dilution and spread plates on Sabouraud dextrose agar. Results are expressed as the percentage of cryptococcal growth relative to the input inoculum and were calculated as ([CFU in experimental well/inoculum] − 1) × 100 (15). Thus, a value of zero indicates no net fungal growth, 100% indicates an average of one replication, and a negative value indicates that the number of CFU decreased during the experiment and therefore some fungal killing had occurred.
In some experiments, recombinant interleukin-6 (IL-6) (R&D Systems, United Kingdom) at a final concentration of 5 ng/ml was added to wells prior to prestimulation. When the culture medium was replaced at days 3, 6, and 9 of prestimulation, IL-6 was included in the replacement culture medium at the same final concentration.
Cell separations.
In some experiments, PBMC were depleted of specific cell populations by magnetic bead separation using MACS microbeads and the AutoMACS system (Miltenyi Biotec). CD3 and CD25 microbeads were used for depletion of CD3+ and CD25+ T cells (1). In order to deplete PBMC of CD4+ and CD8+ T cells, CD3+ cells were first stained with a fluorescein isothiocyanate-conjugated anti-CD3 antibody and separated using an anti-fluorescein isothiocyanate MultiSort kit (Miltenyi Biotec). The CD3+ fraction was depleted of either CD4+ or CD8+ T cells with CD4 or CD8 microbeads (Miltenyi Biotec), and the remainder of the cells added back to the CD3-negative fraction. In this way, only CD4+ or CD8+ T cells were depleted, and not other cell types bearing these receptors.
Flow cytometry was used to check the purity of cell separations using antibodies against CD3, CD4, CD8, CD14, CD19, CD56 (Beckman Coulter), and CD25 (Dako UK) together with the relevant isotype controls. Three-color staining was performed using directly conjugated fluorescent mouse anti-human monoclonal antibodies. Cells were analyzed on a Beckman Coulter FC 500 flow cytometer running RXP Acquisition and Analysis software. Unseparated PBMC contained 47% ± 9% CD3+ T cells, 31% ± 8% CD4+ T cells, 13% ± 4% CD8+ T cells, 5% ± 2% CD19+ B cells, 23% ± 8% CD14+ monocytes, 16% ± 7% CD56+ NK cells, and 2% ± 1% CD25+ cells. CD3+ cell-depleted PBMC contained 1.9% ± 2.5% CD3+ cells. CD4+ T-cell-depleted PBMC contained 0.5% ± 0.3% CD4+ T cells, CD8+ T-cell-depleted PBMC contained 1.0% ± 1.1% CD8+ T cells, and CD25+ T-cell-depleted PBMC contained 0.1% ± 0.1% CD25+ cells.
Cytokine profiling.
Multiple cytokine levels were determined in supernatant samples using the Luminex 100 technology and Bioplex kits from Bio-Rad Laboratories Ltd. (30). Cytokines measured, with sensitivities for the assay expressed in picograms per milliliter, were as follows: IL-1β, 4; IL-2, 1; IL-6, 1; IL-10, 1; IL-12p70, 1; gamma interferon (IFN-γ), 1; tumor necrosis factor alpha (TNF-α), 1.
Confocal and electron microscopy.
For confocal microscopy, prestimulation and infection were performed as described above but using Labtek II 8-well chamber slides (Nunc) and 2 × 106 PBMC prestimulated with 4 × 105 heat-killed B3501 and infected after 12 days with 1.5 × 103 live organisms. Six days after infection, the cell aggregates were fixed in 2% paraformaldehyde. Cell aggregates were visualized using a Zeiss LSM410 confocal microscope and Zeiss software.
For electron microscopy, for ease of manipulation of cell aggregates, the model was scaled up to 24-well plates. Six million PBMC were cultured with 1.2 × 106 heat-killed B3501 organisms for 12 days and then infected with 9 × 103 live organisms. On day 6 after infection, the cells were fixed in glutaraldehyde. Aggregates of cells were carefully detached from the well and embedded in agar blocks. The agar was trimmed and processed for electron microscopy using a Zeiss EM 900 electron microscope.
Statistics.
Means and standard errors (SE) for cryptococcal growth and cytokine levels of sample groups were calculated and compared using two-tailed, unpaired (with unequal variance), or paired Student t tests.
RESULTS
Control of the growth of encapsulated and acapsular C. neoformans by PBMC prestimulated with heat-killed C. neoformans.
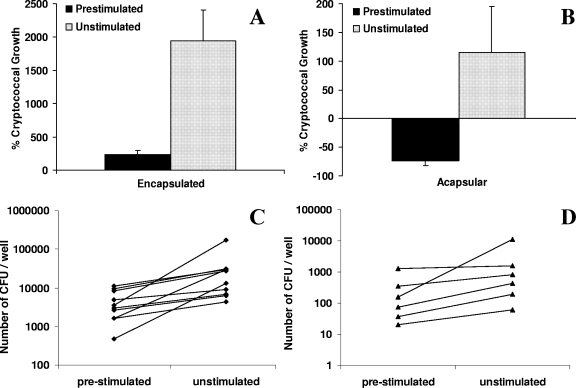
In initial experiments we sought to confirm the findings of Levitz and colleagues (20) that specific stimulation of human PBMC by heat-killed C. neoformans results in cells that more effectively control a subsequent challenge with live C. neoformans than unstimulated PBMC. In preliminary experiments we found that prestimulation for at least 12 days was required for maximal subsequent antifungal activity, consistent with the findings of Levitz and colleagues that such enhancement of activity occurs after 15 but not after 7 days of prestimulation. Using 12 days of prestimulation, in 10 independent experiments with PBMC from different donors, growth of the encapsulated strain B3501 was significantly reduced in the presence of PBMC prestimulated by heat-killed C. neoformans B3501 compared to growth in the presence of unstimulated PBMC: Six days after addition of live organisms, mean cryptococcal growth was 239% ± 61% in the presence of prestimulated PBMC compared with 1,930% ± 466% in the presence of unstimulated PBMC (P = 0.001) (Fig. 1A). Similarly, PBMC prestimulated with the heat-killed isogenic acapsular CAP67 strain were able to kill over half of the inoculum of live CAP67 mutant organisms by day 10 postinfection (−73% ± 9%), whereas CAP67 mutant growth in the presence of unstimulated PBMC was 116% ± 80% at this time point (P = 0.03) (Fig. 1B).
FIG. 1.
Control of in vitro C. neoformans infection by PBMC prestimulated with heat-killed C. neoformans. PBMC (106) were cultured with and without heat-killed organisms (2 × 105), either B3501 (encapsulated) (A) or the CAP67 mutant (acapsular) (B), for 12 days before live organisms of the same strain (1 × 103 to 2 × 103) were added in fresh medium with 10% PHS. The course of infection was followed by CFU counts, expressed as the percentage of cryptococcal growth in relation to the inoculum of live organisms. Data are means + SE from 10 independent experiments with different donors for B3501 and 6 independent experiments with different donors for the CAP67 mutant. Growth of encapsulated (at 6 days postinfection) (A) and acapsular (at 10 days postinfection) (B) C. neoformans was significantly reduced in the presence of stimulated compared with unstimulated PBMC (P = 0.001 and P = 0.03, respectively). (C and D) Corresponding CFU data for B3501 (C) and the CAP67 mutant (D). The mean inocula of live organisms were 1,490 and 1,270, respectively.
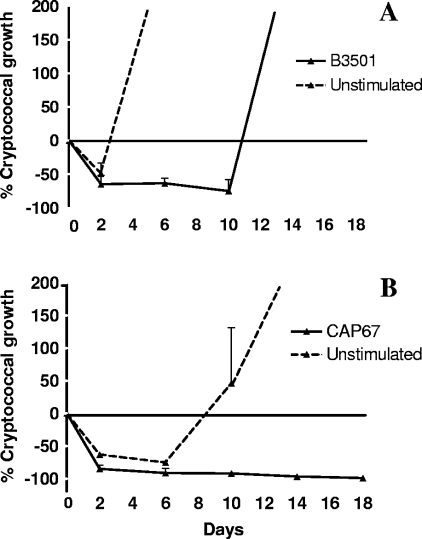
From these initial experiments it was clear that there was significant variation between donors, with cells from some donors better able to control live cryptococcal infection than cells from other individuals. To investigate the reproducibility of results and the degree and time course of immune control using a single donor, triplicate experiments were repeated three times using PBMC from a donor with good control in an initial experiment. The results were highly reproducible and demonstrate effective immune control of cryptococcal infection for at least 10 days following addition of live encapsulated yeast cells (Fig. 2A). In wells with unstimulated PBMC, CFU fell in the first 48 h after addition of live yeast (mean cryptococcal growth, 49% at day 2), but uncontrolled fungal replication soon followed so that mean cryptococcal growth was 618% and 2,760% at 6 and 10 days, respectively. In contrast, in wells with prestimulated PBMC, the fall in cryptococcal CFU at 2 days was maintained, with mean cryptococcal growth of −65%, −63%, and −74% at 2, 6, and 10 days, respectively. Prestimulation and infection with acapsular organisms resulted in even more profound and prolonged immune control (Fig. 2B). In wells containing unstimulated PBMC, C. neoformans CFU fell initially, remained suppressed for 6 days, and then increased. In contrast, after prestimulation with killed organisms, infection with this acapsular isolate was virtually eradicated (mean percent cryptococcal growth, −85%, −91%, −92%, −97%, and −99% at 2, 6, 10, 14, and 18 days, respectively). Indeed, in two further triplicate experiments, at day 35 after infection, a time point at which PBMC were no longer viable, the CAP67 mutant could not be cultured from any of six prestimulated wells, compared with cryptococcal growth of 3,260% ± 975% in wells with unstimulated PBMC.
FIG. 2.
Reproducibility and duration of control of C. neoformans growth by PBMC from a single donor. PBMC (106) were cultured with and without heat-killed organisms (2 × 105), either B3501 (encapsulated) (A) or the CAP67 mutant (acapsular) (B), for 12 days before live organisms of the same strain (1 ×103 to 2 ×103) were added in fresh medium with 10% PHS. The course of infection was followed by CFU counts expressed as a percentage of cryptoccocal growth in relation to the inoculum of live organisms. Data are means + SE from four independent experiments.
Organization of host cell aggregates formed in response to prestimulation and infection with C. neoformans.
Over the course of the experiments, wells were serially examined by light microscopy. In wells with unstimulated PBMC, following infection with live C. neoformans, extracellular fungi became apparent, coincident with the early onset of unrestricted cryptococcal growth and subsequent destruction of the cell monolayer. In wells with prestimulated PBMC, aggregates of host cells, initially formed during prestimulation with C. neoformans, became larger and more distinct after addition of live organisms. During the period of effective immune control, no isolated extracellular fungal cells were seen, suggesting that all the fungal cells were associated with these aggregates. At these time points, when host cells were lysed, prior to dilution and plating out of cryptococcal cells to determine fungal growth, sparse numbers of cryptococcal cells were seen only in association with partially lysed aggregates of host cells. Furthermore, at the time of loss of immune control, prior to the complete destruction of host cell aggregates, budding cryptococcal cells were seen to be emanating from host cell aggregates.
The organization of host cell aggregates was studied by confocal and electron microscopy at 6 days after addition of live encapsulated C. neoformans. Aggregates of host cells were seen to contain some intracellular degraded cryptococcal cells. In addition, however, a few abnormally large, extracellular fungal cells were seen, completely surrounded and in close association with host immune cells (Fig. 3A and B). Protrusions of the plasma membrane of host cells were seen in intimate contact with the cryptococcal capsule, and the internal organization of these fungal cells appeared abnormal (Fig. 3B). Fluorescent staining confirmed the presence of CD68-positive phagocytic cells and CD3-positive lymphocytes within the aggregates.
FIG. 3.
(A) Confocal microscopy image of an aggregate of host immune cells 6 days after live infection of PBMC prestimulated with B3501. In addition to intracellular C. neoformans, two enlarged extracellular cryptococcal cells are seen completely surrounded by mononuclear phagocytes and lymphoid host cells. (B) Transmission electron microscope image of a enlarged, extracellular cryptococcal cell surrounded by host cells, protrusions of which are seen to be in close association with the fungal capsule.
T-cell requirement for restriction of cryptococcal growth in vitro.
The requirement or lack of requirement for T cells for effective immune control of cryptococcal infection in the model was analyzed by depletion of specific cell populations using antibody-labeled magnetic microbeads prior to prestimulation. PBMC were depleted of CD3+ T cells and of either CD4+ T cells (Fig. 4A) or CD8+ T cells (Fig. 4B). In both sets of experiments, depletion of the CD3+ fraction was associated with a significant loss of control of cryptococcal growth at 6 days after addition of live organisms. Cryptococcal growth in wells selectively depleted of either CD4+ or CD8+ T cells was intermediate between that in wells with unseparated PBMC and that in wells with CD3+ cell-depleted PBMC, suggesting that both CD4+ and CD8+ cells may be contributing to the requirement for T cells for restriction of fungal growth in the model.
FIG. 4.
T-cell requirement for restriction of cryptococcal growth and effect of capsule during prestimulation on control of growth. (A and B) PBMC, unseparated, depleted of CD3+ T cells by magnetic bead separation, or depleted similarly of either CD4+ (A) or CD8+ (B) T cells, were prestimulated with heat-killed B3501 and then infected with live B3501, and cryptococcal growth was determined after 6 days, as described in Materials and Methods. Results are means ± SE from three independent experiments in each case. Growth of C. neoformans was significantly increased in wells with CD3+-depleted PBMC compared to unseparated PBMC (P = 0.006). (C) PBMC were prestimulated with either heat-killed encapsulated B3501 or heat-killed acapsular CAP67 mutant yeasts for 12 days, then infected with live encapsulated organisms, and cryptococcal growth was determined after 6 days. Results are means + SE from five independent experiments. Cryptococcal growth was significantly reduced in wells with PBMC prestimulated with acapsular compared to encapsulated C. neoformans (P < 0.02).
Effects of capsule on immune control and the cytokine response.
In the initial experiments, more effective and prolonged immune control was seen after prestimulation and infection with acapsular compared with encapsulated C. neoformans. This may reflect reduced resistance of acapsular strains to host effector mechanisms and/or, given the known deleterious effects of capsule on the host immune response (33), the development of a more effective immune response with prestimulation in the absence of capsule. Therefore, to determine the separate effect of the presence or absence of capsule in the prestimulation phase of the immune response in the model, PBMC from different donors were prestimulated with either heat-killed encapsulated or isogenic acapsular C. neoformans before infection with live encapsulated organisms. On day 6 following live infection, mean cryptoccocal growth was significantly reduced in wells with PBMC prestimulated with the acapsular mutant compared with wells prestimulated with encapsulated C. neoformans (39% ± 33% versus 167% ± 7%, respectively; P < 0.02) (Fig. 4C).
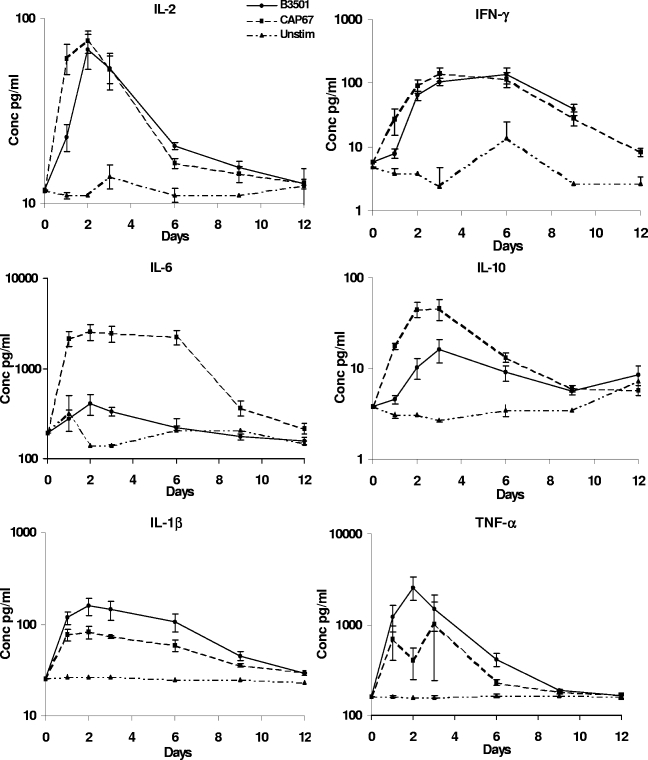
A possible mechanism whereby prestimulation of PBMC with acapsular organisms leads to a more effective immune response is through induction of a different cytokine profile. Therefore, the pattern of cytokine release in response to prestimulation with encapsulated and acapsular strains was investigated by measuring cytokine levels in cell culture supernatants. IL-12 levels were consistently low (<10 pg/ml). IL-1β, IL-2, TNF-α, and IFN-γ levels were not significantly different in wells prestimulated with acapsular or encapsulated C. neoformans. IL-10 levels were significantly higher in wells prestimulated with acapsular compared with encapsulated C. neoformans for the first 3 days of prestimulation (P ≤ 0.02 at days 1, 2, and 3). However, the striking difference in cytokine release concerned IL-6. Mean levels of IL-6 were 6- to 10-fold higher in wells prestimulated with acapsular compared with encapsulated C. neoformans, and this difference was maintained throughout the first week of prestimulation (P ≤ 0.01 at days 1, 2, 3, and 6) (Fig. 5).
FIG. 5.
Cytokine profiles of supernatants from PBMC prestimulated with either heat-killed encapsulated B3501 or heat-killed acapsular CAP67 mutant organisms. PBMC were prestimulated with either heat-killed encapsulated B3501 or heat-killed acapsular CAP67 mutant yeasts for 12 days before addition of live encapsulated organisms. Supernatant samples were taken on days 1, 2, 3, 6, 9, and 12 for cytokine analysis. Results are means ± SE from five independent experiments. Supernatants from PBMC prestimulated with the CAP67 mutant had 6- to 10-fold-higher mean IL-6 levels than those from cells prestimulated with B3501 (P ≤ 0.01 at days 1, 2, 3, and 6).
Effects of addition of recombinant IL-6 and depletion of CD25+ T cells on immune control of infection with encapsulated C. neoformans.
To further investigate the role of IL-6 in immune control in this model, and in view of data from other systems suggesting that IL-6 may allow antigen-specific T cells to overcome suppression by CD25+ regulatory T cells (25), the effect of adding recombinant IL-6, and of depleting PBMC of CD25+ T cells, on immune control of C. neoformans was examined. With PBMC prestimulated with encapsulated organisms, addition of IL-6 was associated with a reduction in cryptococcal growth that reached borderline statistical significance. Growth in wells with addition of IL-6 was 0% ± 39% versus 154% ± 72% with no added IL-6 (P = 0.06). In contrast, addition of IL-6 had no effect on cryptococcal growth following prestimulation with acapsular organisms (Fig. 6). Cryptococcal growth in wells with PBMC depleted of CD25+ cells and prestimulated with encapsulated organisms was 21% ± 48% compared to 189% ± 111% in wells with undepleted prestimulated PBMC (P = 0.08) (Fig. 7A). With control, unstimulated PBMC, which are normally rapidly destroyed by unrestricted cryptococcal growth following live infection, depletion of CD25+ cells resulted in PBMC that could significantly restrict cryptococcal growth (mean growth, 325% ± 101% with CD25+ cell-depleted PBMC compared with 3,970% ± 1,470% with undepleted PBMC; P = 0.04) (Fig. 7B).
FIG. 6.
Effect of addition of IL-6 on control of cryptococcal growth by PBMC prestimulated with encapsulated C. neoformans. PBMC were prestimulated with either heat-killed encapsulated B3501 or heat-killed acapsular CAP67 mutant organisms, with or without the addition of IL-6, for 12 days before addition of live encapsulated organisms. Cryptococcal growth was determined 6 days after live infection. Results are means ± SE from five independent experiments. Growth in wells with PBMC prestimulated with encapsulated B3501 with addition of IL-6 was 0% ± 39% versus 154% ± 72% with no added IL-6 (P = 0.06).
FIG. 7.
Effect of depletion of CD25+ T lymphocytes on control of cryptococal growth. Resting PBMC were depleted of CD25+ T cells by magnetic separation. Unseparated and depleted PBMC were either prestimulated with heat-killed encapsulated B3501 (A) or left unstimulated (B) for 12 days before addition of live encapsulated organisms. Cryptococcal growth was determined 6 days after live infection. Results are means ± SE from three independent experiments. Cryptococcal growth in wells with prestimulated PBMC depleted of CD25+ T cells was 21% ± 48% compared with 189% ± 111% in wells with undepleted prestimulated PBMC (P = 0.08) (A). Growth in wells with unstimulated PBMC depleted of CD25+ T cells was 325% ± 101% compared with 3,970% ± 1,470% with undepleted PBMC (P = 0.04) (B).
DISCUSSION
The exact circumstances and frequency of human exposure to C. neoformans are not clearly understood. However, serological evidence suggests that most individuals are exposed, perhaps during early childhood (12). Thus, the model presented here likely reflects the development and effector phases of a secondary immune response to C. neoformans. Differences in the degree of immune control of in vitro cryptococcal infection by PBMC from different individuals may reflect differences in the time since and/or the intensity of exposure. Further work will analyze the immunological basis of this difference. However, in preliminary experiments, the degree of immune control did not correlate with the degree of lymphoproliferation in response to C. neoformans (unpublished observations), suggesting that more subtle, qualitative differences in immune response underlie these differences between individuals.
The model was designed to reflect some of the complexities of the in vivo human immune response to C. neoformans. On a number of issues, the results are consistent with prior data from different systems. Thus, it appears that inhibition of cryptococcal growth may occur extracellularly as well as intracellularly, as suggested by prior in vitro studies (11, 13). Consistent with epidemiological and animal model data, T cells are essential for effective immune control in the model, and both CD4+ and CD8+ T cells appear to contribute to this immune control, consistent with the findings of studies using CD4+ and CD8+ T-cell-depleted or -deficient mice (16, 36) or human CD8+ T cells (23).
In addition, the results suggest new insights into human immune control of C. neoformans infection. In particular, high levels of IL-6 were associated with the greater restriction of cryptococcal growth seen after prestimulation with acapsular compared with encapsulated organisms. Thus, suppression of IL-6 production may be an additional important mechanism whereby the cryptococcal capsule, the major virulence factor of C. neoformans (26, 33), subverts a protective immune response.
In the setting of the immunosuppression of late-stage HIV-infection, we previously found that higher levels of the trio of proinflammatory cytokines IL-6, TNF-α, and IFN-γ at the site of infection in the cerebrospinal fluid (CSF) were associated with survival (30). Levels of these three cytokines were highly correlated. Nevertheless, in multivariate modeling, it appeared that IFN-γ was most important in determining the rate of clearance of infection, as assessed by serial quantitative CSF cultures (30). Absolute levels of IL-6 were high and those of IFN-γ were low, consistent with evidence that IL-6 production is well preserved (5), but IFN-γ secretion severely reduced, in late-stage HIV infection (37). In this circumstance, IFN-γ levels may be critical in restricting cryptococcal growth. The situation may be different in the setting of apparent immunocompetence. Results from this in vitro model using cells from immunocompetent donors suggest that IL-6 may play an important role, independently of IFN-γ, in the control of cryptococcal growth in immunocompetent individuals.
Few studies have focused on the role of IL-6 in host defense against C. neoformans. Nevertheless, IL-6 has previously been shown to be produced in response to C. neoformans by whole blood and monocyte cultures. In agreement with our findings, higher levels of IL-6 were secreted after stimulation with acapsular compared with encapsulated C. neoformans, but the effect on the anticryptococcal activity of the cells was not examined (10). Although the capsule polysaccharides glucoronoxylomannan (GXM) and galactoxylomannan (GalXM) both elicited IL-6 production, the highest levels of IL-6 were seen after stimulation with cryptococcal mannoprotein. Since mannoprotein may be less exposed on the surfaces of encapsulated organisms, the effect of capsule may be to mask interaction of cell wall-associated mannoproteins with mononuclear phagocyte and dendritic cell mannose receptors. Macrophages and dendritic cells have been shown to bind and phagocytose acapsular, but not encapsulated, C. neoformans via macrophage mannose receptors (9, 19, 21, 31), engagement of which, at least in some settings, has been linked to IL-6 production (35). Acapsular organisms and cryptococcal mannoprotein have been shown to induce dendritic cell maturation and activation (27, 34), and mannoprotein has been shown to interact with multiple mannose receptors (24).
A protective role for IL-6 in the immune response to C. neoformans is supported by the finding that IL-6 knockout mice have reduced survival following intravenous infection with C. neoformans compared with the parental strain (2). In an intracerebral model of infection, local (central nervous system [CNS]) expression of, as well as local exogenous administration of, IL-6 or IL-1β (but not TNF-α) was associated with enhanced survival (4). A CNS-specific and TNF-α-dependent role for IL-6 and IL-1β in protection against cryptococcosis is suggested by findings with TNF/lymphotoxin-α-deficient mice. The markedly reduced survival of TNF/lymphotoxin-α-deficient mice was associated with a marked reduction in brain levels of IL-6 and IL-1β, while levels of these cytokines in plasma and other organs were similar in knockout and parental-strain mice, and plasma and tissue levels of IFN-γ and IL-12 were higher in knockout than in parental-strain mice (28).
The mechanism by which IL-6 is associated with enhanced restriction of cryptococcal growth in the model remains to be elucidated. However, in the setting of a primary immune response, IL-6 has been shown to render responding T cells refractory to the effect of CD4+ CD25+ regulatory T cells (25), involved in controlling the activation of pathogen-specific as well as autoreactive T cells (3). Immune control of cryptococcal growth was enhanced in the absence of the CD25+ population, suggesting that regulatory T cells also play an inhibitory role in this secondary immune response to C. neoformans and that IL-6 could act by abrogating this inhibitory effect. To investigate this further, studies are planned to determine the relative contributions of different T and B lymphocyte (32) and mononuclear phagocyte populations to the aggregates and the production of IL-6, the effect of CD25+ T-cell depletion on cytokine release, and the effect of addition and neutralization of IL-6 in the presence and absence of CD25+ T cells.
In conclusion, a long-term in vitro model of human immune control of cryptococcal infection has demonstrated a protective role for IL-6 and, through suppression of IL-6 release, an additional mechanism whereby the cryptococcal capsule, the major virulence factor of C. neoformans, subverts a protective immune response. Further in vitro and in vivo work is required to clarify the mechanism of action of IL-6 in this setting and any interaction with regulatory T cells.
Acknowledgments
This work was supported by an Advanced Training Fellowship to T.S.H. from The Wellcome Trust.
We thank Diane Irving for help with the Luminex analyses.
Editor: A. Casadevall
REFERENCES
- 1.Baecher-Allan, C., V. Viglietta, and D. A. Hafler. 2004. Human CD4+ CD25+ regulatory T cells. Semin. Immunol. 16:89-97. [DOI] [PubMed] [Google Scholar]
- 2.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 69:6445-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 4.Blasi, E., R. Barluzzi, R. Mazzolla, L. Pitzurra, M. Puliti, S. Saleppico, and F. Bistoni. 1995. Biomolecular events involved in anticryptococcal resistance in the brain. Infect. Immun. 63:1218-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breen, E. C., A. R. Rezai, K. Nakajima, G. N. Beall, R. T. Mitsuyasu, T. Hirano, T. Kishimoto, and O. Martinez-Maza. 1990. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 144:480-484. [PubMed] [Google Scholar]
- 6.Brouwer, A. E., A. Rajanuwong, W. Chierakul, G. E. Griffin, R. A. Larsen, N. J. White, and T. S. Harrison. 2004. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363:1764-1767. [DOI] [PubMed] [Google Scholar]
- 7.Chariyalertsak, S., T. Sirisanthana, O. Saengwonloey, and K. Nelson. 2001. Clinical presentation and risk behaviors of patients with acquired immunodeficiency syndrome in Thailand, 1994-1998: regional variation and temporal trends. Clin. Infect. Dis. 32:955-962. [DOI] [PubMed] [Google Scholar]
- 8.Corbett, E. L., G. Churchyard, S. Charalambos, B. Samb, V. Moloi, T. C. Clayton, A. D. Grant, J. Murray, R. J. Hayes, and K. M. De Cock. 2002. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin. Infect. Dis. 34:1251-1258. [DOI] [PubMed] [Google Scholar]
- 9.Cross, C. E., and G. J. Bancroft. 1995. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and β-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect. Immun. 63:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delfino, D., L. Cianci, E. Lupis, A. Celeste, M. L. Petrelli, F. Curro, V. Cusumano, and G. Teti. 1997. Interleukin-6 production by human monocytes stimulated with Cryptococcus neoformans components. Infect. Immun. 65:2454-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flesch, I. E., G. Schwamberger, and S. H. Kaufman. 1989. Fungicidal activity of IFN-γ-activated macrophages. Extracellular killing of Cryptococcus neoformans. J. Immunol. 142:3219-3224. [PubMed] [Google Scholar]
- 12.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L.-A. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66. [DOI] [PubMed] [Google Scholar]
- 13.Granger, D. L., J. R. Perfect, and D. T. Durack. 1986. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J. Immunol. 136:672-680. [PubMed] [Google Scholar]
- 14.Hakim, J. G., I. T. Gangaidzo, R. S. Heyderman, J. Mielke, E. Mushangi, A. Taziwa, V. J. Robertson, P. Musvaire, and P. R. Mason. 2000. Impact of HIV infection on meningitis in Harare: a prospective study of 406 predominantly adult patients. AIDS 14:1401-1407. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, T. S., G. E. Griffin, and S. M. Levitz. 2000. Conditional lethality of the diprotic weak bases chloroquine and quinacrine against Cryptococcus neoformans. J. Infect. Dis. 182:283-289. [DOI] [PubMed] [Google Scholar]
- 16.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T-cells in a protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35-42. [DOI] [PubMed] [Google Scholar]
- 17.Imwidthaya, P., and N. Poungvarin. 2000. Cryptococcosis in AIDS. Postgrad. Med. J. 76:85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami, K. 2005. Innate immunity in the lungs to cryptococcal infection, p. 135-155. In P. L. Fidel and G. B. Huffnagle (ed.), Fungal immunology: from an organ perspective. Springer, New York, N.Y.
- 19.Kelly, R. M., J. Chen, L. E. Yauch, and S. M. Levitz. 2005. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect. Immun. 73:592-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitz, S. M., and R. T. Farrell. 1991. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J. Infect. Dis. 163:1108-1113. [DOI] [PubMed] [Google Scholar]
- 21.Levitz, S. M., and A. Tabuni. 1991. Binding of Cryptococcus neoformans by human cultured macrophages: requirements for multiple complement receptors and actin. J. Clin. Investig. 87:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindell, D., and G. B. Huffnagle. 2005. Pulmonary cell-mediated immunity (CMI) to Cryptococcus neoformans, p. 157-180. In P. L. Fidel and G. B. Huffnagle (ed.), Fungal immunology: from an organ perspective. Springer, New York, N.Y.
- 23.Ma, L. L., J. C. L. Spurrell, J. F. Wang, G. G. Neely, S. Epelman, A. M. Krensky, and C. H. Mody. 2002. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J. Immunol. 169:5787-5795. [DOI] [PubMed] [Google Scholar]
- 24.Mansour, M. K., E. Latz, and S. M. Levitz. 2006. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J. Immunol. 176:3053-3061. [DOI] [PubMed] [Google Scholar]
- 25.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033-1036. [DOI] [PubMed] [Google Scholar]
- 26.Perfect, J. R. 2005. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol. Med. Microbiol. 45:395-404. [DOI] [PubMed] [Google Scholar]
- 27.Pietrella, D., C. Corbucci, S. Perito, G. Bistoni, and A. Vecchiarelli. 2005. Mannoproteoins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect. Immun. 73:820-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayhane, N., O. Lortholary, C. Fitting, J. Callebert, M. Huerre, F. Dromer, and J.-M. Cavaillon. 1999. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-α-deficient mice to C. neoformans infection despite increased levels of nitrite/nitrate, interferon-γ, and interleukin-12. J. Infect. Dis. 180:1637-1647. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, P. A., M. Bauer, M. E. Leal, S. G. Evans, P. D. Holtom, D. M. Diamond, J. M. Leedom, and R. A. Larsen. 1999. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 28:82-92. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui, A., A. E. Brouwer, V. Wuthiekanun, S. Jaffar, R. Shattock, D. Irving, J. Sheldon, W. Chierakul, S. Peacock, N. Day, N. J. White, and T. S. Harrison. 2005. Interferon-γ at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J. Immunol. 174:1746-1750. [DOI] [PubMed] [Google Scholar]
- 31.Syme, R. M., J. C. L. Spurrell, E. K. Amankwah, F. H. Y. Green, and C. H. Mody. 2002. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcγ receptor II for presentation to T lymphocytes. Infect. Immun. 70:5972-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai, M. C., S. Chakravarty, G. Zhu, J. Xu, K. Tanaka, C. Koch, J. Tufariello, J. Flynn, and J. Chan. 2006. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol. 8:218-232. [DOI] [PubMed] [Google Scholar]
- 33.Vecchiarelli, A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38:407-417. [DOI] [PubMed] [Google Scholar]
- 34.Vecchiarelli, A., D. Pietrella, P. Lupo, F. Bistoni, D. C. McFadden, and A. Casadevall. 2003. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J. Leukoc. Biol. 74:370-378. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan, R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, M., J. Gong, D. V. Iyer, B. E. Jones, R. L. Modlin, and P. F. Barnes. 1994. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J. Clin. Investig. 94:2435-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]