Abstract
Inhalational pneumonic tularemia, caused by Francisella tularensis, is lethal in humans. F. tularensis is phagocytosed by macrophages followed by escape from phagosomes into the cytoplasm. Little is known of the phagocytic mechanisms for Francisella, particularly as they relate to the lung and alveolar macrophages. Here we examined receptors on primary human monocytes and macrophages which mediate the phagocytosis and intracellular survival of F. novicida. F. novicida association with monocyte-derived macrophages (MDM) was greater than with monocytes. Bacteria were readily ingested, as shown by electron microscopy. Bacterial association was significantly increased in fresh serum and only partially decreased in heat-inactivated serum. A role for both complement receptor 3 (CR3) and Fcγ receptors in uptake was supported by studies using a CR3-expressing cell line and by down-modulation of Fcγ receptors on MDM, respectively. Consistent with Fcγ receptor involvement, antibody in nonimmune human serum was detected on the surface of Francisella. In the absence of serum opsonins, competitive inhibition of mannose receptor (MR) activity on MDM with mannan decreased the association of F. novicida and opsonization of F. novicida with lung collectin surfactant protein A (SP-A) increased bacterial association and intracellular survival. This study demonstrates that human macrophages phagocytose more Francisella than monocytes with contributions from CR3, Fcγ receptors, the MR, and SP-A present in lung alveoli.
Francisella tularensis, a highly infectious gram-negative coccobacillus and potential infectious agent of bioterrorism, is most devastating to humans in its pneumonic form (16), where it is phagocytosed by alveolar macrophages (AM). Whereas ingested microbes are normally degraded within macrophage phagolysosomes, it was recently shown that F. tularensis escapes from its phagosome into the cytoplasm within 4 hours of phagocytosis by human macrophages (11, 24). The avoidance of phagosome-lysosome fusion and escape into the host cytoplasm are potential virulence mechanisms, as Francisella mutants which are defective in escape demonstrate reduced intramacrophage growth (33, 40). Little is understood of either the mechanisms of uptake of Francisella into macrophages or its subsequent evasion of macrophage-directed killing. The severity and rapidity of the disease caused by Francisella, especially in the lungs, are likely the result of an exploitation and/or failure of one or more components of innate immunity, leading ultimately to the inability of macrophages to control infection. Failure of the innate immune response to control F. tularensis infection of human macrophages is corroborated by the short incubation time of 1 to 2 days in human volunteers for developing symptomatic disease following aerosol challenge of F. tularensis (3, 41) and in nonhuman primates challenged with the virulent Schu 4 strain (54).
Macrophage receptors mediate phagocytosis and initiate signaling cascades, intracellular trafficking, inflammatory responses, and antigen presentation (51). While most receptor-ligand interactions between macrophages and microbial pathogens lead to the destruction of the pathogen, certain receptor-ligand interactions allow for permissive environments in which the pathogen can thrive and even proliferate. For example, Mycobacterium tuberculosis is initially taken up by AM in the lung via complement receptors (CR) and specific pattern recognition receptors (PRR) such as the mannose receptor (MR) and scavenger receptors (19). Uptake via the MR appears to be permissive for M. tuberculosis growth; more bacteria were found in fused phagolysosomes when the MR was competitively inhibited (30).
The macrophage receptors which are important for the uptake and survival of Francisella have been poorly characterized. Recent studies by Clemens et al. have indicated a role for complement and CR in an unusual form of phagocytosis involving spacious asymmetric pseudopod loops (11, 12). Within the lung alveolus, bacterium-macrophage interactions are also regulated by the pulmonary collectins surfactant proteins A and D (SP-A and SP-D) (20). These proteins along with the MR are calcium-dependent lectins that bind to an array of microbial surface carbohydrates (19, 49) and have several immunomodulatory effects on macrophages (14, 15, 22, 55). SP-A has been shown to increase the MR phagocytic pathway in human macrophages (5, 23).
There are three main subspecies of Francisella tularensis: F. tularensis subsp. tularensis (type A), the highly virulent form found in North America; F. tularensis subsp. holarctica (type B), a less-virulent form found both in North America and Europe; and F. tularensis subsp. mediasiatica (17). Francisella novicida and the live vaccine strain (LVS) of F. tularensis (type B strain) are the two most commonly used model strains for study. F. novicida, which causes lethal disease in mice, shares near genetic identity with F. tularensis subsp. tularensis by 16S rRNA gene sequencing (17, 21). F. novicida causes occasional cases of human disease and has been speculated to cause infection more frequently than previously thought (10, 26, 53). In this report we evaluated the role of the major classes of human macrophage phagocytic receptors in the interaction with F. tularensis. We provide evidence for involvement of complement, antibody, SP-A, and the macrophage MR in these interactions. We also directly compared the macrophage association of F. novicida with the LVS.
MATERIALS AND METHODS
Isolation of monocyte-derived macrophages.
Institutional Review Board approval was obtained for isolating cells from human blood via venipuncture, and informed consent was obtained from individual blood donors. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood in 0.9% saline by centrifugation over Ficoll-Paque (Amersham Biosciences) as previously described (44). PBMC were then washed twice in RPMI 1640 with l-glutamine (Gibco) and cultured in Teflon wells at a concentration of 2.0 × 106 cells/ml in RPMI 1640 plus l-glutamine with 20% autologous human serum at 37°C in 5% CO2 for 5 to 6 days to produce monocyte-derived macrophages (MDM). In the case of monocytes, incubation in Teflon wells was for 24 h. On the day of harvest, PBMC were collected from Teflon wells, washed, counted, and placed in monolayer culture in 24-well tissue culture plates for 2 h at 37°C in 5% CO2. Nonadherent lymphocytes were washed away, leaving adherent monocyte or MDM monolayers at a density of approximately 1.5 × 105 to 2.0 × 105 cells/well.
Human serum preparation.
Autologous sera from healthy adult volunteers with no known exposure to Francisella or Francisella-infected patients were used in all experiments except where noted. Sera were obtained from donors by venipuncture and processed to maintain complement activity (28). Briefly, whole blood was kept at room temperature for 1 h to allow for clot formation and then at 4°C to allow for clot retraction and then centrifuged at 500 × g for 15 min. The serum fraction was collected, filter sterilized, aliquoted, and stored at −80°C. Serum was heat inactivated (HI) on the day of experimentation at 56°C for 30 min.
Preparation of Francisella.
Strains of F. novicida U112 and the F. tularensis subsp. holarctica LVS ATCC 29684 were used in this study. A destabilized form of green fluorescent protein (GFP) plasmid was constructed in pKK214 as described previously (1). The GFP plasmid was introduced into the LVS and F. novicida by the rubidium chloride cryotransformation technique. Briefly, 109 bacteria were suspended in chilled cryotransformation buffer (10 mM HEPES, 100 mM CaCl2, 10 mM RbCl2, 15% glycerol; pH adjusted to 6.5 with diluted NaOH), 100 ng of plasmid was added to the cells, and cells were left on ice for 30 min. Cells were flash frozen in liquid nitrogen for 5 min, warmed to room temperature, and plated on chocolate agar with tetracycline (10 μg/ml) at 37°C. Bacteria were plated on chocolate agar for 1 to 2 days and harvested in RPMI 1640 with l-glutamine. The multiplicity of infection (MOI) for each experiment was approximated by measuring the optical density at 600 nm and confirmed by plating the inocula and counting CFU. Macrophage infections were performed at the MOIs listed in the figure legends. Other strains of Francisella were cultivated as described above on chocolate agar.
Microscopy assays of Francisella association with monocytes and macrophages.
Monocyte and MDM monolayers were formed on Chromerge-cleaned glass coverslips in 10% autologous serum in RPMI 1640 at 37°C with 5% CO2 for 2 to 3 h, as previously described (46). The cells were then washed extensively with warm RPMI 1640 and incubated with RHH (RPMI 1640 with l-glutamine, 10 mM HEPES, and 0.25% human serum albumin [HSA]) or RH (RPMI 1640, l-glutamine, and 10 mM HEPES) medium and 2.5% autologous serum. Fifty microliters of appropriately diluted bacterial stock was then added to each well. MDM were incubated on a rotating platform for 30 min and then under stationary conditions for an additional 90 min, both at 37°C in 5% CO2. After incubation, the cells were washed extensively with warm media to remove nonadherent bacteria and fixed in 2% paraformaldehyde and coverslips were allowed to dry.
Three complementary microscopy assays were used to assess phagocyte-associated bacteria. First, in experiments using GFP-expressing Francisella, coverslips were mounted on glass slides and the phagocyte-associated bacteria were counted and enumerated using fluorescence microscopy. Second, in experiments with nonfluorescent bacteria, MDM on coverslips were permeabilized after paraformaldehyde fixation with 100% methanol for 5 min, washed, and stained with 300 μl of 300 nM DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes) for 20 min. Phagocyte-associated bacteria were enumerated by fluorescence microscopy. Third, phagocyte-associated bacteria were visualized by indirect immunofluorescence microscopy. In this assay, monocytes and MDM on coverslips were permeabilized, washed, and then incubated with a monoclonal mouse anti-F. novicida lipopolysaccharide primary antibody (Immuno-Precise Antibodies Limited, Victoria, B.C., Canada) (diluted 1:100 in blocking buffer composed of 5% HI human AB serum [Cambrex] and 1% bovine serum albumin [Sigma] in buffer) or a monoclonal mouse anti-F. tularensis lipopolysaccharide primary antibody (Abcam, Cambridge, MA) (diluted 1:1,000 in blocking buffer) for 4 h at room temperature with gentle rotation. After being washed extensively, MDM were incubated with Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin G (IgG; Molecular Probes) (diluted 1:1,000 in blocking buffer) for 90 min at room temperature. Coverslips were mounted on glass slides. In all assays, the average number of bacteria per monocyte or MDM on each coverslip was determined by counting a minimum of 200 cells per coverslip using a 100× oil immersion objective with a wide-bandwidth 570-nm dichroic mirror on a BX51 Olympus fluorescence microscope and pictures were taken with an Color 3 digital camera (Olympus, Melville, NY). Triplicate coverslips were used for each test group.
In certain experiments soluble mannan (TCI, Tokyo, Japan) was used to block the MR on MDM as previously described (42). Mannan (2.5 mg/ml) was incubated with MDM for 30 min at 37°C prior to the addition of bacteria.
Macrophage FcγR down-modulation.
To assess the role of Fcγ receptors (FcγR) on MDM in the cell association of Francisella, FcγR were down-modulated as described previously (35, 44). Briefly, Chromerge-cleaned glass coverslips in 24-well tissue culture plates were incubated with 300 μl of 0.1-mg/ml poly-l-lysine (Sigma) for 60 min, washed, and treated with 300 μl of 2.5% glutaraldehyde for 60 min. After further washing, the coverslips were transferred to wells of another tissue culture plate and incubated with 300 μl of 1-mg/ml HSA (ZLB Bioplasma AG) for 30 min. Coverslips were then washed and incubated with 500 μl of 0.2 M glycine to quench the glutaraldehyde groups and incubated overnight at room temperature. Next, 300 μl of 1-mg/ml rabbit IgG anti-HSA (Sigma) was added to certain coverslips to form immune complexes, whereas control coverslips were incubated in medium only. After a 30-min incubation and wash, MDM were adhered in monolayer culture onto the treated coverslips as described above for 2 h. For MDM infections, bacteria were opsonized in 10% autologous fresh or HI serum or media alone for 30 min at 37°C with gentle rocking every 5 to 10 min, washed twice, and then added to the MDM. Bacterial concentrations of the inocula used in tissue culture experiments were verified by CFU.
In order to test the efficacy of FcγR down-modulation, IgG-coated sheep red blood cells (E-IgG; Advanced Research Technologies) were incubated on treated coverslips (with or without anti-HSA treatment) for 60 min at 37°C in 5% CO2, after which the coverslips were fixed in 2.5% glutaraldehyde. Ingested E-IgG were counted and enumerated after hypotonic lysis of extracellular E-IgG (44).
Determination of antibody in donor serum to Francisella.
To ascertain the presence of antibody in human serum for F. novicida, 1 × 108 bacteria were incubated for 30 min at 37°C on an Adams Nutator (Becton Dickinson, Franklin Lakes, NJ) in fresh and HI serum from multiple donors at different concentrations (0.625%, 1.25%, 2.5%, 10%, and 50%) or in HSA at concentrations approximating those in the serum (31.25 mg/dl, 62.5 mg/dl, 125 mg/dl, 500 mg/dl, and 2.5 g/dl). After vigorous washing, bacteria were dried overnight onto medium-binding polystyrene Costar enzyme-linked immunosorbent assay (ELISA) plates (Corning, Pittsburgh, PA), blocked for 12 h with 3% ovalbumin (Sigma, St. Louis, MO) at 4°C on a Nutator, and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-human polyvalent immunoglobulin (IgG, IgA, or IgM; 1:3,500 dilution; Sigma, St. Louis, MO) in 0.3% ovalbumin for 3 h at 24°C (47). Substrate was added for 10 min at room temperature (ABTS HRP substrate kit; Bio-Rad, Hercules, CA), after which the reaction was stopped with 2% oxalic acid in sterile water. Absorbance at 415 nm was then measured on a 96-well plate reader (Molecular Devices, Sunnyvale, CA). For detection of antibody to F. novicida using fluorescence microscopy, nonimmune serum from one donor was incubated with bacteria for 30 min at 37°C as described above, blocked in 3% ovalbumin for 1 h at 24°C, and then washed and incubated for 2 h at 24°C with fluorescein isothiocyanate (FITC)-conjugated goat anti-human polyvalent immunoglobulin (IgG, IgA, or IgM; 1:128 dilution; Sigma, St. Louis, MO) in 0.3% ovalbumin in triplicate Eppendorf tubes. After a final wash, 10 μl of each sample was placed on glass slides, and the presence or absence of fluorescent bacteria was assessed qualitatively by immunofluorescence microscopy.
Determination of the effect of SP-A on Francisella association with MDM.
SP-A was purified from the bronchoalveolar lavage fluid from patients with pulmonary alveolar proteinosis according to a previously described protocol using mannose-Sepharose affinity chromatography (15). Purified protein was verified using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. The endotoxin concentration was <0.25 pg/μg protein. Fifty microliters of bacteria at a concentration of ∼1.8 × 109 bacteria per ml was incubated with 5 μg SP-A or buffer for 30 min at 37°C and then washed twice in media by centrifugation (10,000 × g for 10 min each wash). The bacteria were resuspended in RHH for a final concentration of ∼1.8 × 109 bacteria per ml, and 50 μl of the bacterial suspension was added to MDM at an MOI of ∼600:1. To examine the direct effect of SP-A on macrophages, MDM were incubated with 5 μg SP-A for 30 min prior to the addition of bacteria at 37°C in 5% CO2 and washed or added simultaneously with bacteria.
Flow cytometry assay for cell association of Francisella with macrophages.
MDM monolayers (6 × 105) in six-well plates were incubated with GFP-expressing F. novicida for 2 h as in the microscopy assays. After being washed extensively with warm RPMI 1640, the cells were placed on ice for 30 min, gently lifted using a rubber policeman, placed in a conical tube, and washed with cold RPMI 1640. Cells were adjusted to 1 × 107 cells/ml and fixed with 2% paraformaldehyde without permeabilization. Samples were read on a Becton Dickinson (San Jose, CA.) LSRII flow cytometer, and data were analyzed using FACSDiva software (Becton Dickinson). Uninfected macrophages were used in each experiment to set gates for analysis. Macrophages were gated according to their forward and side scatter profiles, and GFP-expressing F. novicida-infected MDM were identified by fluorescence in the FL1 channel.
CFU assay to assess Francisella growth in MDM.
After a 2-h incubation of Francisella with MDM monolayers, MDM were washed vigorously and incubated with 50 μg/ml of gentamicin for 40 min at 37°C in 5% CO2 to kill extracellular and attached bacteria, washed again, and lysed with 0.1% Triton X-100 for 2 to 5 min immediately prior to plating for CFU. In control experiments, F. novicida and the LVS showed no significant differences in viability when 1 × 106 bacteria were directly incubated with 0.1% Triton X-100 for 5 to 10 min immediately prior to plating for CFU.
Assay for Francisella association with Chinese hamster ovary CR3 cells.
Chinese hamster ovary cells expressing CR3 (CHO-CR3) were kindly provided by Douglas Golenbock at the University of Massachusetts Medical Center. CHO-CR3 cells were grown to confluence in tissue culture plates using Geneticin (Gibco)-supplemented selection media containing 10% HI fetal calf serum and 10 mM HEPES. Tissue culture plates were placed on ice, and cells were harvested by gentle pipetting 24 h prior to experimentation. After a washing, the cells were adhered onto glass coverslips in 24-well tissue culture plates at 2.0 × 105 per well and incubated at 37°C in 5% CO2 overnight. The next day the cells were ensured to be 80 to 100% confluent in tissue culture plates and then washed extensively prior to use. Bacteria were preopsonized in single-donor fresh serum or HI serum before addition to CHO-CR3 cells or wild-type control CHO cells for 2 h. Cells were then extensively washed free of nonadherent bacteria, and cell-associated bacteria were immunostained as described above. Coverslips were dried and mounted on slides. Five hundred consecutive cells per coverslip on triplicate coverslips were counted and enumerated using immunofluorescence microscopy.
EM.
In order to determine whether bacteria associated with MDM were internalized, MDM were plated on plastic coverslips, infected with F. novicida at an MOI of 500:1, and then washed, fixed, and prepared for electron microscopy (EM), as previously described (27).
Statistics.
Independent experiments were performed on separate occasions in triplicate or quadruplicate with a minimum of two different donors unless otherwise noted. Controls were included in each experiment. Paired two-tailed Student t tests were used to determine associations of significance between groups unless otherwise specified. Differences between groups were considered statistically significant for P values <0.05. Means and standard errors of means (SEM) are reported in Results.
RESULTS
F. tularensis association with monocytes and MDM.
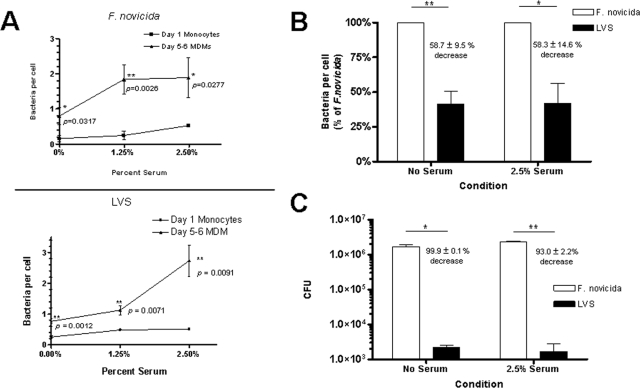
Monocytes and MDM are in part distinguished by the array of phagocytic receptors expressed on their respective surfaces (43). We assessed the cell association of F. novicida and the LVS with monocytes and MDM in the presence or absence of serum in order to provide insight into which phagocytic receptors are used for the recognition of Francisella. Monocytes and MDM were incubated with GFP-expressing F. novicida and the LVS at an MOI of 10:1 for 2 h, and bacterial association was measured using fluorescence microscopy. MDM exhibited a 5.36-fold ± 2.54-fold increase in bacterial association with F. novicida compared with monocytes (n = 3; Fig. 1A, top panel). The mean number of bacteria per monocyte at this MOI was consistently found to be much lower for the LVS (below 0.05 bacteria per monocyte). At an MOI of 60:1, MDM exhibited a 3.95-fold ± 1.41-fold increase in bacterial association with the LVS compared to monocytes (n = 2; Fig. 1A, bottom panel). These results provide evidence that (i) cell differentiation is important for F. novicida and the LVS cell association, indicating that F. tularensis preferentially enters macrophages via receptors which are either not expressed or expressed in lower abundance on monocytes; (ii) there was a serum-dependent increase in association for both F. novicida and the LVS with MDM; and (iii) the LVS association with human monocytes is much lower than for F. novicida. Further comparisons of F. novicida and the LVS association with MDM were made. Even at an MOI of 600:1, the association of the LVS with MDM was consistently less than F. novicida both in the presence and absence of serum (Fig. 1B). In order to determine the effect of the observed serum-dependent increase in F. tularensis association on its survival in MDM, CFU from cell lysates were obtained after a 2-h incubation with F. novicida and the LVS. The increase in association after serum opsonization correlated with an increase in survival for F. novicida: serum-opsonized F. novicida demonstrated a 1.45-fold ± 0.10-fold increase in viability at 2 h compared to the nonopsonic condition (n = 8, P = 0.004). Compared to F. novicida, the LVS exhibited 99.9% ± 0.1% and 93.0% ± 2.2% decreases in viability in the absence and presence of serum, respectively (Fig. 1C). This difference in viability was greater than that predicted by the difference in uptake between strains, suggesting that, in addition to potential differences in phagocytic pathways for F. novicida and the LVS, F. novicida possesses an additional virulence factor(s) that enhances intracellular viability. Given the increase in cell association and marked increase in viability, we chose to perform subsequent experiments with F. novicida as a potentially more useful model for studying human macrophage interactions.
FIG. 1.
Cell differentiation-dependent association of F. novicida and the LVS with monocytes and MDM. (A) Monocytes or MDM were harvested from human PBMC at either day 1 or day 5 to 6, respectively, adhered to glass coverslips, and incubated for 2 h with F. novicida at an MOI of 10:1 or the LVS at an MOI of 60:1 in the absence or presence of increasing concentrations of autologous serum. Cell association was quantified by counting F. novicida cells associated with 150 to 200 consecutive cells. Data are the means ± standard deviations of bacteria per cell (triplicate wells) in representative experiments for F. novicida (n = 3) and the LVS (n = 2). Paired one-tail Student t tests were used to generate P values. (B) MDM monolayers on glass coverslips were incubated with either GFP-expressing F. novicida or LVS for 2 h, and bacteria per macrophage were enumerated using fluorescence microscopy. Results were normalized to the mean number of F. novicida cells per macrophage in both the “no-serum” and “serum” groups. The results shown are the means ± SEM from six independent experiments, three at an MOI of 10:1 and three at an MOI of 600:1. *, P = 0.0163; **, P = 0.0016 (paired two-tailed Student t test). (C) MDM monolayers were incubated with F. novicida or the LVS for 2 h and then incubated with gentamicin and lysed in 0.1% Triton X-100, and MDM lysates were plated on chocolate agar for CFU. Results shown are the means ± SEM from two independent experiments. *, P = 0.0194; **, P = 0.0023 (unpaired Student t test).
The role for complement and CR3 in the recognition of F. novicida by macrophages.
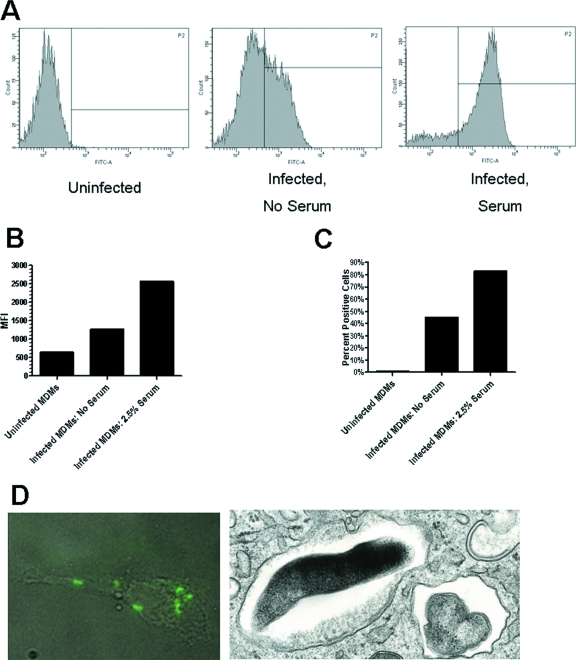
Recent studies provide evidence for the role of complement C3 and CR in the uptake of a clinical isolate of F. tularensis and the LVS by macrophages (11, 12). To further evaluate the role of serum components in the uptake of F. tularensis by monocytes and macrophages, cell monolayers were incubated with GFP-expressing F. novicida in the presence or absence of different concentrations of fresh autologous serum for 2 h and bacterial association was assessed by fluorescence microscopy. Both monocytes and MDM showed serum-dependent increases in bacterial association that were evident at low concentrations of serum (Fig. 1A). In the case of MDM, there was a 2.07-fold ± 0.26-fold increase in bacterial association in the presence of 2.5% serum (n = 14, P = 0.001), providing evidence for the importance of serum opsonins in enhancing the uptake of F. novicida. These findings were confirmed using indirect immunofluorescence and flow cytometry assays (Fig. 2A to D). Transmission EM was used to determine whether cell-associated bacteria were internalized (Fig. 2D, right panel). By 2 h all visualized bacteria were intracellular in membrane-bound vacuoles.
FIG. 2.
Serum-dependent cell association of F. novicida with MDM. (A to C) GFP-expressing F. novicida-infected MDM were analyzed by flow cytometry. Uninfected MDM were used to set the gates. Shown is an analysis of histograms (A) and bar graphs showing the mean fluorescence intensities (MFI) (B) and percentages of positive cells infected (C) of uninfected MDM and GFP-expressing F. novicida-infected MDM in the absence and presence of 2.5% serum. Data shown are representative of two experiments. (D, left panel) Following incubation of MDM for 2 h, nonadherent F. novicida cells were washed away, and MDM on coverslips were permeabilized, washed, and then incubated with mouse anti-F. novicida antibody followed by Alexa Fluor 488-conjugated goat anti-mouse IgG. Bacterial association was visualized by indirect immunofluorescence microscopy. Shown is a representative image of an overlay of light- and fluorescence-microscopic images of an MDM with associated fluorescent F. novicida (magnification, ×1,000). (D, right panel) After a 2-h incubation, MDM were fixed and prepared for transmission EM. A representative cross section showing ingested F. novicida cells contained within phagosomal membranes is shown (magnification, ×28,000).
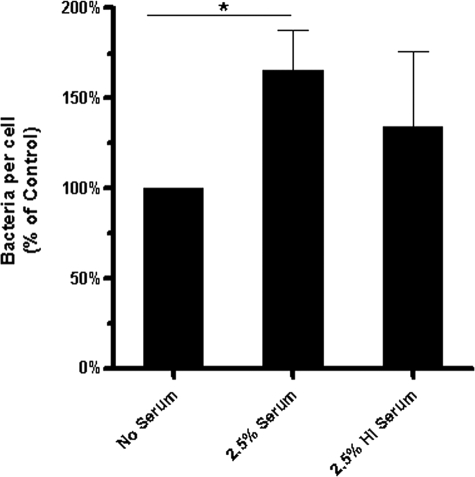
To determine the contribution of heat-labile components in serum such as complement in Francisella cell association, GFP-expressing F. novicida and wild-type F. novicida were incubated with MDM in the presence of 2.5% fresh or HI serum (Fig. 3). Heat inactivation of serum resulted in a level of bacterial association that was lower than the fresh-serum condition but still higher than that of the no-serum condition, suggesting that both heat-labile and stable components of serum such as complement and antibody, respectively, are important in bacterial association. However, there was an unexpectedly large variation in the level of bacterial association in the presence of HI serum in individual experiments, suggesting that the contribution of heat-stable serum components to cell association was donor dependent (see below).
FIG. 3.
Heat-labile and stable components of serum contribute to Francisella association with MDM. GFP-expressing F. novicida or wild-type F. novicida cells were opsonized in media alone, 2.5% serum, or 2.5% HI serum for 30 min at 37°C. Bacteria were then added to MDM monolayers for 2 h at an MOI of 50:1. Cells were fixed and, in the case of wild-type F. novicida, permeabilized and immunostained, and the bacteria per MDM were enumerated by fluorescence microscopy. One hundred fifty to 200 consecutive MDM on each coverslip of triplicate coverslips/test group were counted. Results shown are the means ± SEM from three independent experiments. *, P<0.05 (paired one-tailed Student t test).
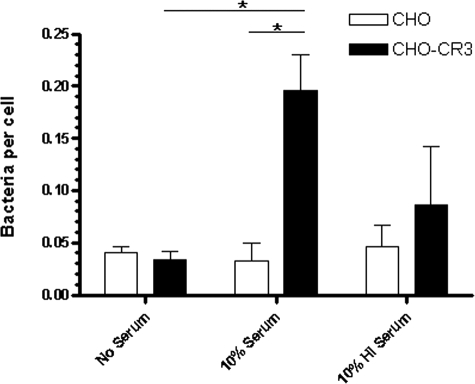
The fresh-serum-dependent increase in F. novicida association with MDM was consistent with a role for CR3 in host cell recognition. To explore this further, CHO-CR3 cells or wild-type control CHO cells were incubated with F. novicida cells that were preopsonized in 10% fresh or HI serum or in medium alone (mock preopsonized). Bacteria per cell were enumerated by indirect immunofluorescence microscopy. There was a 5.91-fold ± 1.02-fold increase in the association of serum-preopsonized bacteria with CHO-CR3 cells compared to mock-preopsonized F. novicida (n = 2, P < 0.05) (Fig. 4). In contrast, preopsonization of F. novicida with HI serum did not significantly increase bacterial association with CHO-CR3 cells compared to the mock-preopsonized group (1.84-fold ± 1.27-fold increase, P > 0.05). Bacterial association with wild-type CHO cells was negligible. Taken together, our data provide supportive evidence that F. novicida is opsonized with complement C3 in serum, which in turn increases opsonophagocytosis by macrophage CR such as CR3.
FIG. 4.
CR3 is important in the association of Francisella. Wild-type CHO and CHO-CR3 cells were adhered to glass coverslips overnight and then incubated with F. novicida cells that had been preopsonized in media alone, 10% fresh serum, or 10% HI serum at MOIs of 100:1 and 50:1. After fixation, permeabilization, and immunostaining, >500 consecutive cells per coverslip were counted and enumerated by immunofluorescence microscopy. Results shown are means ± SEM from two independent experiments. *, P < 0.05 (unpaired two-tailed Student t test).
Fcγ receptors are involved in the recognition of F. novicida by macrophages.
As noted above, there was a relatively high degree of variability in the level of bacterial association of F. novicida by MDM in the presence of HI serum. This suggested that, for some donors, heat-stable opsonins such as antibody play a role in mediating the recognition of bacteria via FcγR. Donors had no history of tularemia or known exposure to F. tularensis.
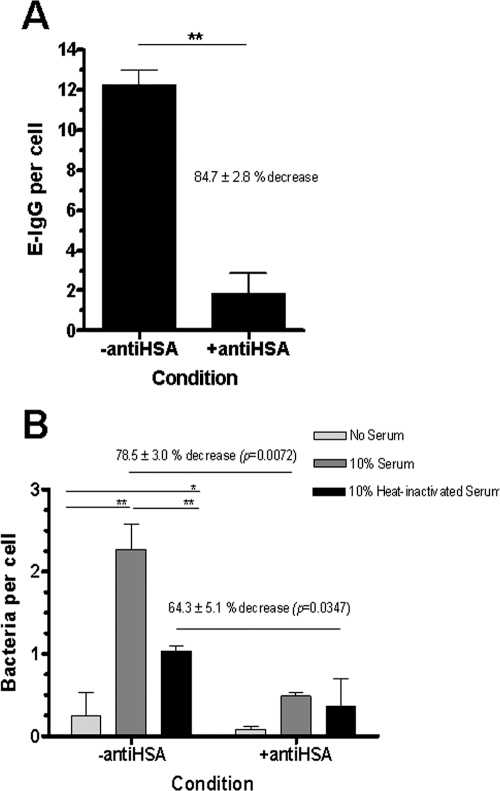
In order to ascertain the role of macrophage FcγR in the association of Francisella, MDM were plated on immune complexes composed of HSA and anti-HSA to down-modulate all three classes of FcγR (CD64, -32, and -16) to the undersurface of the cell (35, 44). Control MDM were placed on a substrate of HSA only. Down-modulation of FcγR was confirmed by incubation of E-IgG with MDM. There was an 84.7% ± 2.8% decrease in E-IgG binding to MDM on immune complexes, confirming efficient down-modulation of FcγR (Fig. 5A). Next, F. novicida that had been preopsonized in 10% fresh serum, HI serum, or media alone was added to MDM with or without FcγR down-modulation. After 2 h the MDM were washed and fixed, and bacterial association was enumerated by fluorescence microscopy (Fig. 5B). As expected, for MDM without down-modulation of FcγR, fresh-serum-preopsonized F. novicida demonstrated a marked increase in association while there was also a smaller but significant increase in association of bacteria that had been preopsonized in HI serum (Fig. 5B). FcγR down-modulation led to a significant decrease in the association of fresh-serum- and, in some cases, HI-serum-preopsonized bacteria (Fig. 5B and Table 1). After FcγR down-modulation there was little difference between the association of fresh-serum-preopsonized bacteria and that of HI-serum-preopsonized bacteria (Fig. 5B, right bars, and Table 1). Cumulative data showed a modest decrease in the association of HI-serum-preopsonized bacteria after FcγR down-modulation (Table 1), consistent with the variation seen from donor to donor. Thus, these data indicate that optimal opsonophagocytosis of F. novicida occurs via both functional CR and FcγR, that there is likely to be cooperativity between them in bacteria uptake, and, finally, that involvement of FcγR is donor dependent.
FIG. 5.
FcγR on human macrophages mediate the association of Francisella. MDM were adhered to coverslips that had been coated with HSA (control) or HSA and rabbit anti-HSA antibody to down-modulate FcγR. (A) E-IgG were incubated with control or FcγR-down-modulated MDM for 1 h at an MOI of 10:1. After fixation, E-IgG per MDM were counted by light microscopy. A representative experiment is shown (means ± standard deviations [SD] of triplicate wells). **, P = 0.0025 (paired two-tailed Student t test). (B) GFP-expressing F. novicida cells were preopsonized in media alone, 10% fresh serum, or 10% HI serum and added to control or FcγR-down-modulated MDM for 2 h at an MOI of 500:1. After fixation, GFP-expressing F. novicida cells per MDM were counted by fluorescence microscopy. Results shown are from one representative experiment (n = 4). Means ± SD of triplicate wells are shown. *, P < 0.01; **, P < 0.001 (calculated by two-way analysis of variance comparing multiple samples and Bonferroni posttests). External comparisons, e.g., “10% serum, −antiHSA” versus “10% serum, +antiHSA,” were made using a two-tailed Student t test, with P values as indicated.
TABLE 1.
FcγR contribute to the association of Francisella with human macrophagesa
| Cell treatment | No. of bacteria/cell after preopsonization with:
|
||
|---|---|---|---|
| No serum | 10% fresh serum | 10% HI serum | |
| No anti-HSA | 1.00 | 4.44 ± 1.71 | 2.55 ± 1.28 |
| HSA + anti-HSA | 1.00 | 2.02 ± 1.34** | 2.43 ± 1.78 |
MDM were adhered to coverslips that had been coated with HSA (control) or HSA and rabbit anti-HSA antibody to down-modulate FcγR. Results shown are normalized to the no-serum group for both the control and FcγR-down-modulated test groups. Results are means ± SEM from four independent experiments, each with triplicate wells per group. **, P = 0.0086 (paired two-tailed Student t test) compared to “10% serum, no-anti-HSA” case.
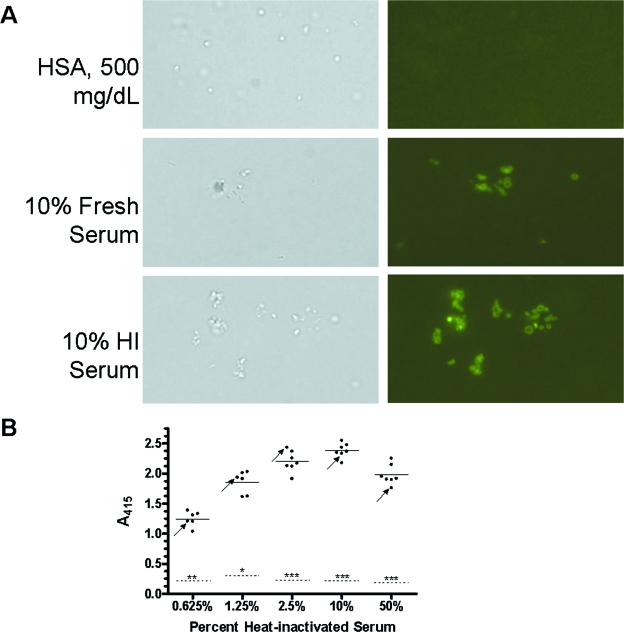
Antibody in nonimmune human serum recognizes F. novicida.
Given the variability in HI-serum-dependent bacterial association with MDM and the role for FcγR, we next sought to determine whether nonimmune human serum contained antibody to F. novicida. Bacteria were incubated in various fresh and HI sera from nonimmune donors or HSA control, washed, and then incubated with a FITC-conjugated anti-human IgG, IgA, or IgM antibody to detect the presence of antibody on the bacterial surface. While there was no fluorescence seen in the HSA group, serum-opsonized bacteria were all brightly fluorescent (Fig. 6A). To quantify the amount of antibody in nonimmune serum to F. novicida, serum- or HSA-incubated bacteria were analyzed by a whole-cell ELISA. Sera were from seven donors (six who had no known contact with any Francisella species and one who had a history of cutaneous F. tularensis infection). There was a dose-dependent increase in detectable antibody (Fig. 6B, fresh serum data not shown). In 10% HI serum, there was a 12.5-fold ± 1.2-fold increase in absorbance relative to the HSA group (P < 0.0005). These data show that there is antibody to F. novicida even in low concentrations of nonimmune human serum which can mediate opsonophagocytosis of F. tularensis by human macrophages.
FIG. 6.
Antibody in nonimmune human serum recognizes F. novicida. F. novicida cells (1 × 108) were incubated with various concentrations of fresh nonimmune human serum, HI serum, or HSA control for 30 min at 37°C, washed, and blocked with 3% ovalbumin. (A) Triplicate samples were then incubated with FITC-conjugated anti-human IgG, IgA, or IgM antibody, washed, and placed on slides. The presence or absence of fluorescent bacteria was assessed by immunofluorescence microscopy. Approximately 10 fields per coverslip were examined in each group. The photomicrographs shown are representative high-power fields (×1,000). (B) Serum- or HSA-treated samples (1 × 107 bacteria, in triplicate) were dried in ELISA plate wells overnight. Wells were then blocked, washed, and incubated with HRP-conjugated anti-human IgG, IgA, or IgM antibody. Subsequently, wells were incubated with HRP substrate and reactions were terminated with 2% oxalic acid. A415 was measured. The results from six or seven donors, one immune donor (arrows) and five or six nonimmune donors, are shown. Each dot represents the mean of triplicate wells for each donor. The solid horizontal lines are the means for donors, and the dashed horizontal lines are the means for the HSA groups. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 (paired two-tailed Student t test for HSA relative to the serum groups). The immune donor's result was excluded from the statistical comparisons.
The MR is a mediator of nonopsonic recognition of F. novicida by human macrophages.
Although serum increases the association of F. novicida with macrophages, bacteria associate in the absence of serum opsonins. The MR is a C-type lectin PRR that is abundantly expressed on macrophages, particularly AM, and plays an important role in the uptake of pathogens (19, 42, 49). To determine the contribution of the MR in Francisella recognition, MDM were preincubated with soluble mannan, a competitive inhibitor of the MR (42). These experiments were performed in the presence and absence of 2.5% fresh serum to assess the impact of serum opsonins on MR involvement. In the absence of serum, mannan preincubation resulted in a significant decrease in the association of F. novicida with MDM when compared to the control group (Fig. 7A). When serum was present, mannan resulted in a 30.2% ± 11.6% (n = 4, P = 0.081) decrease in bacterial association (Fig. 7B). These results are consistent with involvement of the MR, which appears to play a relatively more important role in the absence of serum opsonins.
FIG. 7.
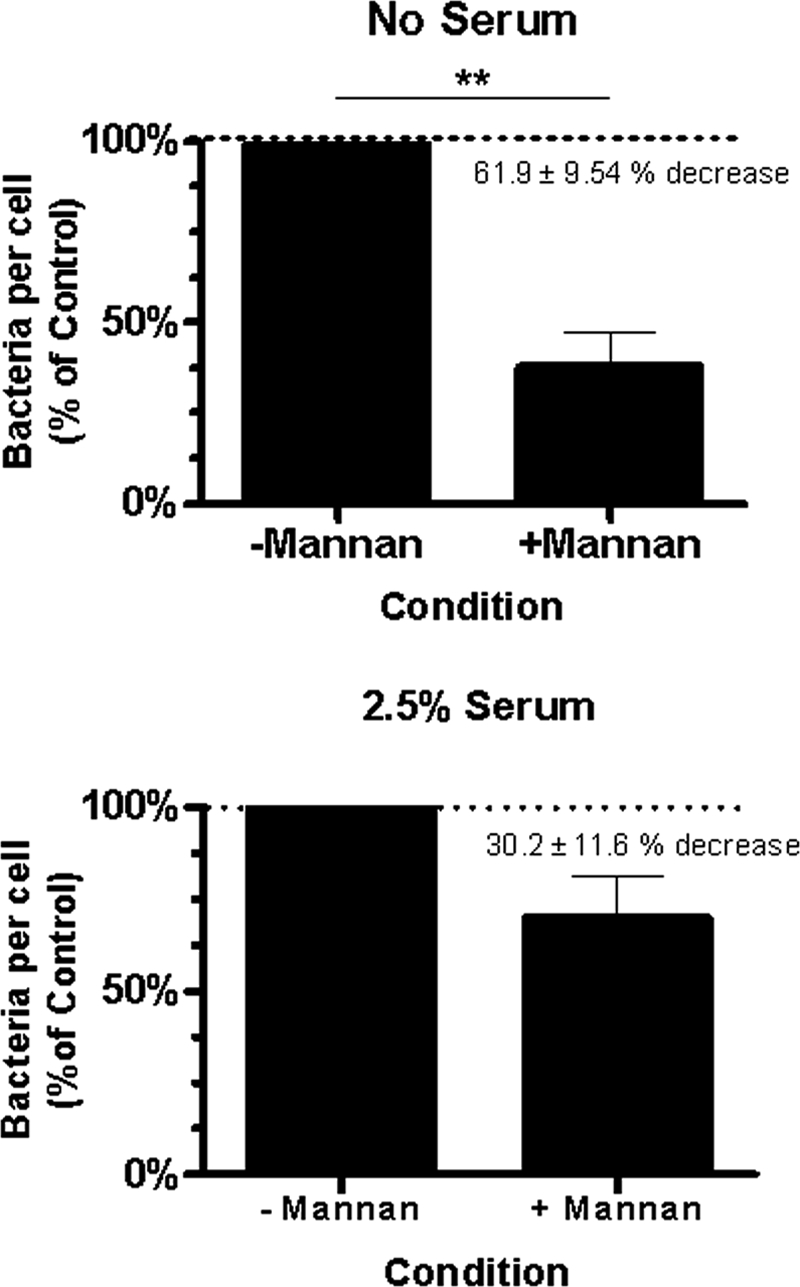
The macrophage MR mediates nonopsonic association of F. novicida. MDM monolayers on glass coverslips were preincubated with either soluble mannan or media alone for 30 min at 37°C in 5% CO2 and then incubated with GFP-expressing F. novicida at an MOI of 600:1 for 2 h. After fixing, F. novicida cells per MDM were enumerated using fluorescence microscopy. Infections were performed in the absence (A) and presence (B) of 2.5% autologous serum. Results from four independent experiments are shown (means ± SEM). **, P = 0.0074 (paired two-tailed Student t test).
SP-A preopsonization leads to increased association of F. novicida with macrophages.
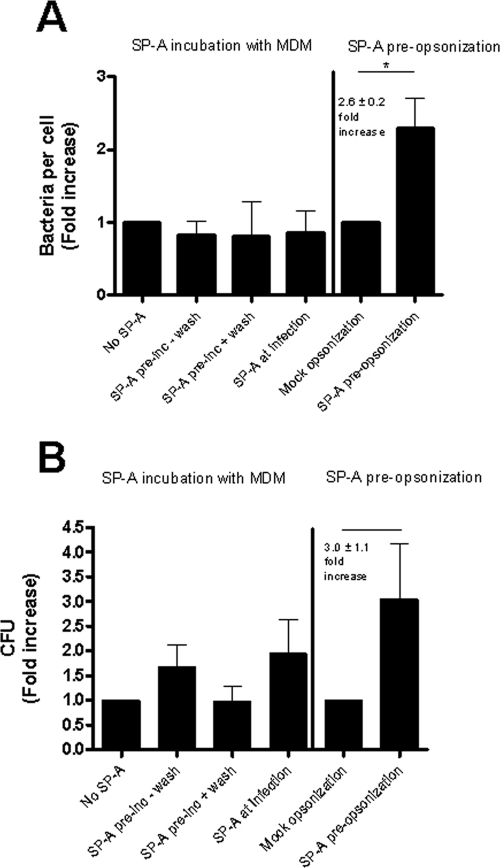
Macrophages in the lung are bathed in and ingest SP-A, an abundant surfactant protein that plays an important role in the recognition of pathogens by macrophages (55). SP-A can enhance phagocytosis by various mechanisms: as a direct opsonin (34), as an activator of phagocytosis via its own receptor (55), or as an up-regulator of other PRR, such as scavenger receptor A (32) or the MR (5). As Francisella causes severe infection when it enters via the airborne route, we sought to determine whether SP-A plays an immunoregulatory role in macrophage receptor-mediated association of F. novicida. In the first set of experiments, MDM were incubated without SP-A (control condition) or with SP-A either before infection or at the time of infection. SP-A had no detectable effect on bacterial association when MDM were preincubated with SP-A or when SP-A was added concomitantly with Francisella (Fig. 8A). However, it did have a discernible effect on bacterial viability under these conditions (Fig. 8B). There were 1.91-fold ± 0.29-fold and 2.35-fold ± 0.46-fold increases in viability when SP-A was added to MDM before or at the time of addition of bacteria, respectively. Importantly, the increase in viability was lost when MDM that had been preincubated with SP-A were washed prior to the addition of bacteria (Fig. 8B). This result provided a clue that the effect of SP-A was primarily a result of an interaction between SP-A and the bacterium.
FIG. 8.
Preopsonization of F. novicida with SP-A increases the association of bacteria with MDM. MDM monolayers on glass coverslips were incubated without (control) or with SP-A before or at the time of infection with GFP-expressing F. novicida at an MOI of 600:1. As indicated, some MDM monolayers were washed prior to adding bacteria. After 2 h and a washing, MDM were fixed and bacteria per MDM were enumerated using fluorescence microscopy (A, left four bars). Results shown are the means ± SEM normalized to the “no-SP-A” group (n = 2). (A, right two bars) GFP-expressing F. novicida cells were preopsonized with or without SP-A and added to MDM. After 2 h and a washing, MDM were fixed and bacteria per MDM were enumerated using fluorescence microscopy. Results shown are the means ± SEM normalized to the “mock-opsonization” group (n = 3). *, P = 0.0107 (paired two-tailed Student t test). (B) SP-A treatment of MDM and bacteria was identical to that for panel A. After 2 h and a washing, extracellular bacteria were killed with gentamicin and CFU of MDM lysates were obtained. Results shown are the means ± SEM from three independent experiments.
Next, F. novicida was preincubated with SP-A and washed prior to its addition to MDM. Preincubation of F. novicida with SP-A led to a 2.6-fold ± 0.2-fold (n = 3, P = 0.0107) increase in bacterial association compared to control bacteria (Fig. 8A), providing further evidence that the effect of SP-A was primarily the consequence of an interaction between SP-A and the bacterium. SP-A preincubation also led to a 3.0-fold ± 1.1-fold increase (n = 3) in the survival in MDM compared to mock-preincubated bacteria. Taken together, these data indicate that SP-A serves as a bacterial opsonin for Francisella, a finding that is of particular importance for bacterial uptake by macrophages in the alveoli of the lung.
DISCUSSION
F. tularensis has a short incubation period and is readily transmissible to humans from mammalian or arthropod vectors. It is highly virulent, with a 50% lethal dose of 0.5 to 4 organisms (6), and the rapidity of onset of clinical disease, especially of the pneumonic form, suggests a primary failure of the innate arm of immunity to control initial infection in this locale. This observation suggests that macrophages in the lung are particularly permissive for F. tularensis. In phagocytosing microbial pathogens, macrophage receptors bind to pathogen-associated ligands, which in turn leads to specific macrophage functions. Since F. tularensis is an intracellular pathogen of macrophages, knowledge of the phagocytic pathway will be important for understanding disease pathogenesis. Our data extend recent observations on the role of complement C3 and CR, particularly CR3, in the phagocytosis of F. tularensis by demonstrating the importance of this pathway for the genetically related F. novicida. Further, we provide the first evidence for the role of antibody and FcγR in phagocytosis. Finally, our data support involvement of the macrophage MR and SP-A in phagocytosis and intracellular survival, two important C-type lectin PRR that are highly active in the lung alveolus.
Our study highlights significant differences in the macrophage interaction between F. novicida and the LVS. We found a nearly 60% decrease in bacterial association of the LVS with MDM compared to F. novicida. To our knowledge this is the first report of the difference in recognition between F. novicida and the LVS by human macrophages. Growth of F. novicida and that of the LVS have been compared in mouse peritoneal and bone marrow-derived macrophages, as well as in peritoneal macrophages from rats and guinea pigs (4). In that report it was found that, despite similar growth curves for mouse and guinea pig macrophages, F. novicida had 100-fold less growth in rat macrophages at 24 h. Interestingly, rats are relatively resistant to death by infection with both F. tularensis subsp. tularensis and F. novicida. The authors suggested that a possible reason for the differences between the LVS and F. novicida was in receptor-ligand interactions.
The LVS has recently been reported to infect human macrophages at a considerably lower MOI than we used (8). However, in that report the investigators used centrifugation to enhance the interaction between bacteria and macrophages and their results were reported in terms of the percentage of macrophages infected, not distinguishing between heavy and light infections of individual cells. We used a higher MOI without centrifugation to more closely simulate physiologic conditions and to enable quantification of differences in the number of bacteria per cell. Of interest, the authors showed that LVS-infected human macrophages expressed more interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α) than murine macrophages. In contrast, the LVS has been shown to inhibit TNF-α and IL-1β production in a murine macrophage-like cell line (50). One can speculate that, given the anti-inflammatory phenotype of the LVS in murine macrophages and its virulence in mice, the proinflammatory phenotype of the LVS in human macrophages might contribute to its effective control and limited virulence in humans. Given that phagosomes containing both Francisella tularensis subspecies show limited phagosome-lysosome fusion (4), the differences in cytokine production by macrophages infected with F. novicida and the LVS may be a result of earlier events such as those occurring during receptor-mediated phagocytosis. Together, our data suggest that F. novicida may be a better model bacterium for studying human macrophage interactions.
We found increases in bacterial association with MDM compared to monocytes, indicating that receptors which recognize Francisella are more abundantly or newly represented on mature macrophages compared to monocytes. Monocytes express less CR3 (CD11b/CD18) and do not express CR4 (CD11c/CD18) or the MR (36, 48). CR3 and CR4 bind to iC3b-coated targets, an interaction which is sufficient to trigger phagocytosis (29). CR are important in mediating the uptake of several intracellular pathogens (7, 9, 37, 45, 46).
Experiments with CHO-CR3 cells confirm that CR3 is a major receptor involved in host cell recognition of Francisella. These findings are consistent with recently published work by Clemens et al. in which the authors used antibody blocking strategies and C3-depleted serum to provide evidence for a role for C3 and CR3 in the phagocytosis of a clinical isolate of F. tularensis and the LVS (12). The same authors reported that serum was a prerequisite for entry into human macrophages (11). Our findings provide evidence that despite a marked increase in phagocytosis in the presence of serum, serum opsonization is not obligatory for macrophage entry, especially at higher MOIs. However, increased association of F. novicida with MDM in the presence of serum correlated with increased survival; thus opsonophagocytosis enhances the ability of this pathogen to reach its intracellular niche.
It is clear that heat-labile components in serum such as complement are important in Francisella uptake by macrophages. However, during the course of our studies we noted that there was marked variability in the level of association of F. novicida with macrophages in the presence of HI serum, which is devoid of complement activity. This finding was donor dependent, and this variability almost always led to a level of association greater than that seen in the absence of serum (Fig. 3). These studies support involvement of antibody in uptake. With removal of available macrophage FcγR (35, 44), we observed a marked decrease in association of bacteria that had been preopsonized in both fresh serum with functional complement and antibody and, in most cases, HI serum containing only functional antibody. FcγR down-modulation reduced the association of fresh-serum-preopsonized bacteria almost to the level seen with bacteria that had been preopsonized in HI serum. This result indicates that CR and FcγR cooperate during phagocytosis.
Our data provide evidence that nonimmune human serum contains variable amounts and types of circulating anti-Francisella antibody. Although this could be taken for evidence of the presence of natural cross-reactive antibody which mediates opsonophagocytosis by activating complement, natural antibody is typically inefficient in engaging FcγR (29, 45). Francisella seroprevalence has been reported infrequently but has ranged from 0.19% in an unexposed human population (25) to 9.1% in a small group of people with an occupational risk for contracting Francisella (18). Surprisingly, all of our donors had detectable antibody to Francisella, despite none having had a prior infection or known exposure to Francisella tularensis subspecies. Our findings suggest that exposure to related environmentally derived Francisella species with conserved antigenic epitopes and consequent positive Francisella serology may be higher than what has been reported. In support of the role of antibody and FcγR, neutrophils were unable to phagocytose Francisella in the absence of immune serum (38).
On human phagocytes, there are three general classes of FcγR with different affinities and functions: Fcγ RI (CD64), Fcγ RII (CD32), and Fcγ RIII (CD16) (39). FcγR function as either activating or inhibitory receptors depending on the presence of either an Ig tyrosine-activating motif or an Ig tyrosine-inhibitory motif sequence in their respective cytosolic domains (39). We nonspecifically down-modulated all three classes of FcγR to study their contribution to opsonophagocytosis. Further definition of the antibody types and FcγR involved in the phagocytosis of Francisella as well as the downstream effects of Francisella-mediated FcγR ligation awaits future studies.
Nonopsonic phagocytosis of microbes occurs via the MR and other surface lectins, CR3 and other integrins, and scavenger receptors (51). A number of pathogens have been shown to be recognized by the MR (31, 42, 49, 56). It is a prototypic PRR C-type lectin which binds terminal mannose, fucose, and N-acetylgalactosamine residues (2); mediates both recycling endocytosis and phagocytosis of larger particles (51); and may preferentially enhance the ability of certain pathogens to avoid standard killing mechanisms in macrophages (30). Our results are consistent with involvement of the MR in phagocytosis, particularly in the absence of serum opsonins. Francisella binding to the MR implicates specific constituents of the Francisella cell wall in this interaction such as the core regions of the lipopolysaccharides of Francisella subspecies, which are mannose containing (52). In addition, the capsule of Francisella may contain mannose. AM and human lung dendritic cells express significant MR activity, suggesting a possible mechanism for the increased pulmonary susceptibility to Francisella (13).
We found that preincubation of F. novicida with SP-A led to a marked increase in association and intracellular survival of the bacterium in macrophages. Preincubation of macrophages with SP-A had lesser effects and only when the protein was present during the time the bacteria were added. These findings indicate that SP-A functions primarily as a bacterium-bound ligand for Francisella in enhancing phagocytosis. Together with the MR results, these data point towards the importance of Francisella cell wall carbohydrates in mediating interactions with C-type lectins enabling host cell recognition.
Defining the molecular mechanisms of host-pathogen interactions is important for understanding the early events which lead to the success or failure of an innate immune response. In this study we have elucidated major receptor-ligand pathways that are involved in the recognition and survival of Francisella in primary human macrophages, including those highly active in the lung alveolus. Future studies will be aimed at linking these pathways to regulation of intracellular trafficking and triggering of bactericidal and cytokine responses. Better characterization of these pathways will lead to identifying molecular targets for drug design as well as to revealing potential candidate antigens for vaccine discovery.
Acknowledgments
This work was supported by the National Institute for Allergy and Infectious Diseases, grants T32 0155411 (A.B.), U56AI057164 (L.S.S. and J.S.G.), and U54AI057156 (J.S.G.).
We thank Joy Crowther for the purification of SP-A, Abul Azad for his assistance in working with CHO cells, Bridget Vesosky for assistance with flow cytometry and analysis, Jordi Torrelles for his assistance in editing the manuscript, Michail Gavrilin for assistance in obtaining serum from a donor with a history of tularemia, and Karen Elkins and Fran Nano for providing Francisella strains.
Editor: J. T. Barbieri
REFERENCES
- 1.Abd, H., T. Johansson, I. Golovliov, G. Sandstrom, and M. Forsman. 2003. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 69:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allavena, P., M. Chieppa, P. Monti, and L. Piemonti. 2004. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit. Rev. Immunol. 24:179-192. [DOI] [PubMed] [Google Scholar]
- 3.Alluisi, E. A., W. R. Beisel, P. J. Bartelloni, and G. D. Coates. 1973. Behavioral effects of tularemia and sandfly fever in man. J. Infect. Dis. 128:710-717. [DOI] [PubMed] [Google Scholar]
- 4.Anthony, L. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beharka, A. A., C. D. Gaynor, B. K. Kang, D. R. Voelker, F. X. McCormack, and L. S. Schlesinger. 2002. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J. Immunol. 169:3565-3573. [DOI] [PubMed] [Google Scholar]
- 6.Bell, J. F., C. R. Owen, and C. L. Larson. 1955. Virulence of Bacterium tularense. I. A study of the virulence of Bacterium tularense in mice, guinea pigs, and rabbits. J. Infect. Dis. 97:162-166. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell, J., R. A. B. Ezekowitz, M. B. Roberts, J. Y. Channon, R. B. Sim, and S. Gordon. 1985. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J. Exp. Med. 162:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolger, C. E., C. A. Forestal, J. K. Italo, J. L. Benach, and M. B. Furie. 2005. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukoc. Biol. 77:893-897. [DOI] [PubMed] [Google Scholar]
- 9.Bullock, W. E., and S. D. Wright. 1987. Role of the adherence-promoting receptors, CR3, LFA-1, and p150, 95, in binding of Histoplasma capsulatum by human macrophages. J. Exp. Med. 165:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarridge, J. E., III, T. J. Raich, A. Sjosted, G. Sandstrom, R. O. Darouiche, R. M. Shawar, P. R. Georghiou, C. Osting, and L. Vo. 1996. Characterization of two unusual clinically significant Francisella strains. J. Clin. Microbiol. 34:1995-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2005. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect. Immun. 73:5892-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochand, L., P. Isler, F. Songeon, and L. P. Nicod. 1999. Human lung dendritic cells have an immature phenotype with efficient mannose receptors. Am. J. Respir. Cell Mol. Biol. 21:547-554. [DOI] [PubMed] [Google Scholar]
- 14.Crouch, E. C. 1998. Collectins and pulmonary host defense. Am. J. Respir. Cell Mol. Biol. 19:177-201. [DOI] [PubMed] [Google Scholar]
- 15.Crowther, J. E., V. K. Kutala, P. Kuppusamy, J. S. Ferguson, A. A. Beharka, J. L. Zweier, F. X. McCormack, and L. S. Schlesinger. 2004. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J. Immunol. 172:6866-6874. [DOI] [PubMed] [Google Scholar]
- 16.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 17.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman, K. A., D. Stiles-Enos, K. Julian, B. T. Matyas, S. R. Telford III, M. C. Chu, L. R. Petersen, and E. B. Hayes. 2003. Tularemia on Martha's Vineyard: seroprevalence and occupational risk. Emerg. Infect. Dis. 9:350-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton, M. J., L. W. Riley, and L. S. Schlesinger. 2005. Receptor-mediated recognition of Mycobacterium tuberculosis by host cells, p. 405-426. In S. T. Cole, K. D. Eisenach, D. N. McMurray, and W. R. Jacobs, Jr. (ed.), Tuberculosis and the tubercle bacillus. ASM Press, Washington, D.C.
- 20.Ferguson, J. S., and L. S. Schlesinger. 2000. Pulmonary surfactant in innate immunity and the pathogenesis of tuberculosis. Tubercle Lung Dis. 80:173-184. [DOI] [PubMed] [Google Scholar]
- 21.Forsman, M., G. Sandstrom, and A. Sjostedt. 1994. Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. Int. J. Syst. Bacteriol. 44:38-46. [DOI] [PubMed] [Google Scholar]
- 22.Gardai, S. J., Y. Q. Xiao, M. Dickinson, J. A. Nick, D. R. Voelker, K. E. Greene, and P. M. Henson. 2003. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13-23. [DOI] [PubMed] [Google Scholar]
- 23.Gaynor, C. D., F. X. McCormack, D. R. Voelker, S. E. McGowan, and L. S. Schlesinger. 1995. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J. Immunol. 155:5343-5351. [PubMed] [Google Scholar]
- 24.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjostedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez, M. P., M. A. Bratos, J. I. Garrote, A. Duenas, A. Almaraz, R. Alamo, M. H. Rodriguez, M. J. Rodriguez Recio, M. F. Munoz, A. Orduna, and A. Rodriguez-Torres. 2003. Serologic evidence of human infection by Francisella tularensis in the population of Castilla y Leon (Spain) prior to 1997. FEMS Immunol. Med. Microbiol. 35:165-169. [DOI] [PubMed] [Google Scholar]
- 26.Hollis, D. G., R. E. Weaver, A. G. Steigerwalt, J. D. Wenger, C. W. Moss, and D. J. Brenner. 1989. Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J. Clin. Microbiol. 27:1601-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz, M. A. 1984. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell 36:27-33. [DOI] [PubMed] [Google Scholar]
- 28.Horwitz, M. A., and S. C. Silverstein. 1980. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J. Clin. Investig. 65:82-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janeway, C. A., P. Travers, M. J. Walport, and J. D. Capra. 1999. Immunobiology. Current Biology Publications, New York, N.Y.
- 30.Kang, B. K., A. K. Azad, J. B. Torrelles, T. M. Kaufman, A. A. Beharka, E. Tibesar, L. E. Desjardin, and L. S. Schlesinger. 2005. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202:987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo, K., H. Sano, H. Takahashi, K. Kuronuma, S. Yokota, N. Fujii, K. Shimada, I. Yano, Y. Kumazawa, D. R. Voelker, S. Abe, and Y. Kuroki. 2004. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J. Immunol. 172:7592-7602. [DOI] [PubMed] [Google Scholar]
- 32.Kuronuma, K., H. Sano, K. Kato, K. Kudo, N. Hyakushima, S. Yokota, H. Takahashi, N. Fujii, H. Suzuki, T. Kodama, S. Abe, and Y. Kuroki. 2004. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J. Biol. Chem. 279:21421-21430. [DOI] [PubMed] [Google Scholar]
- 33.Lindgren, H., I. Golovliov, V. Baranov, R. K. Ernst, M. Telepnev, and A. Sjostedt. 2004. Factors affecting the escape of Francisella tularensis from the phagolysosome. J. Med. Microbiol. 53:953-958. [DOI] [PubMed] [Google Scholar]
- 34.McNeely, T. B., and J. D. Coonrod. 1994. Aggregation and opsonization of type A but not type B Haemophilus influenzae by surfactant protein A. Am. J. Respir. Cell Mol. Biol. 11:114-122. [DOI] [PubMed] [Google Scholar]
- 35.Michl, J., M. M. Pierczorka, J. C. Unkeless, and S. C. Silverstein. 1979. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycolate-elicited mouse peritoneal macrophages. J. Exp. Med. 150:607-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myones, B. L., J. G. Dalzell, N. Hogg, and G. D. Ross. 1988. Neutrophil and monocyte cell surface p150,955 has iC3b-receptor (CR4) activity resembling CR3. J. Clin. Investig. 82:640-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne, N., and M. A. Horwitz. 1987. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J. Exp. Med. 166:1377-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proctor, R. A., J. D. White, E. Ayala, and P. G. Canonico. 1975. Phagocytosis of Francisella tularensis by Rhesus monkey peripheral leukocytes. Infect. Immun. 11:146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 40.Santic, M., M. Molmeret, K. E. Klose, S. Jones, and Y. A. Kwaik. 2005. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell. Microbiol. 7:969-979. [DOI] [PubMed] [Google Scholar]
- 41.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702-714. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 43.Schlesinger, L. S. 1996. Role of mononuclear phagocytes in M. tuberculosis pathogenesis. J. Investig. Med. 44:312-323. [PubMed] [Google Scholar]
- 44.Schlesinger, L. S., C. G. Bellinger-Kawahara, N. R. Payne, and M. A. Horwitz. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771-2780. [PubMed] [Google Scholar]
- 45.Schlesinger, L. S., and M. A. Horwitz. 1990. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J. Clin. Investig. 85:1304-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlesinger, L. S., and M. A. Horwitz. 1991. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1(CD35), CR3(CD11b/CD18), and CR4(CD11c/CD18) and interferon gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147:1983-1994. [PubMed] [Google Scholar]
- 47.Schlesinger, L. S., and M. A. Horwitz. 1994. A role for natural antibody in the pathogenesis of leprosy: antibody in nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect. Immun. 62:280-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speert, D. P., and S. C. Silverstein. 1985. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J. Leukoc. Biol. 38:655-658. [DOI] [PubMed] [Google Scholar]
- 49.Taylor, P. R., L. Martinez-Pomares, M. Stacey, H. H. Lin, G. D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901-944. [DOI] [PubMed] [Google Scholar]
- 50.Telepnev, M., I. Golovliov, T. Grundstrom, A. Tarnvik, and A. Sjostedt. 2003. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-α and IL-1 from murine macrophages. Cell. Microbiol. 5:41-51. [DOI] [PubMed] [Google Scholar]
- 51.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]
- 52.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 53.Whipp, M. J., J. M. Davis, G. Lum, J. de Boer, Y. Zhou, S. W. Bearden, J. M. Petersen, M. C. Chu, and G. Hogg. 2003. Characterization of a novicida-like subspecies of Francisella tularensis isolated in Australia. J. Med. Microbiol. 52:839-842. [DOI] [PubMed] [Google Scholar]
- 54.White, J. D., J. R. Rooney, P. A. Prickett, E. B. Derrenbacher, C. W. Beard, and W. R. Griffith. 1964. Pathogenesis of experimental respiratory tularemia in monkeys. J. Infect. Dis. 114:277-283. [DOI] [PubMed] [Google Scholar]
- 55.Wright, J. R. 2005. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 5:58-68. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, J., J. Zhu, A. Imrich, M. Cushion, T. B. Kinane, and H. Koziel. 2004. Pneumocystis activates human alveolar macrophage NF-κB signaling through mannose receptors. Infect. Immun. 72:3147-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]